- *Corresponding Author:
- V. Alagarsamy
Medicinal chemistry R&D laboratory, Arulmigu kalasalingam college of pharmacy, Anand nagar, Krishnankoil - 626 190, India
E-mail: samy_veera@yahoo.com
| Date of Submission | 21 October 2005 |
| Date of Revision | 17 August 2006 |
| Date of Acceptance | 14 April 2007 |
| Indian J Pharm Sci, 2007, 69 (2): 304-307 |
Abstract
The title compounds 2-methyl-3-(substituted methylamino)-(3 H )-quinazolin-4-ones were synthesized by condensing the active hydrogen atom of the amino group of 3-amino-2-methyl-(3 H )-quinazolin-4-one with formaldehyde and appropriate amines. Their structures were confirmed by spectral data (IR, 1H-NMR and Mass) and the purity was ascertained by elemental analysis. Investigation of antimicrobial activity of the test compounds was performed by agar dilution method against 7 pathogenic bacteria, 3 pathogenic fungi and antiHIV activity against replication of HIV-1(IIIB) and HIV-2 (ROD) in MT-4 cells. The compounds exhibited significant antibacterial and antifungal activities.
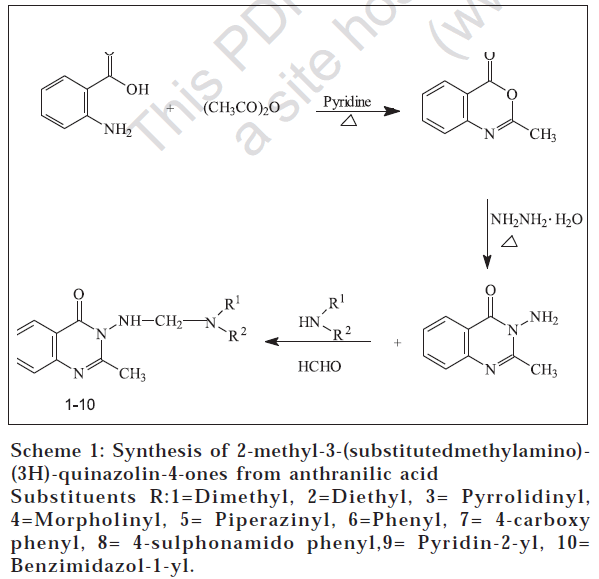
Quinazolines and condensed quinazolines are reported to show potent cytotoxic [1,2] and antimicrobial activities, such as antibacterial [3], antifungal [4] and antiHIV [5,6]. In view of these facts and as a continuation of our earlier studies [5-7] on quinazolines, a series of 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones were synthesized. The title compounds were synthesized by condensing the active hydrogen atom of the amino group of 3-amino-2-methyl-(3H)-quinazolin-4-one with formaldehyde and appropriate amines (Scheme 1). The starting material was synthesized from anthranilic acid using methods reported from our laboratory [8]. The title compounds were screened for antibacterial and antifungal activities by agar dilution method and antiHIV activity against HIV-1(IIIB), HIV-2(ROD) in MT-4 cells.
Melting points were determined in open capillary tubes on a electrically heated melting point apparatus and are uncorrected. IR spectra were recorded in KBr on a Perkin Elmer-841 grating spectrometer (cm-1), Mass spectra on a varian Atlas CH-7 mass spectrometer at 70 eV and NMR Spectra on a varian A-60 or EM-360 spectrometer, using TMS as internal standard. Elemental analysis were performed on a Carlo Erba 1108 CHN analyzer.
The starting material, 3-amino-2-methyl-(3H)-quinazolin-4-one was synthesized by the following method. A mixture of anthranilic acid (1.4g, 0.01mol) and acetic anhydride (9.4 ml, 0.1 mol) was refluxed on gentle flame for 1 h. The excess acetic anhydride was distilled off under reduced pressure and the residue was dissolved in petroleum ether and kept aside for 1 h, to yield 2-methyl-3,1-benzoxazin-one. Yield: 1.2 g (75%); mp: 182°; IR (KBr): 1700 (C=O) and 1640 (C=N); NMR (CDCl3) (δ ppm): 2.5 (s, 3H, CH3), 6.9-7.4 (m, 4H, ArH); MS (m/z) 161 (M+); Anal. (C9H7NO2) C, H, N. A mixture of the above prepared 2-methyl-3,1-benzoxazin-one (1.5 g, 0.01 mol) and hydrazine hydrate (1.4 ml, 0.03 mol) in ethanol was refluxed for 3 h and cooled. The separated solid was recrystallized from ethanol. Yield: 1.3 g (80%); mp: 140-142°; IR (KBr): 3300-3260 (NH2), 1680 (C=O), 1640 (C=N) and 1600 (C=C); NMR (CDCl3) (δ ppm): 2.6 (s, 3H, CH34.6 (s, 2H, NH2), 6.6-7.2 (m, 4H, ArH); Ms (m/z) 175 (M+); Anal. (C9H9N3O) C, H, N.
