- *Corresponding Author:
- D. H. Ahn
Institute of Fisheries Sciences,
Pukyong National University,
Busan 46041,
Republic of Korea
E-mail: dhahn@pknu.ac.kr
| Date of Received | 14 December 2020 |
| Date of Revision | 19 August 2021 |
| Date of Acceptance | 22 February 2022 |
| Indian J Pharm Sci 2022;84(1):232-239 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study investigated the effects and the anti-inflammatory activity of Sargassum muticum ethanol extract in lipopolysaccharide-induced RAW 264.7 murine macrophage cells and in a croton oil-induced mouse ear edema model. Pretreatment of lipopolysaccharide-induced RAW 264.7 cells with Sargassum muticum ethanol extract (0.1-100 μg/ml) inhibited lipopolysaccharide-induced production of nitric oxide, interleukin-6 and tumour necrosis factor alpha in a dose-dependent manner. The expression of lipopolysaccharide-induced, inducible nitric oxide synthase and cyclooxygenase-2 in the Sargassum muticum ethanol extract-treated group was also suppressed in a dose-dependent manner. Furthermore, we found that Sargassum muticum ethanol extract induced anti-inflammatory effects by inhibiting Mitogenactivated protein kinases (extracellular signal-regulated kinase, c-Jun N-terminal kinase and p38) and nuclear factor kappa B, p65 phosphorylation in lipopolysaccharide-stimulated RAW 264.7 cells. The antiinflammatory activity of Sargassum muticum ethanol extract in vivo was evaluated in the ear edema model of a croton oil-treated mouse. Compared to the untreated control, croton oil-induced ear edema was found to be reduced by about 33 % upon treatment with 250 mg/kg Sargassum muticum ethanol extract.
Keywords
Anti-inflammation, Sargassum muticum, nuclear factor kappa B, mitogen-activated protein kinases, ear edema
Inflammation is a complex biological process caused by activated immune cells and is an innate immune response to protect the body from infection and foreign antigens[1,2]. Persistent inflammation, however, promotes mucosal injury causing pain, edema, fever and other dysfunctions. Chronic inflammation can cause diseases such as cancer and arthritis[3]. Macrophages play a central role in the activation of inflammatory mediators such as pro-inflammatory cytokines and control their overproduction under inflammatory conditions[1,4]. In the early stages of external infection, Nitric Oxide (NO) and cytokines are secreted, which play an important role in biological defense. Macrophages are reactivated by these secretions and are involved in inflammatory reactions[5].
Lipopolysaccharide (LPS) is an extracellular membrane component of gram-negative bacteria that activates macrophages[6]. LPS stimulates Toll-Like Receptor 4 (TLR4) on the macrophage surface, leading to the activation of Mitogen-Activated Protein Kinase (MAPK), a subcellular signaling pathway. These signaling pathways in turn activate Nuclear Factor kappa B (NF-κB), a nuclear transcription factor, which induces transcription of inflammation regulators such as various pro-inflammatory cytokines, inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase-2 (COX- 2) and increases secretion of Interleukin (IL)-6, IL-1 beta (β), Tumor Necrosis Factor alpha (TNF-α), NO and Prostaglandin E2 (PGE2) . Therefore, inhibition of NF-κB and MAPK activities is important for the treatment of inflammatory responses.
Seaweed has been widely consumed and used in Asia as a functional food and medicinal herb[9]. Seaweeds are rich in lipids, minerals, dietary fiber and vitamins. They are considered as functional food as they contain various bioactive substances such as proteins, polysaccharides and polyphenols that have been shown to have beneficial effects on oxidative stress, cancer, diabetes, inflammation[10], thrombosis[11], allergies[12] and obesity[13].
Sargassum muticum (S. muticum) is a brown algae belonging to the genus Sargassum[14] and is widely distributed in the southern and eastern coasts of Korea. S. muticum is a polyphenol-rich seaweed that has been shown to have biological activities such as antioxidant, antiproliferative and anti-angiogenesis activities[15]. Therefore, the present study further examined the anti- inflammatory activity of S. muticum Ethanol Extract (SMEE) in a croton oil-induced ear edema mouse model and the impact of not only NF-κB but also MAPKs in inflammatory pathways.
S. muticum was collected at Song-Jung, Busan, Korea in 2016. It was washed with tap water to remove salt and sand. After air-dry at room temperature for 1 d, it was lyophilized, powdered, packed in vacuum and stored at -20°. Powdered S. muticum was extracted with 95 % ethanol at room temperature for 24 h with an agitator (H-0820, Dongwon Science Co., Busan, Korea). The extraction process was carried out twice. The extract was then centrifuged at 2090×g for 10 min and the supernatant was filtered. The filtrate was concentrated using a rotary evaporator (RE200, Yamato Co., Tokyo, Japan).
Male Institute of Cancer Research (ICR) mice (8 w old) and female Bagg and Albino (BALB)/c mice (10 w old) were purchased from Orient Bio (Seongnam, Korea). Mice were raised in an animal facility maintained at a temperature of 20°±2°, humidity of 50 %±10 % and 12/12 h light-dark cycles for a week before the experiment. All experimental protocols for animal care were performed in accordance with the rules and regulations of the Animal Ethics Committee, Pukyong National University, Busan, Korea (Approval No. 201504).
