- *Corresponding Author:
- U. Tutar
Cumhuriyet University, Faculty of Health Sciences, Department of Nutrition and Dietetic, Kayseri, Turkey
E-mail: ututar5@gmail.com
| Date of Submission | 07 March 2017 |
| Date of Revision | 14 February 2018 |
| Date of Acceptance | 13 August 2018 |
| Indian J Pharm Sci 2018;80(5):868-874 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Dried leaves of the Thymbra sintenisii have long been used traditionally in the form of herbal tea and as an antiseptic against flu in Anatolia. In the present study, Thymbra sintenisii extract’s antimicrobial, antioxidant, cytotoxic and wound healing effects were investigated. Aerial parts of Thymbra sintenisii were extracted with ethanol and the chemical composition was analysed using gas chromatography-mass spectrometry. Antimicrobial activity of the ethanol extract of Thymbra sintenisii was evaluated using micro-well dilution method and radical scavenging activity was measured using a spectrophotometric method. Cytotoxic activity of ethanol extract of Thymbra sintenisii in MCF-7 and MG63 cell lines were evaluated using the XTT assay. Results revealed that thymol was the major component by 64.97 %. Ethanol extract of Thymbra sintenisii displayed strong antimicrobial activity against Candida tropicalis (0.06 mg/ml) and moderate activity against Shigella boydii and Pseudomonas aeruginosa (0.5 mg/ml) strains. IC50 values of the ethanol extract of Thymbra sintenisii were against MG63, 37.28 μg/ml, MCF-7, 44.40 μg/ml and L929 44.84 μg/ml at 24 h. Significantly faster healing was observed in wound area of the treatment group when compared to the control group. Wound area measurements showed that ethanol extract of Thymbra sintenisii reduced the total wound area by 30 % while that of the untreated reduced by 7 % on end of the seventh day. Antimicrobial, antioxidant, cytotoxic and wound healing activities of Thymbra sintenisii appeared to be quite remarkable. The results indicated that ethanol extract of Thymbra sintenisii could be of therapeutic potential.
Keywords
Antimicrobial, antioxidant, cytotoxic activity, Thymbra sintenisii, wound healing
Plants and bioactive substances from plants were used as traditional medicine to treat diseases throughout history. Nowadays, treatment with plants still continues significantly in various cultures. According to the World Health Organization, approximately 65 % of the world population uses plants for treatment purposes [1]. There have been many studies related to the biological effects of plants. Therefore, extracts and essential oils obtained from plants have been used as anticancer agents in addition to antimicrobial, antiviral and antiparasitic effects. Thymbra sintenisii is thought as an important branch of Lamiaceae family [2-4].
Thymbra species are represented in the flora of Turkey by two types and 4 taxon, which are T. spicata L. (var. spicata, var. intricata P.H. Davis) and T. sintenisii Bornm. and Aznav. (subsp. sintenisii, subsp. isaurica P.H. Davis). The dried leaves of the plant, known as “white zahter” colloquially, are used in the form of a herbal tea in Anatolia and as an antiseptic against flu [5,6]. The biological and antimicrobial activities of Thymbra species have been reported in several studies [7-9]. However, only a few reports were published on T. sintenisii. Therefore, the aim of the study was to evaluate antimicrobial, antioxidant, cytotoxic and wound healing activities, as well as the phytochemical composition of ethanol extract of T. sintenisii (TSEE).
Materials and Methods
Ethanol, trypan blue solution, dimethyl sulfoxide, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and other chemicals were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from Biochrom (Berlin, Germany). Penicillin and streptomycin were purchased from Gibco (Paisley, UK) and trypsin ethylenediaminetetraacetic acid solution from Biological Industries (Kibbutz Beit Haemek, Israel). Dulbecco's modified eagle medium was obtained from Lonza (Verviers, Belgium). TNF-α ELISA kit was purchased from Yehau (China).
Plant material and extract preparation
Aerial parts of T. sintenisii were collected during August 2016 from Siirt city located in the Southeastern Anatolia region of Turkey. The plant material was identified at the Pamukkale University and verified using Flora of Turkey. Dried samples of specimen were deposited at the Pamukkale University herbarium, Turkey (M. Çiçek 2014-36 Herb) for future reference. Plant samples were dried at room conditions. Aerial parts of T. sintenisii were ground in a Waring blender and extracted with ethanol using a Soxhlet apparatus. Solvent was evaporated using a rotary evaporator (Stuart RE300) under reduced pressure at 30° [10].
