- *Corresponding Author:
- B. A. Rather Bio-Organic and Photochemistry Laboratory, Department of Pharmaceutical Sciences, Guru Nanak Dev University Amritsar-143 005, India E-mail: rather_bilu@rediffmail.com
| Date of Submission | 13 July 2009 |
| Date of Revision | 11 January 2010 |
| Date of Acceptance | 15 May 2010 |
| Indian J. Pharm. Sci., 2010, 72 (3): 375-378 |
Abstract
Schiff bases (9a-l) of 3-amino-6,8-dibromo-2-phenyl-quinazolin-4-(3H)-ones (8) with various substituted aldehydes were obtained by refluxing 1:1 molar equivalents of the reactants in dry ethanol for 6 h. The aminoquinazoline (8) was inturn obtained from 3,5-dibromoantharlinic acid via intermediate (7). All the synthesized compounds (9a-l) were evaluated for their anticonvulsant activity on albino mice by maximal electroshock method using phenytoin as a standard. The compound (9l) bearing a cinnamyl function displays a very high activity (82.74 %) at dose level of 100 mg/kg b.w.
Keywords
Anticonvulsant, maximal electroshock method, Schiff base, Quinazolin-4-(3H)-one, 3-aminoquinazolines
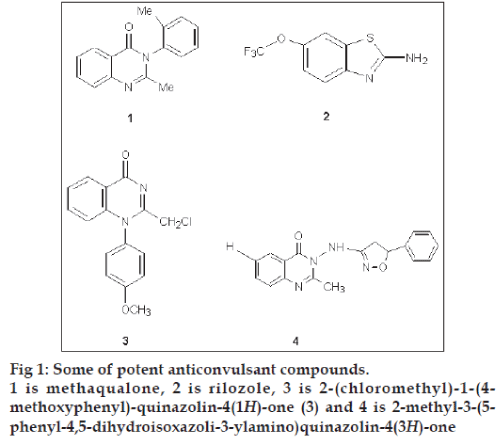
Quinazolinones and their Schiff bases are an important class of heterocyclic systems, enjoying considerable interest on account of their diverse range of biological activities [1-9] such as antimicrobial, analgesic and antiinflammatory, anticonvulsant, anticancer, antitubercular, antimalarial, antiviral, antihelmintic and, in particular, very high anticonvulsant activity. Anticonvulsant quinazolinones include methaqualone (1) [10], which acts on voltage dependant sodium channels in a manner similar to rilozole (2) [11,12], 2-(chloromethyl)-1- (4-methoxyphenyl)-quinazolin-4(1H)-one (3) and 2-methyl-3-(5-phenyl-4,5-dihydroisoxazoli-3-ylamino) quinazolin-4(3H)-one (4, fig 1). Structural modification of the quinozolines nucleus i.e., introduction of Ph- and -CH3 group at C2 position [13], bromination of benzene ring at C6 and C8, introduction of various substituted phenyl moieties [14], bridged phenyl rings [15], heterocyclic rings [16] and aliphatic moieties [17] at position-3 are reported to enhance the anticonvulsant activity. The high anticonvulsant activity of quinazolines is also a consequence of their high membrane permeability [18].
Earlier, we had reported [19,20] synthesis and antimicrobial activity of Schiff bases of 3-amino- 6,8-dibromo-2-phenyl-quinazolin-4-(3H)-ones (9a-l). Taking cognizance of the reported high anticonvulsant activity of the quinazolines, it was decided to synthesize the Schiff bases of 3-amino-quinazolines according to the earlier reported procedure [19,20] and evaluate their anticonvulsant activity.