The murine macrophage cell line RAW 264.7 was purchased from Korean Cell Line Bank (KCLB 40071). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; GIBCO, Grand Island, New York, United States of America (USA)) supplemented with 10 % inactivated Fetal Bovine Serum (FBS; Hyclone, Logan, Utah, USA) and 1 % penicillin-streptomycin (Hyclone, Logan, Utah, USA) in a 5 % Carbon Dioxide (CO2) incubator (MCO15AC, Sanyo, Osaka, Japan) at 37°. Cells were sub-cultured every 3 d and counted with a hemocytometer. The number of viable cells was determined by trypan blue dye exclusion staining.
The cytotoxicity of SMEE was measured using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) reagents (Sigma-Aldrich Co., St. Louis, Missouri, USA). RAW 264.7 cells (1×106 cells/ ml) were seeded on 96-well plates and pre-incubated for 20 h. The cells were then cultured with different concentrations of SMEE (0.1, 1, 10, 50 and 100 μg/ ml) for 22 h at 37° and 5 % CO2. MTT reagent (5 mg/ ml) was added and the cells were incubated for 2 h. The medium was then discarded and Dimethylsulfoxide (DMSO; Sigma-Aldrich Co., St. Louis, Missouri, USA) was added to each well and absorbance was measured at 540 nm with a microplate reader (Model 550, Bio- Rad, Richmond, Virginia, USA). The cell proliferation ability was calculated according to the following formula: Proliferation index (%)=(Absorbance of the sample/Absorbance of the control)×100.
RAW 264.7 cells (2.5×105 cells/ml) were pre-cultured for 20 h and then incubated for 24 h in presence of 1 μg/ml LPS (Sigma-Aldrich Co., St. Louis, Missouri, USA) and SMEE (0.1, 1, 10, 50 and 100 μg/ml). After that, 100 μl of cell culture medium and the same amount of Griess reagent (1 % sulfanilamide and 0.1 % naphthalene diamine dihydrochloride in 5 % phosphoric acid) were mixed and incubated at room temperature for 10 min. The absorbance was measured at 540 nm using a microplate reader (Model 550) and the amount of nitrite was calculated using a standard curve of Sodium Nitrite (NaNO2).
RAW 264.7 cells (2.5×105 cells/ml) were incubated for 24 h in presence of LPS (1 μg/ml) and different concentrations of SMEE (0.1, 1, 10, 50 and 100 μg/ml). The levels of TNF-α and IL-6 in culture medium were determined using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (Mouse ELISA set; BD Biosciences, San Diego, California, USA) according to the manufacturer’s instructions.
RAW 264.7 cells were incubated for 24 h with SMEE (0.1, 1, 10, 50 and 100 μg/ml) and LPS (1 μg/ml) and cells were collected. The cells were washed three times with cold Phosphate Buffered Saline (PBS) and lysed in lysis buffer (50 mM 4-(2-Hydroxyethyl)-1- Piperazineethanesulfonic Acid (HEPES) (pH 7.4), 150 mM Sodium Chloride (NaCl), 5 mM Ethylenediamine Tetraacetic Acid (EDTA), 1 % deoxycholate, 5 mM Phenylmethylsulfonyl Fluoride (PMSF), 1 μg/ml aprotinin, 1 % Triton X-100 and 0.1 % NP-40) and kept on ice for 30 min. In the case of analyses involving NF-κB p65, nucleus lysis buffer (10 mM HEPES, 100 mM NaCl, 1.5 mM Magnesium Chloride (MgCl2), 0.1 mM EDTA, 0.1 mM dithiothreitol) was added and the cells were lysed on ice for 30 min. The cell lysates were centrifuged at 15 520×g for 20 min and the protein concentration in the supernatant was measured by Pierce Bicinchoninic Acid (BCA) protein assay kit (Thermo Scientific, NY, USA). Protein samples were separated by 10 % Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and then transferred on to Polyvinylidene Fluoride (PVDF) membranes (Bio-rad, USA) with transfer buffer (25 mM Tris-HCl (pH 8.8), 192 mM glycine, 20 % methanol). After blocking nonspecific sites with 5 % skim milk (Fluka, Switzerland) in 0.1 % Tris-Buffered Saline (TBS)-Tween 20 for 2 h, the membranes were incubated with specific primary anti-mouse iNOS, COX-2, NF-κB p65, phosphorylated c-Jun N-Terminal Kinase (p-JNK), phosphorylated Extracellular Signal-Regulated Kinase (p-ERK) and p-p38 antibodies in TBS (1:500) at 4°, overnight. Each membrane was further incubated for 1 h with secondary antibody, Horseradish Peroxidase (HRP)- conjugated anti-mouse Immunoglobulin G (IgG) and anti-rabbit IgG (1:2000). The immune-active proteins were detected using an Enhanced Chemiluminescence (ECL) detector (Pierce, USA) and the signal intensity of each protein band was measured by densitometry, employing the Gene Tools from Syngene software (Synoptics Ltd., Cambridge, United Kingdom (UK).