Analysis and identification of T. sintenisii extract
Chemical composition of TSEE was evaluated according to the method by Abay et al [11]. Gas chromatography-mass spectrometry (GC-MS) analyses were performed on an Agilent Technologies GC 7890A equipped with 5975 Triple Axis Detector mass spectrometer. For GC-MS detection, DBWAXETR column (60×320×0.25 m), electron ionization system and ionization energy of 70 eV were used. Helium was the carrier gas at a flow rate of 1 ml/min. The column temperature was operated under the same conditions as described above.
Antimicrobial assay
Bacterial and fungal strains used were as follows, Klebsiella pneumoniae (ATCC 10031), Shigella boydii (ATCC 9905), Pseudomonas aeruginosa (ATCC 27853), Proteus vulgaris (ATCC 7829), Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 10987), Candida tropicalis (ATCC 750) were obtained from American Type Culture Collection (ATCC) and clinical isolates of P. aeruginosa, Acinetobacter baumannii, S. aureus were obtained from clinical microbiology laboratories of Cumhuriyet University Hospital. Microorganisms were identified using the BD Phoenix 100 (Becton Dickinson, Sparks, MD, USA) automatized microbiology system.
The antimicrobial activity of TSEE was evaluated on test bacteria using Kirby-Bauer disc diffusion method [12]. In this method 100 μl of suspension containing 108 CFU/ml of bacteria on Mueller-Hinton broth (MHB) was used. After the impregnation of the disc (6 mm in diameter) with 20 μl at 50 mg/ml extract, was placed on the inoculated agar. The solvent in which the extract was dissolved was used as the negative control. Commercially available cefoperazone/sulbactam (105 μg) and fluconazole (25 μg) discs were used as positive control for bacteria and fungi, respectively. The zones of inhibition around the disks were measured after 24 h of incubation at 37° for bacteria and 48 h for fungi at 28°. All the assays were done in triplicate. The samples exhibiting antibacterial activity ≥7 mm in preliminary screening were used to determine minimum inhibitory concentration (MIC).
The broth microdilution method was employed for the determination of antimicrobial activities of TSEE according to the protocol of Clinical and Laboratory Standards Institute [13]. MIC values of the TSEE were determined using the micro-well dilution method for the bacterial and fungal strains, which were found to be sensitive to TSEE in the disc diffusion assay. MIC values were determined by the serial dilution technique using 96-well microtiter plates. Serial two-fold dilutions were prepared in 96-well plates with MHB at a concentration varying between 0.03 to 2 mg/ml. The inocula of the strains were prepared from overnight broth cultures and suspensions were adjusted to 0.5 McFarland standard turbidity. The 96-well plates were prepared by adding 95 μl of MHB and 5 μl of the inocula into each well. One hundred microlitres of TSEE dilutions were added to the wells and incubated at 37o, for 24 h. The last well containing 195 μl of nutrient broth without the compound and 5 μl of the inocula on each strip was served as the negative control. Piperacillin/tazobactam (8/1) and fluconazole were used as positive control for bacteria and fungi, respectively. A microplate reader (Thermo Scientific microplate photometer, Multiskan FC, USA) was used to measure the absorbance of the plates at 570 nm after incubation for 24 h at 37°. The optical density at the 24th h of the inoculum remained the same or decreased at the MIC, which was detected as the lowest concentration of the compounds, when compared to the reading at the beginning.
Radical scavenging activity
The modified method of DPPH of Yu et al. [14] was used for radical scavenging activity of TSEE. Varying concentrations of TSEE were prepared. Equal volumes (1000 μl) of DPPH and sample solutions were incubated for 30 min with stirring. After incubation, DPPH was measured using spectrophotometer at 517 nm wavelength. DPPH solution and solvent were used as negative controls while the ascorbic acid used as the positive control. Samples and radical scavenging activity of the standard were calculated accordingly.
In vivo wound healing activity
Male Wistar rats (n=16) weighing between 250 and 275 g were used. This study was conducted within strict adherence to the principles of laboratory animal rules of Cumhuriyet University and the standards for Animal Experiments and Animal Care. All animals were maintained on standard pellet diet and water throughout the experiments and were housed 8 animals per cage. Rats were capable of normal activity in cages at 22 ± 2°, with humidity (50-70 %) under a 12 h light/ dark cycle [15].