The title compound 2-methyl-3-(dimethylamino)methylamino-(3H)-quinazolin-4-one (1) was synthesized by adding a mixture of formalin (37-41%; 1 ml) and dimethylamine (0.26 ml, 0.005 mol) drop by drop with stirring to a slurry of 3-amino-2-methyl-(3H)-quinazolin-4-one (0.81 g, 0.005 mol) in dimethylformamide (15 ml). The reaction mixture was heated on a water bath for about 25 min. After cooling, it was poured into ice-water, the solid obtained was filtered, washed with water, dried and recrystallized from ethanol. Yield: 0.82 g (71%); mp: 116°; IR (KBr): 3280 (NH), 2860 (-CH2), 1700 (C=O); NMR (CDCl3) (δ ppm): 2.3 (s, 6H,N(CH3)2), 2.6 (s, 3H, CH3), 5.1 (s, 2H, CH2), 7.2-7.7 (m, 4H,Ar-H), 9.0 (t, 1H, NH); MS (m/z) 232 (M+); Anal. (C12H16N4O) C, H, N. The other compounds 2-10 were synthesized similarly.
The title compounds 1-5 were tested for antiHIV activity against replication of HIV-1(IIIB) and HIV-2(ROD) in MT-4 cells [5]. The MT-4 cells were grown in RPMI-1640 DM (Dutch modification) medium (Flow lab, Irvine, Scotland), supplemented with 10% (v/v) heat inactivated fetal calf serum and 20 μg/ml gentamicin (E. Merck, Darmstadt, Germany). HIV-1 (IIIB) and HIV-2 (ROD) were obtained from the culture supernatant of HIV-1 infected MT-4 cell lines and the virus stocks were stored at -70° until used. The antiHIV assay was carried out in microtiter plates filled with 100 μl of medium and 25 μl volumes of compounds in triplicate so as to allow simultaneous evaluation of their effects on HIV and mock infected cells. Fifty microlitres of HIV at 100 CCID50 medium was added to either infected or mock infected part of microtiter tray. The cell cultures were incubated at 37° in a humidified atmosphere of 5% CO2 in air. Five days after infection the viability of mock and HIV-infected cells were examined spectrophotometrically by the MTT method. The effective dose of compound achieving 50% protection of MT-4 cells against the cytopathic effect (Viruses cause cell degeneration or cell death, which can be seen by microscopical examination of cultures. Cell degeneration is manifested by certain pathological changes) of HIV (EC50), the cytotoxic dose of compound, required to reduce the viability of normal uninfected MT-4 cells by 50% (CC50) were calculated.
The results of antiHIV data (Table 1) indicates that the compound 1 showed 25% protection against HIV-2 (ROD) and the compound 4 showed 21% protection against HIV-1 (IIIB) while the compounds 2 and 3 showed moderate protection at sub toxic concentration.
| Compound code | Strain | ECa 50 (µg/ml) | CCb 50 (µg/ml) | Max protection |
|---|---|---|---|---|
| 1 | HIV-1(IIIB) | >15.4 | 15.4 | 2 |
| >25.0 | >25.0 | 0 | ||
| HIV-2(ROD) | >25.0 | >25.0 | 25 | |
| >52.9 | >52.9 | 1 | ||
| 2 | HIV-1(IIIB) | >54.9 | 54.9 | 2 |
| >41.4 | 41.4 | 0 | ||
| HIV-2(ROD) | >77.9 | 77.9 | 12 | |
| >62.0 | 62 | 0 | ||
| 3 | HIV-1(IIIB) | >14.0 | 14 | 1 |
| >15.2 | 15.2 | 1 | ||
| HIV-2(ROD) | >25.0 | 25 | 17 | |
| >35.7 | >35.7 | 0 | ||
| 4 | HIV-1(IIIB) | >50.0 | 50 | 21 |
| >65.9 | 65.9 | 1 | ||
| HIV-2(ROD) | >69.5 | 69.5 | 10 | |
| >71.7 | 71.7 | 3 | ||
| 5 | HIV-1(IIIB) | >12.2 | >12.2 | 0 |
| >13.3 | >13.3 | 0 | ||
| AZT | HIV-2(ROD) | >25.0 | >25.0 | 0 |
| HIV-1(IIIB) | 0.0012 | 65.9 | 0 | |
| HIV-2(ROD) | 0.00062 | 65.9 | 0 |
aEffective concentration of compound, achieving 50% protection of MT-4cells against the cytopathic effect of HIV; bCytotoxic concentration of compound required to reduce the viability of normal uninfected MT-4 cells by 50%.
