- *Corresponding Author:
- S Sahoo
University Department of Pharmaceutical Sciences, Utkal University, Bhubaneswar-751 004 India
E-mail: sabujbiotech@rediffmail.com
| Date of Submission | 01 June 2005 |
| Date of Revision | 23 November 2005 |
| Date of Acceptance | 09 October 2006 |
| Indian J. Pharm. Sci., 2006, 68 (5): 653-655 |
Abstract
Hybanthus enneaspermus Muell, belonging to family Violaceae, was investigated to evaluate in vitro antibacterial activity of aqueous, ethanolic, petroleum ether and chloroform extracts against Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Enterococcus faecalis and Staphylococcus aureus . The major urinary tract infection causing pathogens were tested by disc diffusion assay method, and the minimum inhibitory concentration was evaluated. Ethanol (95%) extract exhibited significant and broader spectrum of inhibition in comparison to aqueous, which showed moderate effect; chloroform and petroleum ether extract showed feeble activity at concentration of 300 mg/disc. An attempt has been made to compare the activity of extracts with standard antibiotics against selected urinary tract pathogens.
Hybanthus enneaspermus Muell, belonging to family Violaceae, is a herb or under shrub distributed in the tropical and subtropical regions of the world. It is a herb, often with woody troches, found in the warmer parts of India. The plant is popularly known as Ratanpurus (Hindi). An infusion of the plant extract is given in case of cholera [1]. The plant has been reported to have antiinflammatory [2], antitussive [3], antiplasmodial [4], anticonvulsant [5] and free radical scavenging activity [5]. The plant is reported to contain aurantiamide acetate, isoarborinol, b-sitosterol and triterpene [6]. In folklore the plant is used in case of pregnant and parturient women, and in case of gonorrhoea and urinary infections. The present study is intended to determine the antibacterial activity of the plant against selected urinary tract pathogens and compare it with eight standard drugs frequently used in the treatment of urinary tract infections.
The plant was collected from the rural belt of Bhubaneswar, Orissa. The plant was authenticated in the Department of Botany, Utkal University, Bhubaneswar. The plant was collected in bulk and washed with tap water to remove the soil and dirt particles and then shade dried. The dried plant materials were milled into coarse powder by a mechanical grinder. The powdered crude drug was successively extracted with petroleum ether, chloroform, ethanol (95%) and water by using Soxhlet extractor. The extracts were concentrated to dryness under vacuum. The in vitro screening was carried out using selected urinary tract infection (UTI) causing pathogens, which include two gram-positive bacteria (Staphylococcus aureus, Enterococcus faecalis) and four gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis). These organisms were identified by following the standard microbiological methods [9]. Five strains each of six UTI-causing bacterial species were used in this study, which were obtained from Post Graduate Department of Microbiology, Orissa University of Agricultural Technology, Bhubaneswar, Orissa. The extracts were screened for their antibacterial activity using disc diffusion method [7-8]. The ethanol (95%), aqueous and chloroform extracts were dissolved in dimethyl formamide (6%), which was previously tested for antibacterial activity against all test bacteria and found to have no antibacterial activity. Petroleum ether extract was solubilized in a mixture of dimethyl formamide and a surfactant SDS (sodium dodecyl sulphate-2%) to make 30 mg/ml solution and finally sterilized by filtration using 0.45 μm millipore filters. The sterile discs (6 mm in diameter) were impregnated with 10 μl of extracts (300 μg/disc) at concentration of 30 mg/ml and placed in inoculated agar. The density of the bacterial suspension was standardized by using McFarland standard method11 . Nitrofurantoin (300 μg/disc), norfloxacin (10 μg/disc), cefotaxime (30 μg/disc), trimethoprim (5 μg/disc), amoxycillin+clavulinic acid (20+10) (30 μg/disc), gentamycin (10 μg/disc), ceftriaxone (30 μg/disc) and ciprofloxacin (25 μg/disc) were used as standards for urinary tract pathogens. The controls were prepared using the same solvents employed to dissolve the plant extracts. The inoculated plates were incubated at 37° for bacteria for 24 h. Antibacterial activity of aqueous, ethanol, petroleum ether and chloroform extracts against the bacterial strains based on disc diffusion method is given in Table 1. An attempt has been made to compare antibacterial activity of H. enneaspermus extracts with the most potent standard effective against the selective UTI pathogens and is illustrated in fig.1. The minimum inhibitory concentration (MIC) values were determined by using twofold serial dilution assay [10,12] for the microorganisms which were determined sensitive to various extracts of H. enneaspermus in disc diffusion assay. The inoculums prepared from 12-hour broth cultures and suspensions were adjusted to 0.5 McFarland standard turbidity [11]. The H. enneaspermus aqueous, ethanolic and chloroform extracts were dissolved in 6% dimethyl formamide; except petroleum ether extract, which was solubilized using a mixture of 6% dimethyl formamide and 2% sodium dodecyl sulphate and then diluted to the highest concentration (500 μg/ml), and subsequently two fold serial dilutions were made in the concentration range of 7.8-500 μg/ml. MIC values of the extracts against urinary tract pathogens were determined with some modifications. The dilutions were performed by dispensing into each tube 1 ml of nutrient broth and 1 ml of each extract in the concentration of 500 μg/ml and then serially diluted. Fifty microlitres of freshly prepared inoculum was then added to all the tubes. Control was chosen using 1 ml broth, 1 ml of solvent and then adding 50 μl inoculum without the extract. Contents of each tube were incubated at 37° for 24 h, and absorbance was read at 600 nm using Digital Photo Colorimeter (Model: 312, Environmental and Scientific Inst. Co.) for determination of microbial growth. The contents of the tube were then subcultured on nutrient agar plates by adding 10 μl of inoculum and incubated at 37° for determination of MIC. The MIC values of the extracts determined by twofold serial dilution assay are given in Table 2.