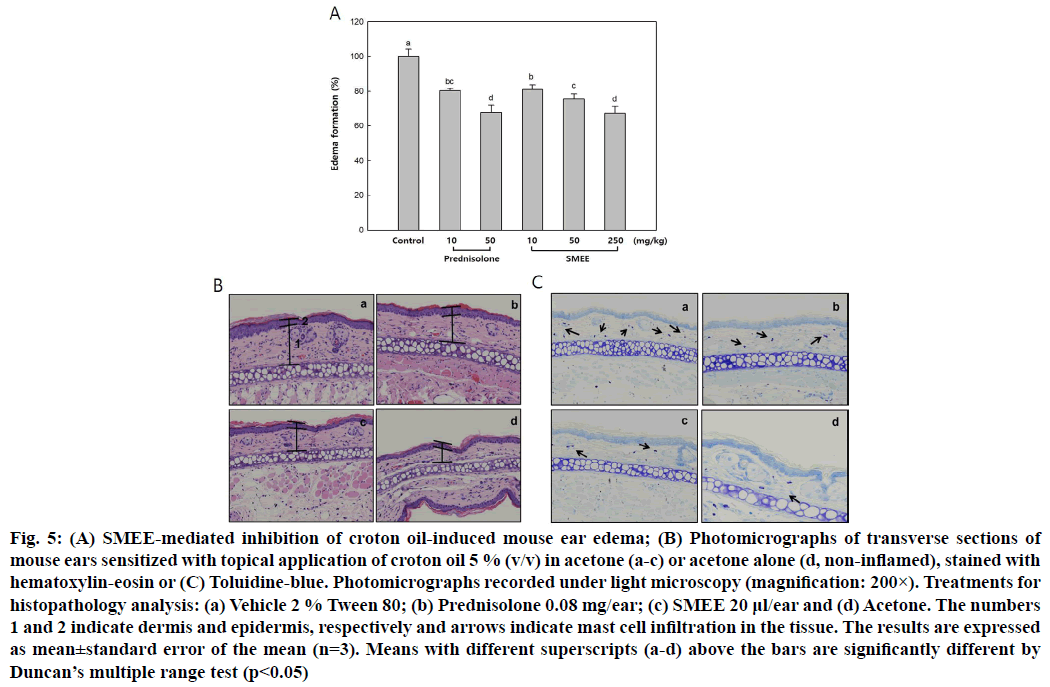
To evaluate the anti-inflammatory effects of SMEE in vivo, ear edema measurements were performed[16]. SMEE (200 μl) was orally administered to 8 w old male ICR mice at concentrations of 10, 50 and 250 mg/kg body weight. After 1 h, 2.5 % croton oil was applied at a concentration of 20 μl/ear on the inner and outer surfaces of the right ear. Ear thickness was measured 5 h after application and an increase in ear thickness after croton oil treatment was considered to be edema formation.
For histopathology analysis, 20 μl/ear of 100 mg/ml SMEE was applied to the right ear of the ICR mouse and was followed by application of 20 μl of 5 % croton oil 15 min later. Mice were sacrificed after 6 h, following which ear tissues were dissected and fixed in 10 % formaldehyde for 72 h. The tissue slices were embedded in paraffin and sections were deparaffinized and stained with hematoxylin-eosin and toluidine-blue stains to allow visualization of the tissue and mast cells.
10 w old female BALB/c mice (n=5) were used for the experiment. The mice were fasted for about 4 to 6 h immediately before the acute toxicity test. SMEE was orally administered at concentrations of 300, 2000 and 5000 mg/kg body weight and 5 % Tween 80 was orally administered for the control group. All mice were observed for general symptoms at least once daily. On the day of administration, observations were made every hour for 30 min after administration and up to 6 h thereafter and general symptoms were observed at least once a day until 14 d after administration.
Data are expressed as mean±standard error of the mean (n=3). Statistical evaluation was carried out using analysis of variance with Statistical Analysis System (SAS) software (SAS Institute, Inc., Cary, North Carolina, USA), according to Duncan’s multiple range test (p<0.05).
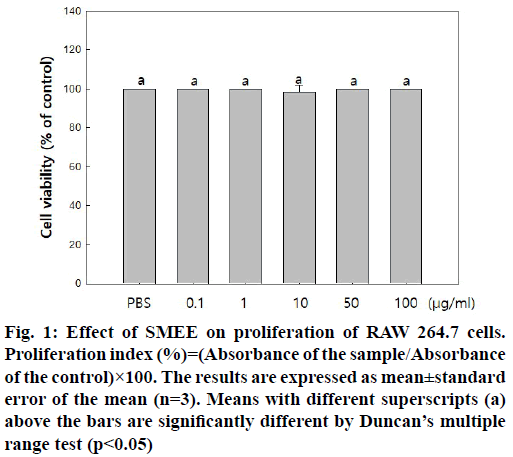
The cytotoxicity of SMEE in RAW 264.7 cells was determined based on MTT assay. Treatment with 0.1 to 100 μg/ml SMEE had no significant effect on MTT- based cell viability (fig. 1). Therefore, SMEE was used at a concentration of 0.1 to 100 μg/ml in subsequent experiments.
