- Corresponding Author:
- P. S. Kalaskar
Departments of Pharmacology and Toxicology, Parel, Mumbai-400 012, India
E-mail: drvikas111081@gmail.com
| Date of Submission | 15 December 2011 |
| Date of Revision | 2 December 2012 |
| Date of Acceptance | 5 December 2012 |
| Indian J Pharm Sci, 2012, 74 (6): 575-579 |
Abstract
The antifungal activity of chloroform extract of leaves of Acanthus ilicifolius was evaluated in Aspergillus fumigatus infected mice. Swiss albino mice (60)] were divided into five groups. All the groups were immunosuppressed with cyclophosphamide and cortisone acetate couple of days prior to intranasal inoculation with Aspergillus fumigatus conidia (10 6 ) ] in all the groups, except the first. Treatment was initiated at 24 h of fungal inoculation and continued up to day 14, and included amphotericin B (1 mg/kg orally) for group III and extract of Acanthus ilicifolius at 250 mg and 500 mg/kg for group IV and V, respectively. Groups I and II received sterile water orally for the same period. From each group, three mice were sacrificed after 1 h and the remaining mice on the 14 th day of inoculation. One hour post-inoculation lung colony forming unit count confirmed the delivery of conidia into the lungs. Colony forming unit count, intensity of gross necropsy changes and histopathological changes were highest in group II. It improved in group III and also in groups IV and V in dose-dependent manner. Lesions were absent in the noninfected group. Lesions included maximum granulomatous inflammation of lung, multifocal diffused necrotic granulomas on kidney and moderate microgranulomas on liver. From this study, it was concluded that chloroform extract of Acanthus ilicifolius contains active principles that are absorbed after oral administration to produce systemic effects when given at 500 mg/kg dose.
Keywords
Antifungal activity, Aspergillus fumigatus, Acanthus ilicifolius
The incidence of fungal infections has dramatically increased in the last few decades with increase in number of immunocompromised patients suffering from malignancy, hematological diseases, HIV as well as those receiving immunosuppressive drug regimens for the management of organ transplants or autoimmune inflammatory conditions [1]. In animals, opportunistic fungal infections like aspergillosis, is becoming more prevalent as a result of increasing number of immunocompromised animals suffering from immunodeficiency virus, infection, prolonged treatment with chemotherapeutic agents, organ transplants [2]. Aspergillosis is caused due to Aspergillus fumigatus and other Aspergillus sp. in canine, equine, cattle and dolphins. A. fumigatus is the most pathogenic organism causing brooder’s pneumonia in birds and it is a major cause of mortality in birds.
A limited number of antifungal agents are available in the market and most of those are expensive. Further these drugs also have the potential to cause adverse effects such as chills, fever, headache, nausea, vomiting and nephrotoxicity [3], neurotoxicity, hepatotoxicities and reproductive disorders [4]. Considering these adverse effects, availability and cost of treatment, there is a need to search for newer, safer, and more potent agents to combat serious fungal infections.
The available literature indicates that mangrove plant Acanthus ilicifolius, exhibits activity against A. fumigatus in vitro [5,6]. Considering the urge for availability of new antifungal drug, the present study was undertaken to validate the antifungal activity of A. ilicifolius in mice against A. fumigatus-induced Aspergillosis.
The study was conducted in 60 healthy swiss albino mice weighing 20-25 g. Mice were kept in their cages for 5 days prior to commencement of the study to allow acclimatisation to the laboratory conditions. They were provided with standard pellet diet, potable water ad libitum and maintained under standard management practices. The study was initiated after the approval of Institutional Animal Ethics Committee.
Plant samples were collected from the coastal region of Vashi, Navi Mumbai. The leaves were separated from the plant, washed thoroughly with distilled water, shade dried for 15 days and then powdered. The thimble made up of Whatman filter paper No. 1 containing the powder was put into the centre of Soxhlet apparatus and was dipped in aqueous chloroform. The temperature was set at 70° and the extract was subjected to solvent distillation eight times. The final extract obtained was strained through single folded cotton cloth and heated in a hot water bath at 70° until it turned semisolid. It was further dried in hot air oven at 55° and packed in airtight glass bottles.