| Conc. (mg/disc) | Zone of Inhibition (in mm)* | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| AQ (300) | 25.0±1.0 | 23.5±0.25 | 20.0±0.25 | 15.2±0.55 | 20.8±0.11 | 19.1±0.55 |
| ET (300) | 17.0±0.41 | 22.0±0.45 | 21.4±0.55 | 20.4±0.05 | 24.0±0.85 | 23.3±0.11 |
| CH (300) | – | 18.0±0.68 | 15.7±0.41 | 13.2±0.57 | – | 14.5±1.37 |
| PE (300) | – | – | 16.0±0.61 | 13.9±0.70 | – | – |
| NF (300) | 18.4±0.45 | 12.6±0.65 | 16.3±0.47 | 17.2±0.17 | 15.3±0.37 | 11.8±0.75 |
| NX (10) | – | 30.5±0.49 | 22.7±0.56 | 20.8±1.07 | 25.2±0.34 | 25.5±0.45 |
| CE (30) | 12.3±0.36 | 24.3±0.49 | 16.1±0.45 | 27.3±0.40 | 9.6±0.61 | 12.3±0.30 |
| TR (5) | 27.1±0.66 | – | 15.2±0.58 | – | – | 10.7±0.70 |
| AC (30) | – | 12.0±0.40 | 17.1±0.36 | 15.6±0.61 | 10.7±0.70 | 25.3±0.35 |
| G (10) | 10.8±0.8 | 26.6±0.60 | 28.3±0.3 | 25.4±0.45 | 25.8±0.55 | 24.5±0.7 |
| CI (30) | 28.2±0.30 | 28.3±0.35 | 25.6±0.80 | 15.5±0.70 | 26.5±0.60 | 22.2±0.40 |
| CF (25) | 28.0±0.15 | 26.4±0.45 | 25.4±0.35 | 24.5±0.47 | 26.4±0.45 | 25.3±0.15 |
Indicates no zone of inhibition. *All the values are mean± standard deviation of three determinations. 1. Escherichia coli; 2. Pseudomonas aeruginosa; 3. Klebsiella pneumoniae; 4. Proteus mirabilis; 5. Enterococcus faecalis; 6. Staphylococcus aureus. AQ, ET, PE and CH stand for aqueous, ethanolic (95%), petroleum ether and chloroform extracts respectively. NF, NX, CE, TR, SE, G, CI and CF stand for nitrofurantoin, norfloxacin, cefotaxime, trimethoprim, amoxycillin+clavulinic acid (20+10), gentamycin, ceftriaxone and ciprofloxacin respectively. Values showed significant difference from those of solvent control at P < 0.001
Table 1: in vitro antibacterial activity of hybanthus enneaspermus extracts against urinary tract pathogens by disc diffusion method
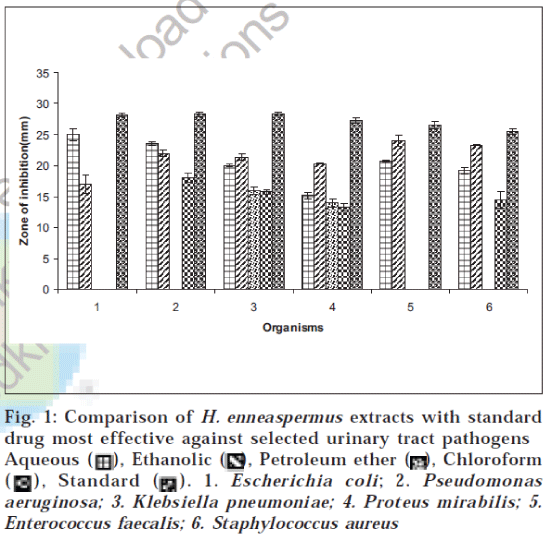
Fig. 1: Comparison of H. enneaspermus extracts with standard drug most effective against selected urinary tract pathogens Aqueous Ethanolic Petroleum ether Chloroform Standard 1. Escherichia coli; 2. Pseudomonas aeruginosa; 3. Klebsiella pneumoniae; 4. Proteus mirabilis; 5. Enterococcus faecalis; 6. Staphylococcus aureus
| Microorganisms | MIC MIC | |||
|---|---|---|---|---|
| Aqueous | Ethanol | Chloroform | Pet ether | |
| Escherichia coli | 125 | 250 | 500 | 500 |
| Pseudomonas aeruginosa | 125 | 125 | 250 | 500 |
| Klebsiellapneumoniae | 125 | 125 | 250 | 250 |
| Proteus mirabilis | 125 | 62.5 | 250 | 250 |
| Enterococcus faecalis | 250 | 125 | 500 | 500 |
| Staphylococcus aureus | 250 | 250 | 125 | 500 |
MIC – Minimum inhibitory concentration (μg/ml)
Table 2: The mic values of hybanthus enneaspermus extracts against the microorganisms tested by twofold serial dilution assay