Fig. 1: Effect of SMEE on proliferation of RAW 264.7 cells. Proliferation index (%)=(Absorbance of the sample/Absorbance of the control)×100. The results are expressed as mean±standard error of the mean (n=3). Means with different superscripts (a) above the bars are significantly different by Duncan’s multiple range test (p<0.05)
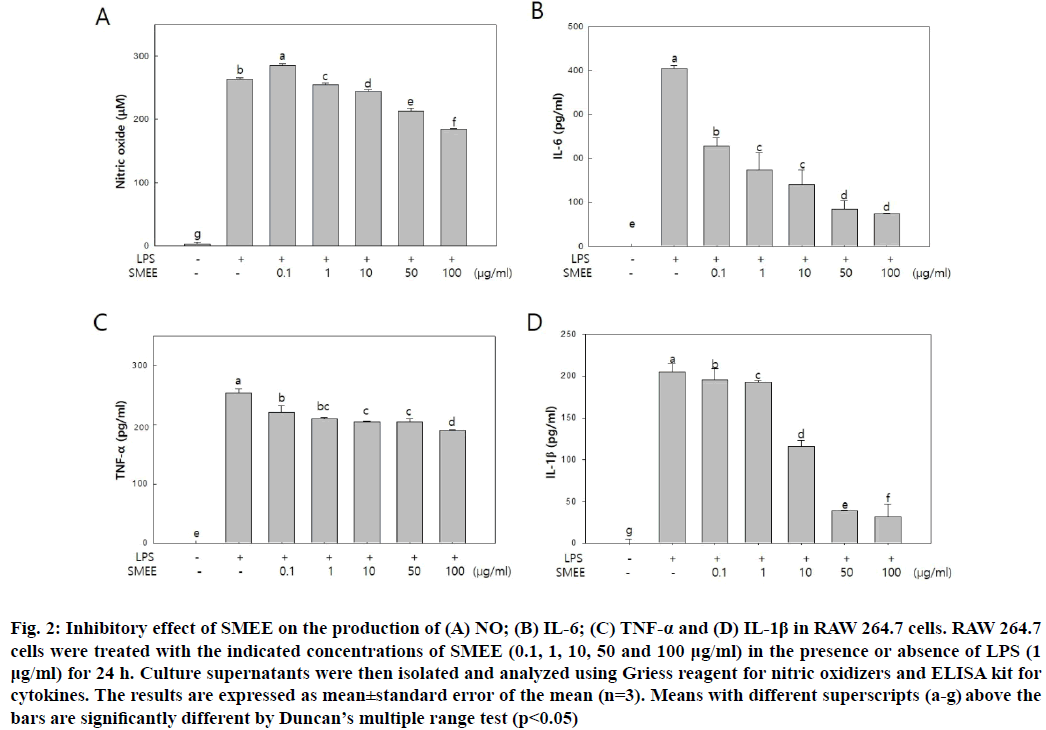
In order to investigate whether SMEE could control NO and pro-inflammatory cytokine production, NO and pro-inflammatory cytokine (IL-6 and TNF-α) secretion was measured in LPS-induced RAW 264.7 cells. As shown in fig. 2A, SMEE suppressed NO production in a dose-dependent manner. According to a previous study that polyphenols extracted from S. muticum significantly inhibited NO release of LPS-induced RAW 264.7 cells[17], it can be inferred that the inhibition of NO is due to polyphenols, a bioactive component of S. muticum.
The secretion of pro-inflammatory cytokines (IL- 6, TNF-α and IL-1β) in the SMEE treated group decreased in a dose-dependent manner compared to that in the LPS-treated group. In particular, IL-6 secretion showed a significant decrease at SMEE concentrations of 0.1~100 μg/ml and decreased about 82.3 % at a concentration of 100 μg/ml (fig. 2B). In case of TNF-α, secretion decreased about 27.5 % at SMEE concentration of 100 μg/ml (fig. 2C). In addition, IL-1β secretion was decreased by 80.90 % and 84.64 % at SMEE concentrations of 50 and 100 μg/ml, respectively (fig. 2D).
Fig. 2: Inhibitory effect of SMEE on the production of (A) NO; (B) IL-6; (C) TNF-α and (D) IL-1β in RAW 264.7 cells. RAW 264.7 cells were treated with the indicated concentrations of SMEE (0.1, 1, 10, 50 and 100 μg/ml) in the presence or absence of LPS (1 μg/ml) for 24 h. Culture supernatants were then isolated and analyzed using Griess reagent for nitric oxidizers and ELISA kit for cytokines. The results are expressed as mean±standard error of the mean (n=3). Means with different superscripts (a-g) above the bars are significantly different by Duncan’s multiple range test (p<0.05)
The macrophage RAW 264.7 cells are activated by the stimulation of antigens such as LPS to promote the secretion of cytokines such as IL-6, TNF-α and IL- 1β to regulate the immune response[18]. The secretion of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β plays an important role in the development of early inflammatory responses[19]. TNF-α produced by LPS-stimulated macrophages and monocytes is cytotoxic to tumor cells and is associated with chronic inflammatory diseases such as autoimmune diseases, rheumatoid arthritis and inflammatory bowel disease[20]. In addition, TNF-α sustains the inflammatory response by inducing the production of other cytokines[21]. Therefore, it is important to reduce pro-inflammatory cytokine secretion in anti-inflammatory treatment.