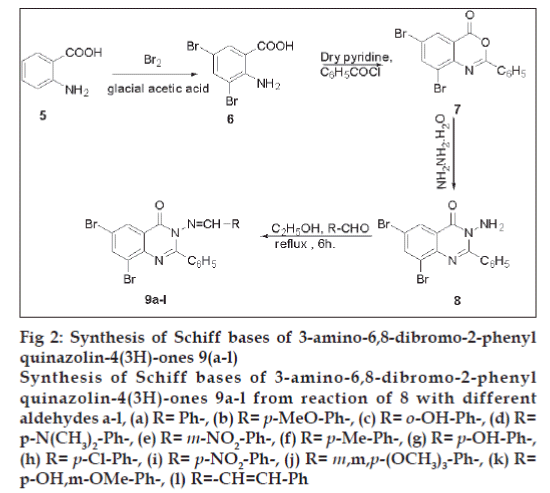
Synthesis of Schiff bases (9a-l) of 3-amino-6,8- dibromo-2-phenyl-quinazolin-4(3H)-ones [19,20] (8) with various substituted aldehydes was achieved by refluxing their equimolar dry ethanol solution for 6 h. The Schiff bases (9a-l) were filtered, dried and recrystallised from absolute ethanol (fig. 2). All compounds (9a-l) were analyzed using detailed spectroscopic (IR, 1H NMR, 13C NMR, Mass) and elemental analysis.
Fig 2: Synthesis of Schiff bases of 3-amino-6,8-dibromo-2-phenyl
quinazolin-4(3H)-ones 9(a-l)
Synthesis of Schiff bases of 3-amino-6,8-dibromo-2-phenyl
quinazolin-4(3H)-ones 9a-l from reaction of 8 with different
aldehydes a-l, (a) R= Ph-, (b) R= p-MeO-Ph-, (c) R= o-OH-Ph-, (d) R=
p-N(CH3)2-Ph-, (e) R= m-NO2-Ph-, (f) R= p-Me-Ph-, (g) R= p-OH-Ph-,
(h) R= p-Cl-Ph-, (i) R= p-NO2-Ph-, (j) R= m,m,p-(OCH3)3-Ph-, (k) R=
p-OH,m-OMe-Ph-, (l) R=-CH=CH-Ph
The animal study protocols were approved by Institutional Animal Ethics Committee’s (IAEC) approval. Anticonvulsant activity of all compounds (9a-l, Table 1) was evaluated by maximal electroshock (MES) method [21]. Swiss mice (n=6) of either sex selected by random sampling technique were used for the study. Phenytoin at the dose of 10 mg/kg (i.p.) was administered as standard drug for comparison. The test compounds were suspended in polyethylene glycol in the ratio of 1:9/ml in water and were given i.p. at doses of 100-200 mg/ kg body weight. Dosing volume was 0.25 ml per 25 g. The animals were held at suitable position and corneal electrodes were placed on the cornea of the mice and applied 50 mA current for 0.2 sec after half an hour administration of the test compounds. Then the time spent by animals in each phase of convulsion was recorded. Animals in which extensor response was abolished were taken as protected mice. Compound 9l (82.74 %) was found to possess high anticonvulsant activity which is followed by 9g (81.61 %), 9i (81.48 %), 9k (81.48 %), 9j (80.29 %) at the dose of 100 mg/ kg. Moderate anticonvulsant activity was observed for the compounds 9e (79.03%) followed by 9d (79.01 %), 9f (77.77 %), 9h (77.77 %), 9c (74.07 %) and 9b (72.88 %). Compound 9a showed very low anticonvulsant activity of (68.14%) at the dose level of 100 mg/kg.
| Compounds | Dose (mg / kg) | Flexion phase | Extensor phase | ||
|---|---|---|---|---|---|
| Mean± SEMa | % protection | Mean± SEMa | % protection | ||
| 9a | 100 | 5.66±0.4375 | 27.99 | 4.3±0.21875 | 68.14 |
| 200 | 4.62±0.3125 | 35.52 | 3.6±0.21875 | 73.33 | |
| 9b | 100 | 5.66±0.4375 | 21.01 | 3.66±0.21875 | 72.88 |
| 200 | 4.82±0.3125 | 32.73 | 3.50±0.21875 | 74.07 | |
| 9c | 100 | 5.66±0.4375 | 27.99 | 4.3±0.21875 | 74.07 |
| 200 | 4.62±0.3125 | 35.52 | 3.6±0.21875 | 76.29 | |
| 9d | 100 | 5.66±0.4375 | 21.01 | 3.66±0.21875 | 79.01 |
| 200 | 4.82±0.3125 | 32.73 | 3.50±0.21875 | 80.59 | |
| 9e | 100 | 4.5±0.21875 | 37.2 | 3.5±0.21875 | 79.03 |