Aqueous extract showed moderate inhibition against all tested UTI pathogens, with maximum inhibition against E. coli (25 mm) and minimum against P. mirabilis (15.2 mm), as indicated in Table 1. Ethanolic extract showed significant activity (P < 0.001) against all tested pathogens, with maximum inhibition (24 mm) against E. faecalis and minimum (17 mm) against E. coli. The chloroform and petroleum ether extracts showed feeble activity when compared to ethanolic and aqueous extracts. The petroleum ether extract showed activity against K. pneumoniae (16 mm) and P. mirabilis (13.9 mm) but was inactive against other microorganisms. Chloroform extract showed inhibition against P. aeruginosa, K. pneumoniae, P. mirabilis and S. aureus (18.0 mm, 15.7 mm, 13.2 mm and 14.5 mm respectively) but showed no inhibition against the other two organisms. Ethanolic and aqueous extracts showed marked activity against all urinary tract pathogens, equating to the standard, and even exhibited better zone of inhibitions in comparison to standard, as indicated in Table 1. Further studies aimed at isolation and purification of active phytoconstituents may yield a few more compounds with greater antibacterial activity. MIC against the tested microorganisms, as indicated in Table 2, showed that aqueous and ethanolic extracts inhibit microbial growth at comparative lesser concentration than chloroform and petroleum ether extracts.
Acknowledgements
The authors are thankful to Director and HOD of University Department of Pharmaceutical Sciences, Utkal University, for providing research facility and valuable guidance.
References
- Kirtikar, K.R. and Basu, B.D., In; Indian Medicinal Plants, 2nd Edn.,determined by twofold serial dilution assay are given in Vol.I, International Book Distributors , Dehradun, 1975, 212
- Boominathan, R., Parimaladevi, B., Mandal, S.C. and Ghoshal, S.K., J.Ethnopharmacol., 2004, 91, 367
- Boominathan, R., Devi, B.P. and Mandal, S.C., Phytotherapy Res., 2003, 17, 838.
- Weniger, B., Lagnika, L., Vonthron-Senecheau, C., Adjabimey, D.,Gbenou, J., Moudachirou, M., Brun, R., Anton, R. and Sanni, A., J.Ethnopharmacol., 2004, 90, 279
- Hemlatha, S.,Wahi, A.K., Singh, P.N. and Chansouria, J.P.N., Indian J. Traditional Knowledge, 2003, 2, 389.
- Majumdar, P.L., Basu, A. and Mal, D., Indian J. Chemistry, 1979,17, 297.
- Murray, P.R., Baron, E.J., Ptaller, M.A., Tenover, F.C. and Yolke, R.H., Manual of Clin. Microbiol., 1995, 6, 45.
- Bauer, A.W., Kirby, W.M.M. and Scherris, J.C., Amer. J. Clin. Pathol., 1966, 36, 493.
- Collins, C.H. and Lyne, P.M. In; Microbiological Methods, 6th Edn.,Butterworths, London, 1970, 66.
- Rath, C.C., Dash, S.K., Mishra, R.K. and Charyulu, J.K., J. Essent. Oil Bear. Plants, 1999, 2, 82.
- McFarland, J., J. Amer. Med. Assoc., 1987, 49, 1176.
- Barry, A.L., In; The Antimicrobial Susceptibility Test; Principles and Practices; Antimicrobial dilution test, Henry Kimpton Publishers, London, 1976, 61.