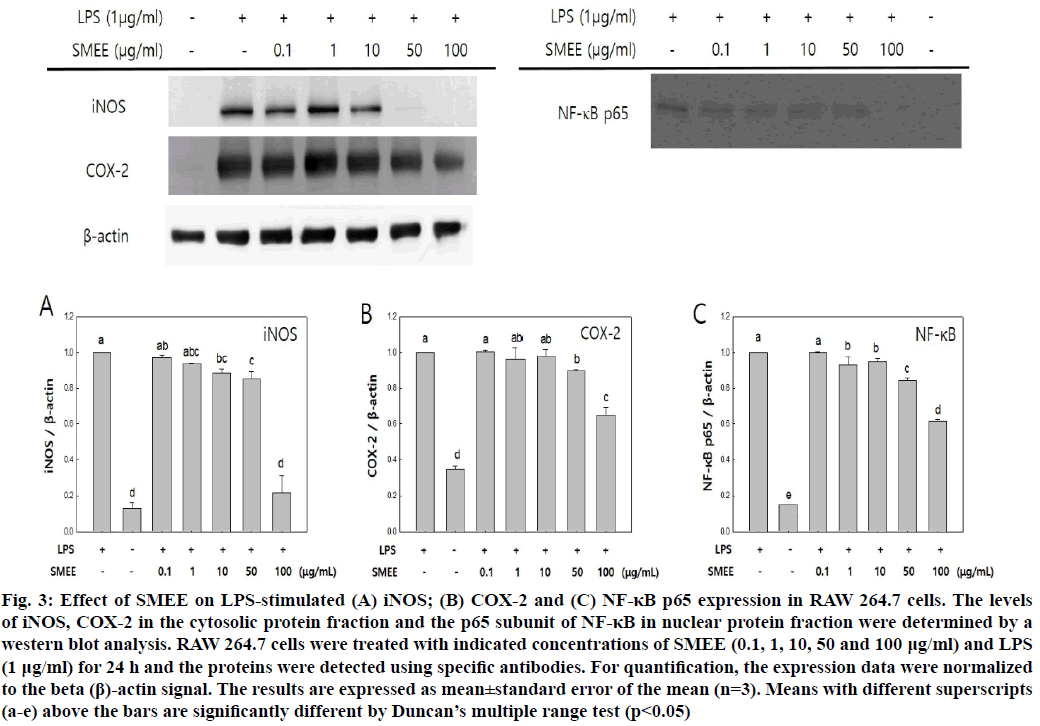
The effect of SMEE on the expression of iNOS and COX-2 in the cytoplasm and NF-κB p65 in the nucleus was investigated. In the control group, expression of each protein was increased on LPS stimulation, but in the SMEE-treated group, the expression of LPS- stimulated iNOS, COX-2 and NF-κB p65 was inhibited in a dose-dependent manner by SMEE (fig. 3). In particular, it was confirmed that the expression level of each protein significantly decreased at SMEE of 100 μg/ml. These results suggest that the inhibition of NO production in fig. 2A may be related to the regulation of iNOS expression. This suggests that inhibition of iNOS, COX-2 and pro-inflammatory cytokines (fig. 2B) may be due to inhibition of NF-κB p65 activation.
Fig. 3: Effect of SMEE on LPS-stimulated (A) iNOS; (B) COX-2 and (C) NF-κB p65 expression in RAW 264.7 cells. The levels of iNOS, COX-2 in the cytosolic protein fraction and the p65 subunit of NF-κB in nuclear protein fraction were determined by a western blot analysis. RAW 264.7 cells were treated with indicated concentrations of SMEE (0.1, 1, 10, 50 and 100 μg/ml) and LPS (1 μg/ml) for 24 h and the proteins were detected using specific antibodies. For quantification, the expression data were normalized to the beta (β)-actin signal. The results are expressed as mean±standard error of the mean (n=3). Means with different superscripts (a-e) above the bars are significantly different by Duncan’s multiple range test (p<0.05)
NF-κB binds to promoters of genes related to the inflammatory response and activated NF-κB is known to be primarily involved in the expression of COX-2 and iNOS[22]. iNOS produces NO when activated by stimuli such as LPS, cytokines and bacterial toxins. Excessive NO production is known to cause inflammation[8,23]. COX-2 is involved in the biosynthesis of PGE2 and causes inflammation by increasing the vascular permeability of free PGE2[24].