Amphotericin B (100 μg) was dissolved in 1 ml of sterile water to get concentration of 100 μg/ml. Ceftazidime (1 g) was dissolved in 100 ml sterile saline whereas cortisone as acetate and cyclophosphamide (Sigma Aldrich life Science, Bangalore, India), 1250 mg each were dissolved in 50 ml sterile water and stored under refrigeration. The extract (1 g) was dissolved in 0.125 ml of dimethyl sulfoxide (DMSO) diluted with 25 ml sterile water.
Cortisone as acetate 250 mg/kg subcutaneously (SC) and cyclophosphamide 250 mg/kg intraperitoneally were administered to all mice for inducing immunosuppression [7]. After 2 days blood was collected randomly from six mice to ensure leucopenia with a white blood cell (WBC) count <1000/mm3 [8] and they were divided in five equal groups (Table 1). Conidia of field isolate of A. fumigatus grown on Sabroud dextrose agar (Hi Media) at 28° for 10 days were harvested with phosphate buffer saline containing 0.1% Tween 80. This conidial inoculum (1×106 concentration) [9] was instilled 5 μl per nostril in both the nostrils in mice from group II to group V. An hour after inducing infection, three mice in each group was sacrificed and colony forming units (CFUs) in lungs were quantified for ensuring delivery of conidia to the lungs. From the next day, mice were subjected to various treatments (Table 1) and the treatments continued for 14 days. Second dose for immunosuppressants was repeated 2 days after commencement of treatments. All mice received ceftazidime (50 mg/kg, SC) from the day of administration of first dose of immunosuppressants until the day of completion of treatments, to prevent secondary bacterial infection.
| Groups | Inoculum used | Treatment (oral) |
|---|---|---|
| Group I | PBS-5 µl/nostril/mouse. (total volume, 10 µl/mouse) | Sterile water containing 0.5% DMSO |
| Group II | A. fumigatus, 1×106 Conidia (10 µl/mouse) | Sterile water containing 0.5% DMSO orally |
| Group III | Amphotericin B 1 mg/kg | |
| Group IV | A. ilicifolius extract 250 mg/kg | |
| Group V | A. ilicifolius extract 500 mg/kg |
DMSO=Dimethyl sulfoxide
Table 1: Grouping of immunosuppressed mice
After fungus inoculations, all mice were observed daily for the development of clinical signs such as rapid or shallow breathing, ruffled fur, hunched posture, impaired ambulation, evidence of muscle atrophy, extensive ulcerative dermatitis and infected tumours. Any obvious illness such as signs of lethargy, drowsiness, aversion to activity, physical or mental alertness, bleeding, central nervous system disturbance and chronic diarrhoea were noted. Mortality was recorded daily for 14 days of treatment for assessing efficacy of antifungal treatment [10].
Quantitative measurement of infection was performed by enumeration of A. fumigatus CFUs in tissue homogenates of lungs, liver and kidneys samples on day 14 of treatment after sacrificing the animals. Triplicate readings were taken for each time point per organ, which were averaged [11]. The results were statistically compared between various groups applying Student’s t test. The mice in all groups whether sacrificed or dead naturally were subjected to necropsy. Organ samples (lung, liver and kidney) were processed for histopathological examination [9].
A. ilicifolius was identified through botanical characters [12]. From 400 g of leaf powder, eight of dried extract were obtained, which was initially dissolved in DMSO and then in sterile water taking care not to let the concentration of DMSO exceed 0.5% as it is reported to be toxic beyond this concentration [13].
Table 2 reveals daily clinical observations as well as mortality recorded upto day 14 of the treatment in different groups. The mortality figures do not take into account mice sacrificed intentionally. Mortality was considered as the sole parameter while assessing antifungal potential of alcoholic extract of P. lyelli in mice [14]. Airborne conidia settle on nasal, tracheal and parabronchial epithelium where they germinate rapidly and disseminate hematogenously to the tissues finally resulting in death.