Table 1: Anti HIV activity and cytotoxicity of test compounds in mt-4 cells
The compounds 1-10 were tested for antibacterial and antifungal activities by agar dilution method [9,10], all bacteria were grown on Muller-Hington Agar (Hi-media) plates (37°, 24 h) and fungi were grown on sabouraud dextrose agar (Hi-media) plates (26°, 48-72 h). The minimum inhibitory concentration (MIC) was considered to be the lowest concentration that completely inhibited the growth on agar plates, disregarding a single colony or faint haze caused by the innoculum. The MIC values of the synthesized compounds against 7 bacteria and 3 fungi are presented in Table 2. The activity of reference compounds, norfloxacin and clotrimazole are also included.
| Microorganism | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Norfloxacin | Clotrimazole |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K. pneumoniae | 156 | 39 | 39 | 156 | 39 | 78 | 39 | 78 | 9 | 156 | 2 | - |
| P. aeruginosa | 78 | 39 | 39 | 78 | 156 | 78 | 9 | 39 | 78 | 78 | 1 | - |
| E. tarda | 39 | 156 | 156 | 39 | 156 | 78 | 78 | 78 | 156 | 39 | 1 | - |
| B. subtilis | 156 | 39 | 39 | 78 | 156 | 39 | 9 | 78 | 9 | 78 | 1 | - |
| S. typhimurium | 39 | 156 | 78 | 39 | 78 | 39 | 9 | 78 | 9 | 78 | 4 | - |
| P. vulgaris | 78 | 39 | 39 | 39 | 156 | 39 | 78 | 156 | 78 | 39 | 1 | - |
| S. paratyphi | 156 | 156 | 39 | 39 | 78 | 156 | 9 | 156 | 9 | 156 | 9 | - |
| C. albicans | 39 | 39 | 156 | 39 | 78 | 39 | 39 | 39 | 78 | 156 | - | 1 |
| A. niger | 78 | 39 | 39 | 156 | 39 | 39 | 156 | 39 | 156 | 39 | - | 2 |
| M. audouinii | 39 | 39 | 39 | 39 | 19 | 78 | 156 | 78 | 19 | 19 | - | 19 |
aMIC. Minimum inhibitory concentration
Table 2: Antibacterial and antifungal activities of test compounds (mic values (?g/ml))a
The results of antibacterial activity (Table 2) revealed that all the test compounds showed moderate activity against the tested bacteria. The compound with 4-carboxyphenylcompound substitution (7) and 2-pyridyl substitution (compound 9) exhibited equipotent activity with norfloxacin against S. paratyphi. The compound 7 exhibited good activity against P. aeruginosa, B. subtilis and S. typhinurium. The compound 9 showed good activity against K. pneumoniae, B. subtilis and S. typhinurium. Compounds with heterocyclic substituents (compound 7, 9 and 10) showed equipotent activity with the reference standard clotrimazole against M. audouinii.
References
- The Merck Index, 13th Edn., Merck & Co., Inc, Whitehouse Station, NJ, 2001, 318.
- Martindale, 32nd Edn., The Pharmaceutical Press, London, 1999, 828.
- European Pharmacopoeia, Supplement 4.1, 4th Edn, Directorate for the Quality of Medicine of the Council of Europe (EDQM), 2001, 2449.
- Thomas, L.C., Colorimetric Chemical Analytical Methods, 8th Edn.,Tintometer Ltd. Salisbury, England, 1974, 27 .
- Diamond, R.M., Solvent Extraction Chemistry, North Holland, Amsterdam, 1967, 349.
- Bera, A. and Pal, D.K., Indian Drugs , 2001, 38, 112.
- Shingbal, D.M. and Tadas, D., J. Inst. Chemists (India), 2002, 74, 43.
- Sankar, G.D., Raju, G.V.H., Satyanarayana, K.N.V. and Ganapaty, S., J. Inst. Chemists (India), 2002, 74, 34.
- Radi, A. and Elmogy, T., Farmaco , 2005, 60, 43. Back to cited text no. 9
- Placek, P., Macek, J. and Klima, J., J. Chromatogr. B., 2003, 789, 405.