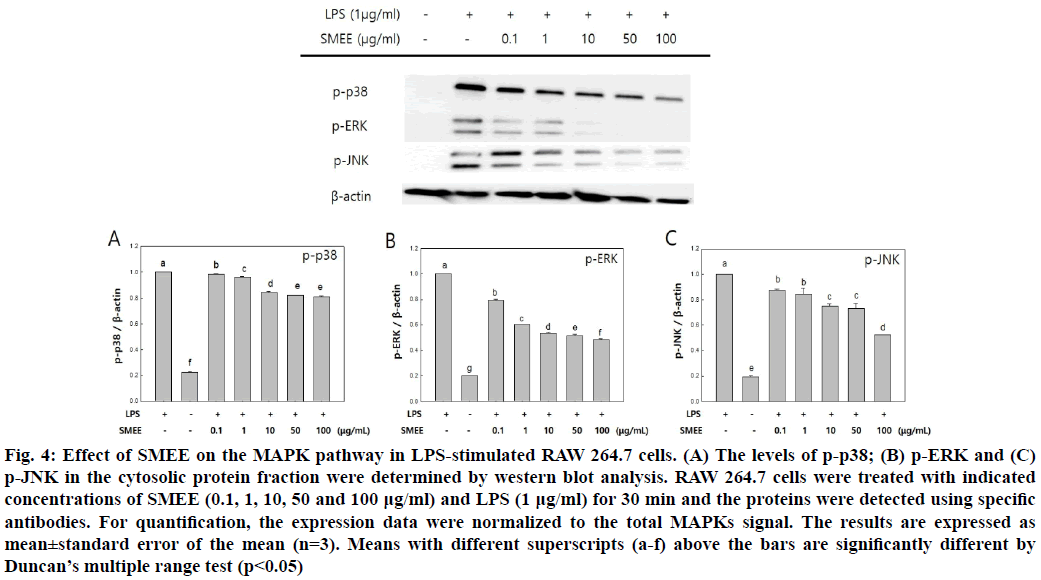
The effect of SMEE on phosphorylation of MAPKs, such as p38, ERK1/2 and JNK subfamilies in LPS- induced RAW 264.7 cells was investigated by western blot analysis. The SMEE treatment was found to reduce the expression levels of p-ERK and p-JNK in LPS-induced RAW 264.7 cells (fig. 4). Especially, the expression level of p-ERK was significantly decreased at SMEE concentrations of 10 μg/ml and higher (fig. 4B).
Fig. 4: Effect of SMEE on the MAPK pathway in LPS-stimulated RAW 264.7 cells. (A) The levels of p-p38; (B) p-ERK and (C) p-JNK in the cytosolic protein fraction were determined by western blot analysis. RAW 264.7 cells were treated with indicated concentrations of SMEE (0.1, 1, 10, 50 and 100 μg/ml) and LPS (1 μg/ml) for 30 min and the proteins were detected using specific antibodies. For quantification, the expression data were normalized to the total MAPKs signal. The results are expressed as mean±standard error of the mean (n=3). Means with different superscripts (a-f) above the bars are significantly different by Duncan’s multiple range test (p<0.05)
MAPKs (ERK, JNK, p-38) are important signaling molecules that mediate macrophage activity. MAPKs can localize to the nucleus and induce the production of other immune activators to further activate the inflammatory response[25]. MAPKs are activated by phosphorylation and are known to play an important role in the expression of iNOS, COX-2 and pro- inflammatory mediators by activating NF-κB[26].
In this study, SMEE inhibited the phosphorylation of NF-κB and MAPKs that were activated by LPS and resulted in reduced production of iNOS, COX-2, NO and pro-inflammatory cytokines (fig. 2-fig. 4). These results are similar to the reports on S. miyabei Yendo extract and S. patens Carl Adolph Agardh extract, known as brown algae in Sargassum, which exhibit anti-inflammatory effects by inhibiting the expression of NF-κB and MAPKs[27,28].
To investigate the anti-inflammatory effects of SMEE on acute inflammation, we performed a croton oil- induced ear edema test using a mouse model. It was confirmed that ear thickness decreased significantly at all concentrations of SMEE when compared to the untreated control group. In particular, it was reduced by about 33 % at 250 mg/kg of SMEE and slightly decreased compared with 50 mg/kg treatment of prednisolone, a steroidal anti-inflammatory drug (fig. 5A). The effect of SMEE on ear edema relaxation was also observed in tissues. In the tissues of mouse ear edema induced by croton oil, treatment with 100 mg/ml SMEE was effective in reducing the dermis and epidermis thickness of tissues to a degree similar to that observed with prednisolone treatment (fig. 5B).
In addition, the degree of mast cell infiltration was confirmed by toluidine-blue staining and treatment with SMEE significantly inhibited mast cell infiltration in the tissues (fig. 5C).
Fig. 5: (A) SMEE-mediated inhibition of croton oil-induced mouse ear edema; (B) Photomicrographs of transverse sections of mouse ears sensitized with topical application of croton oil 5 % (v/v) in acetone (a-c) or acetone alone (d, non-inflamed), stained with hematoxylin-eosin or (C) Toluidine-blue. Photomicrographs recorded under light microscopy (magnification: 200×). Treatments for histopathology analysis: (a) Vehicle 2 % Tween 80; (b) Prednisolone 0.08 mg/ear; (c) SMEE 20 μl/ear and (d) Acetone. The numbers 1 and 2 indicate dermis and epidermis, respectively and arrows indicate mast cell infiltration in the tissue. The results are expressed as mean±standard error of the mean (n=3). Means with different superscripts (a-d) above the bars are significantly different by Duncan’s multiple range test (p<0.05)
Inflammation is a protective reaction against physical and chemical damage of the human body. Representative responses are vasodilation, increased cell membrane fluidity and edema[29]. Mast cells activated by external stimuli secrete vasodilators such as various proteases and histamines. Due to the increase in blood flow, neutrophils in the blood migrate to tissues outside the blood vessels, causing edema and pain due to an increase in prostaglandin at the site of inflammation[30]. Therefore, it is possible to prove the efficacy of antiinflammatory substances by experimentally verifying the effects of inhibiting these responses.