| Groups (n=9) | General signs | Mortality | Necropsy gross observation |
|---|---|---|---|
| I | NAD | 0 | NAD |
| II | Mice were dull and depressed from 3rd day of infection. | 7 | Quantity of fungal nodules per unit area was |
| On the 7th day, feces were pasty. From day 8th, respiration | markedly high as observed with naked eyes | ||
| was rapid and abdominal. 10th day onwards animals showed | |||
| circling movement and emaciation | |||
| III | Signs were not evident | 1 | Quantity of fungal nodules per unit area was very low |
| IV | Sign were evident but the intensity was less and fecal | 5 | Quantity of fungal nodules per unit area was |
| consistency was not affected | moderate | ||
| V | Signs were not evident | 3 | Quantity of fungal nodules per unit area was less |
NAD=Nothing abnormal detected
Table 2: General signs, mortality and gross necropsy observations in mice
It is technically challenging to maintain the balance between achieving immunosuppression upto the desired level and controlling mortality in experimentally infected animals in a way that sufficient number of samples are available at the end of the study. The literature scan revealed that the period of studies pertaining to exploring antifungal potential of drugs following experimental induction of fungal infection in lab animals varied from 7 to 52 days.
It is well known that the immunosuppressed individual is an easy target for fungal attack. Aspergillus is an opportunistic organism which flares up in immunocompromised individual. The design of the experiment, therefore, included induction of immunosuppression (leucopenia) with cyclophosphamide and cortisone injection. Cyclophosphamide is a prodrug requiring metabolic transformation to generate active alkylating species which bind to DNA, induce strand breakage and kill actively replicating WBCs. Cortisone suppresses cell mediated immunity, reduces T cell proliferation, causes apoptosis, suppresses humoral immunity and decreases phagocytosis.
Based on the previous literature and pilot study, the dosage schedule for administration of immunosuppressants as well as the dose of infective conidia was finalized. The dose of A. ilicifolius extract was selected based on previous literature [15]. Mice are reported to be the most preferred and widely used animals for fungal inoculation [16]. The methods of mice inoculation include both, invasive [16-18] as well as noninvasive (viz. aerosols) procedures. The method of instilling inoculums in the nostrils was found simple and did not require any special equipment.
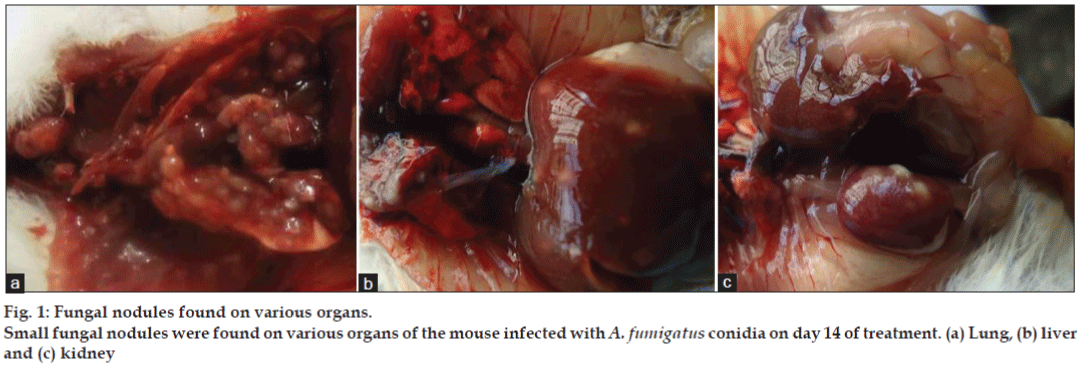
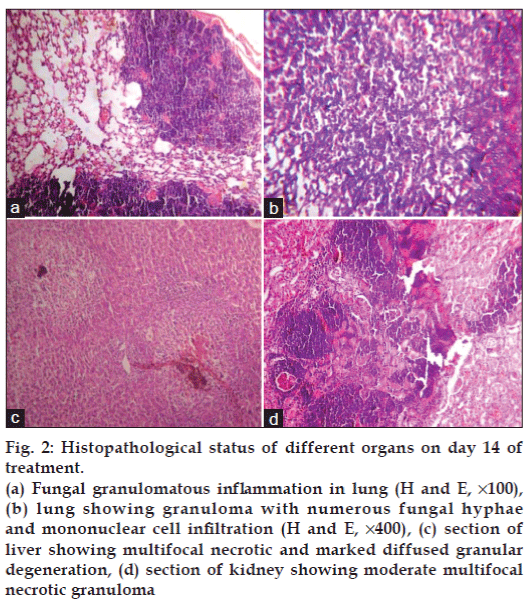
The most prominent characteristic feature observed in fungus inoculated mice during necropsy on day 14 was presence of fungal nodules in lungs, kidneys and liver (figs. 1a-c). Density of nodules in lungs was more than in kidneys followed by liver when observed with the naked eyes. It is quite surprising that the available literature fails to discuss the gross lesions though the discussion on histopathological lesions of aspergillosis is focused. Nodules on the organs is a common feature noted in poultry aspergillosis [19]. Whereas on histopathological examination revealed no abnormalities in group I. In group II (figs. 2a and b) severity of lesions in lungs was more and it included moderately multifocal granulomatous inflammation and necrotic granuloma on day 7 and extensive necrotic pneumonia with multifocal fungal granulomatous inflammatory foci on day 14. In liver (fig. 2c) there was mild multifocal necrotic and marked diffused granular degeneration on day 14. In kidneys (fig. 2d) there were moderate multifocal necrotic granulomas on day 7 and day 14. The intensity of these lesions was less in groups III, IV and V.