| 200 | 3.74±0.2187 | 48.36 | 3.2±0.15625 | 82.07 | |
| 9f | 100 | 4.5±0.21875 | 37.2 | 2.83±0.15625 | 77.77 |
| 200 | 3.9±0.21875 | 45.57 | 2.62±0.15625 | 79.25 | |
| 9g | 100 | 5.33±0.3125 | 25.62 | 2.83±0.15625 | 81.61 |
| 200 | 4.9±0.3125 | 31.62 | 2.42±0.09375 | 83.70 | |
| 9h | 100 | 5.0±0.3125 | 30.22 | 3.0±0.15625 | 77.77 |
| 200 | 4.8±0.3125 | 33.01 | 2.8±0.15625 | 79.25 | |
| 9i | 100 | 5.3±0.3125 | 26.03 | 2.5±0.09375 | 81.48 |
| 200 | 4.98±0.3125 | 30.50 | 2.2±0.09375 | 83.70 | |
| 9j | 100 | 5.83±0.4375 | 18.57 | 3.0±0.15625 | 80.29 |
| 200 | 5.1±0.3125 | 28.83 | 2.8±0.15625 | 82.96 | |
| 9k | 100 | 5.66±0.4375 | 20.94 | 2.5±0.09375 | 81.48 |
| 200 | 5.22±0.3125 | 27.15 | 2.2±0.09375 | 84.44 | |
| 9l | 100 | 6.5±0.4027 | 9.21 | 2.66±0.15625 | 82.74 |
| 200 | 5.31±0.3125 | 25.90 | 2.30±0.09375 | 84.07 | |
| Phenytoin | 10 | 4.8±0.3125 | 32.48 | 0.00±0.00 | 100 |
| Control | - | 7.16±0.5625 | - | 13.5±0.6875 | - |
aSignificant error mean method: significant differences with respect to control was evaluated by ANOVA.
Table 1: Anticonvulsant activity of compounds
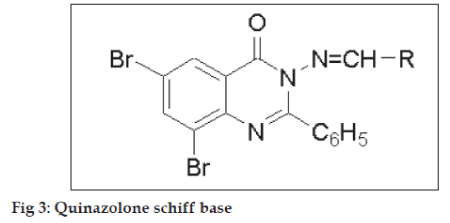
Structure activity relationship (fig. 3, Table 2) for all the compounds was developed on the % protection data of extensor phase at dose of 100 mg/kg. Compounds 9l, g, i, bearing CH=CH-Ph, -p-OH-Ph, -p- NO2-Ph moieties, showed maximum % protection of 82.74, 81.61 and 81.48. Moderate % protection of 79.01, 77.77, 74.07 and 72.88 was observed for compounds 9d, f, c, b, respectively, possessing electron donating groups such –p-N(CH3)2-Ph, -p-Me-Ph, -o-OH-Ph, -p-OMe-Ph. However, very low anticonvulsant activity was observed for 9a bearing unsubstituted aromatic ring.
| R | Activity |
|---|---|
| -CH=CH-Ph, -p-OH-Ph, -NO2-Ph | High |
| -N(CH3)2, -Me-Ph, -o-OH-Ph, -OMe-Ph | Moderate |
| Ph | Low |
Table 2: Structure activity relationship
In conclusion, the present study indicates that compounds 9l, g, i Possessing styryl, p-OH-Ph or -p-NO2-Ph were found to posses high anticonvulsant activity, which may be attributed extended conjugation in case of 9l or hydrogen bonding ability of parasubstituent as in the case of 9g, i. While the compounds 9b, c, d, f bearing electron donating groups (-N(CH3)2, -Me, -o-OH, -OMe), display moderate activity; among the latter para-substituted compound (9d) is most active thereby confirming our earlier inference. On the other hand compound bearing unsubstituted phenyl group showed low activity. These compounds shall serve as ‘Lead’ molecules for further development.
References
- Alagarsamy V, Gridhar R, Yadav MR. Synthesis and H1-Antihistaminic Activity of Some Novel 1-Substituted-4-(3- methylphenyl)-1,2,4-triazolo[4,3-a]quinazolin-5(4H)-ones. Biol Pharm Bull 2005;28:1531-4.
- Alagarsamy V, Rajasolomon V, Meena R, Ramseshe KV. Synthesis, Analgesic, Anti-inflammatory and Antibacterial Activities of Some Novel 2-Butyl-3-substituted Quinazolin-4-(3H)-ones. Biol Pharm Bull 2005;28:1091-4.