In this study, we evaluated the anti-inflammatory activity of SMEE in vivo using the croton oil treated-ear edema model. SMEE (250 mg/kg) relieved ear edema caused by croton oil by about 33 % compared to the control group and significantly inhibited mast cell infiltration in the tissues (fig. 5). According to the study by Jeon et al.[31], treatment of S. muticum extract reduced the expression of inflammatory cytokines IL-6 and TNF-α, contributing to the relief of edema and symptoms in Collagen-Induced Arthritis (CIA) DBA/1J mice model. Therefore, SMEE treatment seems to cause edema relief by reducing the expression of inflammatory cytokines and inhibiting mast cell infiltration in damaged tissues.
The acute oral toxicity of SMEE was assessed by administering 300, 2000 and 5000 mg/kg body weight of SMEE to mice over 2 w. No death and abnormal behaviors were observed among the treated mice (Table 1). According to the World Health Organization (WHO), a medicinal herb is considered toxic if its median Lethal Dose (LD50) is less than 5 g/kg body weight[32]. This indicates that the concentration of SMEE used in this study is nontoxic and safe for human use.
| Groups (mg/kg body weight) | |||||
|---|---|---|---|---|---|
| 0 | 300 | 2000 | 5000 | ||
| Mortalities | Number of dead animals | 0/5 | 0/5 | 0/5 | 0/5 |
| Percentage (%) | 0 | 0 | 0 | 0 | |
| Clinical signs | No abnormalities | 5/5 | 5/5 | 5/5 | 5/5 |
Table 1: Mortality and Clinical Signs of BALB/c Mice Treated Orally With SMEE
In summary, SMEE significantly inhibited the secretion of pro-inflammatory cytokines, iNOS and COX-2 expression and phosphorylation of NF-κB and MAPK in LPS-stimulated RAW 264.7 cells (p<0.05). In addition, SMEE treatment showed edema inhibition in vivo, similar to that of prednisolone, an antiinflammatory agent. Thus, these results suggest the possibility of developing therapeutics using the remarkable anti- inflammatory effects of SMEE.
Acknowledgements:
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A6A1028677).
Conflict of interests:
The authors declare that there are no conflicts of interest.
References
- Lundberg IE. The role of cytokines, chemokines and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep 2000;2(3):216-24.
[CrossRef] [Google Scholar] [Pub Med]
- Lee ST, Jeong YR, Ha MH, Kim SH, Byun MW, Jo SK. Induction of nitric oxide and TNF-α by herbal plant extract in mouse macrophage. J Korean Soc Food Sci Nutr 2000;29:342-348.
- Ljung T, Lundberg S, Varsanyi M, Johansson C, Schmidt PT, Herulf M, et al. Rectal nitric oxide as biomarker in the treatment of inflammatory bowel disease: Responders vs. nonresponders. World J Gastroenterol 2006;12(21):3386-92.
[CrossRef] [Google Scholar] [Pub Med]
- Rochon YP, Frojmovic MM. A model for the recruitment of neutrophils at sites of inflammation. Physiological relevance of in vivo neutrophil aggregation. Med Hypotheses 1992;38(2):132-8.
[CrossRef] [Google Scholar] [Pub Med]
- Kang BK, Kim KB, Kim MJ, Bark SW, Pak WM, Kim BR, et al. Anti-inflammatory activity of an ethanol extract of Laminaria japonica root on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. Korean J Food Sci Technol 2014;46(6):729-33.
- Beutler B, Rietschel ET. Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol 2003;3(2):169-76.
[CrossRef] [Google Scholar] [Pub Med]
- Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, et al. Future research directions in acute lung injury: Summary of a National Heart, Lung and Blood Institute working group. Am J Respir Crit Care Med 2003;167(7):1027-35.
[CrossRef] [Google Scholar] [Pub Med]
- Yun HY, Dawson VL, Dawson TM. Neurobiology of nitric oxide. Crit Rev Neurobiol 1996;10(3-4):291-316.
[CrossRef] [Google Scholar] [Pub Med]
- Liu L, Heinrich M, Myers S, Dworjanyn SA. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J Ethnopharmacol 1996;142:591-619.
[CrossRef] [Google Scholar] [Pub Med]
- Khan MN, Choi JS, Lee MC, Kim E, Nam TJ, Fujii H, et al. Anti-inflammatory activities of methanol extracts from various seaweed species. J Environ Biol 2008;29(4):465-9.
- Nishino T, Fukuda A, Nagumo T, Fujihara M, Kaji E. Inhibition of the generation of thrombin and factor Xa by a fucoidan from the brown seaweed Ecklonia kurome. Thromb Res 1999;96(1):37-49.