Figure 2: Histopathological status of different organs on day 14 of treatment.
(a) Fungal granulomatous inflammation in lung (H and E, ×100), (b) lung showing granuloma with numerous fungal hyphae and mononuclear cell infiltration (H and E, ×400), (c) section of liver showing multifocal necrotic and marked diffused granular degeneration, (d) section of kidney showing moderate multifocal necrotic granuloma
The count of CFUs of lungs at 1 h and of lungs, liver and kidneys on day 14 is given in Table 3. Considering the number of animals and lesion score available at the end of day 14 it was possible to have a statistical comparison in CFU count of lung and kidney between groups III and IV and III and V which differed at 1 and 5% level. In group II only two animals survived by day 14. This indicated that amphotericin B was undoubtedly superior in reducing the fungal count than A. ilicifolius. Numerical comparison between groups II and IV and groups II and V for kidney and lung CFUs indicated efficacy of A. ilicifolius extract over untreated infected control. In liver, fungal CFUs were detected only in group IV on day 14 possibly indicating slow regression of disease or effect of drugs.
| Organ | Day | Groups | ||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | ||
| Lung | 0 (1 h after the inoculation) | 0 | 3184.66 ± 137 | 3133.33 ± 120 | 3043 ± 70 | 3166 ± 132 |
| Lung | 14 | 0 ± 0 | 4659 ± 68 | 25 ± 16 | 3864 ± 103 | 1561 ± 52 |
| Liver | 14 | 0 ± 0 | 200 ± 0 | 0 ± 0 | 63 ± 24 | 0 ± 0 |
| Kidney | 14 | 0 ± 0 | 1068 ± 159 | 75 ± 19 | 1058 ± 11 | 600 ± 41 |
The values are expressed as mean ± SE, SE=Standard error
Table 3: Colony forming unit in various organs of mice
Aspergillus is an airborne infection that enters through the respiratory tract and is carried hematogenously to various organs causing a fungal burden to be present on other visceral organs [9,15,20-22]. Group I mice did not catch the infection despite maintaining them with infected animals as due care was taken while handling and management of mice. CFU is the most relevant parameter for assessing the efficacy of antifungal drug quantified also through polymerase chain reaction and galactomannan assay [7,23].
From this study, it is concluded that when chloroform extracts of leaves of A. ilicifolius is administered orally at 500 mg/kg body weight, it reduces mortality, severity of symptoms and lesion score in Aspergillus infected mice. This indirectly indicates that there are some active principles in A. ilicifolius leaves that are absorbed from gastrointestinal tract after oral administration and produce systemic antifungal effect. Literature has also cited its antifungal potential in in vitro studies. Considering these collectively, further studies are suggested to isolate and identify active principles of A. ilicifolius having antifungal potential and to develop different formulations to arrest the fungal multiplication in vitro and in vivo.
References
- Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, et al. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J RespirCrit Care Med 2011;183:96-128.