- Alagarsamy V, Muruganantham G, Venkateshaperumal R. Synthesis, Analgesic, Anti-inflammatory and Antibacterial Activities of Some Novel 2-Methyl-3-substituted Quinazolin-4-(3H)-ones. Biol Pharm Bull 2003;26:1711-4.
- Srivastava MK, Mishra B, Nizamuddin. Pharmacological studies of some 2-methyl-3-(arylthio-carbamido)quinazol-4-ones and 2-methyl-3-(aryliden-carboxamido)quinazol-4-ones. Indian J Chem 2001;40B: 342-4.
- Alagarsamy V, Rajasolomon V, Vanikavitha G, Pallchamy V, Amuthalakshmi S, Ravi M, et al. Synthesis, Analgesic, Anti-inflammatory and Antibacterial Activities of Some Novel 2-Phenyl-3-substituted Quinazolin-4(3H) ones. Biol Pharm Bull 2002;25:1432-5.
- Pannerselvam P, Pradeepchandran RV, Sridhar SK. Synthesis, characterization and biological activities of novel 2-methyl-quinazolin-4(3H)-ones. Indian J Pharm Sci 2003;65:268-73.
- Alagarsamy V, Thangathirupathy A, Mandal SC, Rajasekaran S, Vijayakumar S, Revathi R, et al. Pharmacological evaluation of 2-substituted (1,3,4) thiadiazoloquinazolines. Indian J Pharm Sci 2006;68:108-10.
- Murgan V, Caroline, Thomas C, Rama Sarma, GVS, Kumar EP. Synthesis of 2-substituted quinayolin-4(3H)-ones as a new class of anticancer agents. Indian J Pharm Sci 2003;65:386-89.
- Nandy P, Vishalakshi M, Bhat AR. Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J HeterocyclChem 2006;15:293-4.
- Kacker IK, Zaheer SH. Potential analgesics. Synthesis of substituted 4-quinazolinones. J Indian ChemSoc 1951;28:344-6.
- Mizoule J, Meldrum B, Mazadier M, Croucher M, Ollat C, Uzan A, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonistof excitatory amino acid neurotransmission-I. Anticonvulsant properties. Neuropharmacol 1985;24:767-73.
- Benavides J, Camelin JC, Mitrani N, Flamand F, Uzan A, Legrand JJ, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonistof excitatory amino acid neurotransmission-II. Biochemical properties. Neuropharmacol 1985;24:1085-92.
- Alyel-Helby AG, Wahab MHA. Design and synthesis of some new derivatives of 3H-quinazolin-4-one with promising anticonvulsant activity. Acta Pharm 2003;53:127-38.
- Lakhan R, Singh, OP, Singh, RL. Studies on 4(3H)-quinazolinone derivatives as anti-malarials. J Indian ChemSoc 1987;64:316-8.
- Rastogi R, Sharma S. Synthesis of 2-substituted quinazolines and quinazolones as potential anthelmintics. Indian J Chem 1982;21B: 744-6.
- Kumar P, Nath C, Bhargava KP, Shanker K. Synthesis and anti-parkinsonian activity of styrylquinazolones. Pharmazie 1982;37:802-4.
- Aono T, Marui S, Itoh F, Yamoaka M, Nakao M. Preparation of heterocyclic compounds as antitumour agents. Eur Patent No. 733633, Chem. Abst 125, 328725U, 1996.
- Sinha D, Tiwari AK, Singh S, Shukla G, Mishra P, Chandra H, etal. Synthesis, characterization and biological activity of Schiff baseanalogues of indole-3-carboxaldehyde. Eur J Med Chem 2008;43: 160-5.
- Panneerselvam P, Bilal AR, Dontrireddy RSR, Natesh RK. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6,8-dibromo-2-phenylquinazolin-4(3H)-ones. Eur J Med Chem 2009;44:2328-333.
- Wheeler S, Oates WM. The Bromination of Anthranilic Acid. J Am ChemSoc 1910;32:770-5.
- El-Helby AGA, Wahab MHA. Design and synthesis of some new derivatives of 3H-quinazolin-4-one with promising anticonvulsant activity. Acta Pharm 2003;53:127-38.