[CrossRef] [Google Scholar] [Pub Med]
- Zuercher AW, Fritsche R, Corthésy B, Mercenier A. Food products and allergy development, prevention and treatment. Curr Opin Biotechnol 2006;17(2):198-203.
[CrossRef] [Google Scholar] [Pub Med]
- Miyashita K. The carotenoid fucoxanthin from brown seaweed affects obesity. Lipid Technol 2009;21(8‐9):186-90.
- Edwards M, Hanniffy D, Heesch S, Hernandez-Kantun J, Queguineur B, Ratcliff J, et al. Microalgae fact-sheets. NUI, Ireland, Galway; 2014.
- Namvar F, Mohamad R, Baharara J, Zafar-Balanejad S, Fargahi F, Rahman HS. Antioxidant, antiproliferative and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). Biomed Res Int 2013;2013:1-9.
[CrossRef] [Google Scholar] [Pub Med]
- Jeong DH, Kim MJ, Kang BK, Ahn DH. Anti-inflammatory activity of methanol extract and n-hexane fraction mojabanchromanol b from Myagropsis myagroides. Life Sci 2014;114(1):12.
[CrossRef] [Google Scholar] [Pub Med]
- Yu Y, Wang L, Fu X, Wang L, Fu X, Yang M, et al. Anti-oxidant and anti-inflammatory activities of ultrasonic-assistant extracted polyphenol-rich compounds from Sargassum muticum. J Oceanol Limnol 2019;37(3):836-47.
- Xu X, Jeong SM, Lee JE, Kang WS, Ryu SH, Kim K, et al. Characterization of Undaria pinnatifida root enzymatic extracts using crude enzyme from Shewanella oneidensis PKA 1008 and its anti-inflammatory effect. J Microbiol Biotechnol 2020;30:79–84.
[CrossRef] [Google Scholar] [Pub Med]
- Lin HI, Chu SJ, Wang D, Feng NH. Pharmacological modulation of TNF production in macrophages. J Microbiol Immunol Infect 2004;37(1):8-15.
- Yang R, Yang L, Shen X, Cheng W, Zhao B, Ali KH, et al. Suppression of NF-κB pathway by crocetin contributes to attenuation of lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol 2012;674(2-3):391-6.
[CrossRef] [Google Scholar] [Pub Med]
- Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression: The role of TATA-binding protein (TBP). J Biol Chem 1999;274(43):30858-63.
[CrossRef] [Google Scholar] [Pub Med]
- Celec P. Nuclear factor kappa B—molecular biomedicine: The next generation. Biomed Pharmacother 2004;58(6-7):365-71.
[CrossRef] [Google Scholar] [Pub Med]
- Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology and pharmacology. Pharmacol Rev 1991;43:109-142.
- Duerksen-Hughes PJ, Day DB, Laster SM, Zachariades NA, Aquino L, Gooding LR. Both tumor necrosis factor and nitric oxide participate in lysis of simian virus 40-transformed cells by activated macrophages. J Immunol 1992;149(6):2114-22.
- Eum WS, Lee KJ, Kim DW, Lim SS, Kang IJ, Park J, et al. Anti-inflammatory effects of extracts from Caesalpinia sappan L. on skin inflammation. J Korean Soc Food Sci Nutr 2013;42(3):384-8.
- Jang BC, Paik JH, Kim SP, Shin DH, Song DK, Park JG, et al. Catalase induced expression of inflammatory mediators via activation of NF-κB, PI3K/AKT, p70S6K and JNKs in BV2 microglia. Cell Signal 2005;17(5):625-33.
[CrossRef] [Google Scholar] [Pub Med]
- Kim MJ, Bae NY, Kim KB, Park SH, Jang MR, Im MH, et al. Anti-inflammatory activity of ethanol extract of Sargassum miyabei Yendo via Inhibition of NF-κB and MAPK activation. Microbiol Biotechnol Lett 2016;44(4):442-51.
- Kim MJ, Kim MJ, Kim KB, Park SH, Choi HD, Park SY, et al. Anti-Inflammatory effect of Sargassum patens C. Agardh ethanol extract in LPS-induced RAW264. 7 cells and mouse ear edema. Microbiol Biotechnol Lett 2017;45(2):110-7.
- Hahm DH, Sur BJ, Han DO, Park JH, Jung ET, Lee HJ, et al. Anti-inflammatory activity of dandelion in mice. J Physiol Pathol Korean Med 2008;22(4):810-4.
- Ju MS, Jeong HU, Kim HG, Park GH, Youn YS, Kim YO, et al. Anti-nociceptive and anti-inflammatory effects of Geranii Herba. Korean J Herbol 2010;25(3):97-101.
- Jeon H, Yoon WJ, Ham YM, Yoon SA, Kang SC. Anti-arthritis effect through the anti-inflammatory effect of Sargassum muticum extract in collagen-induced arthritic (CIA) Mice. Molecules 2019;24(2):276.
[CrossRef] [Google Scholar] [Pub Med]
- Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicine. World Health Organization. Regional Office for Western Pacific, Philippines, Manila; 1992. p. 1-38.