- Tell LA. Aspergillosis in mammals and birds: Impact on veterinary medicine. Med Mycol 2005;43:S71-3.
- Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis 1990;12:308-29.
- Antifungal drugs. In, Sandhu HS, Rampal S, editors. Essentials of Veterinary Pharmacology and Therapeutics. New Delhi: Kalyani Publishers; 2006, p. 1203-22.
- Bose S, Bose A. Antimicrobial Activity of Acanthus ilicifolius (L.). Indian J Pharm Sci 2008;70:821-3.
- Khajure PV, Rathod JL. Antimicrobial activity of extracts of Acanthusilicifoliusextracted from the mangroves of Karwar coast Karnataka.Recent Res SciTechnol 2010;2:98-9.
- Sheppard DC, Marr KA, Fredricks DN, Chiang LY, Doedt T, Filler SG. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis.ClinMicrobiol Infect 2006;12:376-80.
- Hayashi R, Kitamoto N, Iizawa Y, Ichikawa T, Itoh K, Kitazaki T, et al. Efficacy of TAK-457, a novel intravenous triazole, againstinvasive pulmonary Aspergillosis in neutropenic mice. Antimicrob Agents Chemother 2002;46:283-7.
- Sionov E, Mendlovic S, Segal E. Experimental systemic murine aspergillosis: Treatment with polyene and caspofungin combination and G-CSF. J AntimicrobChemother 2005;56:594-7.
- Allendoerfer R, Loebenberg D, Rinaldi MG, Graybill JR. Evaluation of SCH51048 in an experimental model of pulmonary aspergillosis. Antimicrob Agents Chemother 1995;39:1345-8.
- Sionov E, Mendlovic S, Segal E. Efficacy of amphotericin B or amphotericin B-intralipid in combination with caspofungin against experimental aspergillosis. J Infect 2006;53:131-9.
- Dhani RC. A halophyte in the Himalaya.CurrSci 2002;83:1199-200.
- Premanathan M. Studies on antiviral activity of marine plants. Ph.D. Thesis, Submitted to TheAnnamalai University, India; 1991.
- Subhisha S, Subramoniam A. In vivo efficacy of an antifungal fraction from PallaviciniaIyellii, a liverwort. Indian J Pharmacol 2006;38:211-2.
- Babu BH, Shylesh BS, Padikkala J. Antioxidant and hepatoprotective effect of Acanthus ilicifolius. Fitoterapia 2001;72:272-7.
- Lewis RE, Wiederhold NP. Murine model of invasive aspergillosis. Methods Mol Med 2005;118:129-42.
- George D, Miniter P, Andriole VT. Efficacy of UK-109496, a new azole antifungal agent, in an experimental model of invasive aspergillosis.Antimicrob Agents Chemother 1996;40:86-91.
- Chakraborty KK, Naik SR. Therapeutic and hemolytic evaluation of in-situ liposomal preparation containing amphotericin-beta complexedwith different chemically modified beta-cyclodextrins. J Pharm Pharm Sci 2003;6:231-7.
- Wood AM. Fungal Diseases. In, Pattison M, McMullin PF, Bradbury JM, Alexander DJ, editors. Poultry Diseases. 6th ed. Philadelphia, USA: Elsevier Publication; 2009, p. 427.
- Kamberi P, Atsuro H, Takayoshi T, Masaru N. Efficacy of amphotericin B and azoles alone and in combination against disseminated trichosporonosis in neutropenic mice. Chemotherapy 1998;44:55-62.
- Ullmann AJ, Krammes E, Sommer S, Buschmann I, Jahn-Muehl B, Cacciapuoti A, et al. Efficacy of posaconazole and amphotericin B in experimental invasive pulmonary aspergillosis in dexamethasone immunosuppressed rats. J AntimicrobChemother 2007;60:1080-4.
- Ben-Ami R, Lewis RE, Leventakos K, Latgé JP, Kontoyiannis DP. Cutaneous model of invasive aspergillosis.Antimicrob Agents Chemother 2010;54:1848-54.
- Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW, et al. Assessment of Aspergillusfumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob Agents Chemother 2008;52:2593-8.