Rats were anaesthetized with ketamine hydrochloride (90 mg/kg) and were then incised with 3 cm full thickness incision and a punch biopsy was opened 3×3 cm2. Animals were randomly assigned to 2 groups of 8 rats each. Group 1 served as the control, whereas the other group was TSEE treated. Sterile solution of TSEE was applied topically to each wound of the animals at a dose of 50 μl/wound once daily for 7 d. The animals in all groups were euthanized in accordance with the procedures on day 7 and cardiac blood was taken. Tubes were centrifuged at 3000 rpm for 10 min. Serum was stored at –80° until measurement. TNF-α was determined using ELISA according to a previously described method [16]. Animals of all groups were appropriately terminated on d 7 of the experiment and tissue samples were taken from the wounds and were examined histopathologically. The changes occurred in wound area were regularly measured and calculated according to the following Eqn., wound contraction=(healed area/total wound area)×100 and wound healing was compared with the control group graphically (healed area=wound area–present wound area) [17].
Cytotoxic activity and cell culture
MCF-7 (human breast cancer cell line, ATCC HTB-22), MG63 (human osteosarcoma cell line, ATCC CRL- 1427), L929 (mouse fibroblast cell, ATCC CCL-1) cells were purchased from ATCC. Cells were grown at 37° in a humidified incubator (5 % CO2). All media were supplemented with 1 % penicilin (100 U/ml), streptomycin (100 μg/ml), and 10 % FBS. Cytotoxicity was quantitatively evaluated by XTT method. The cells were seeded in 96-well plate in growth medium then treated with different concentrations of test compounds and incubated in a humidified CO2 atmosphere at 37° for 24 h. After the incubation, 100 μl XTT was added to each well for another 4 h incubation. The optical density values were measured at 475 nm using a microplate reader [18].
Statistical analysis
Data were expressed as the arithmetic mean ± standard deviation (SD). One way ANOVA and post-hoc Tukey analyses were used to reveal the relationships between groups. The differences were accepted as significant for p<0.05. Statistical analysis was performed with SPSS for windows 22.0 package. All determinations were computed three times.
Results and Discussion
In Turkey there are many aromatic plant species belonging to Lamiaceae family defined as “thyme”. However, the species containing thymol/carvacrol type of essential oil are regarded as "thyme". Among these species, Thymus, Origanum, Satureja, Thymbra, and Coridothymus types are especially important in terms of their wide distribution and economic benefits [19]. According to the statement of World Health Organization, the increase of the resistance to antibiotics arising in recent years is accepted as one of the most significant problem for human health [20]. In this regard, potential effects of plant extracts on microorganisms have been studied by many researchers [21]. Some studies reported the presence of antibacterial and antifungal activities of several types belonging to Thymbra species. Thymbra species can be considered as a natural antimicrobial agent source [22-24]. The phytochemical composition of TSEE was analysed by gas chromatography. The major components of TSEE were thymol (64.97 %), p-tert-butylcatechol (11.99 %), borneol (2.25 %; Table 1). Khoury et al. reported thymol and borneol were the major components of the essential oil of some species from Lamiaceae collected from different locations in Lebanon [25].
| Compound | Rta (min) | %b | Name |
|---|---|---|---|
| 1 | 8.892 | 0.53 | β-Linalool |
| 2 | 11.241 | 0.19 | Cyclohexanecarboxylic acid, 2-hydroxy-, ethyl ester |
| 3 | 11.879 | 2.25 | Borneol |
| 4 | 14.261 | 0.86 | 7-Ethyl-4-decen-6-one |
| 5 | 15.821 | 64.97 | Thymol |
| 6 | 16.081 | 0.43 | 6-Methyl-cyclodec-5-enol |
| 7 | 16.979 | 0.21 | 1-Butyn-3-one, 1-(6,6-dimethyl-1,2-epoxycyclohexyl)- |
| 8 | 17.189 | 1.49 | 2,6-Di-tert-butylhydroquinone |
| 9 | 17.516 | 0.77 | ??? |
| 10 | 18.329 | 11.99 | p-tert-Butyl catechol |
| 11 | 18.992 | 0.44 | 5-Hepten-3-yn-2-ol, 6-methyl-5-(1-methylethyl)- |
| 12 | 19.135 | 0.15 | 1-Buten-3-one, 1-(2-carboxy-4,4-dimethylcyclobutenyl)- |
| 13 | 19.261 | 0.60 | 4-tert-Butyl-O-phenylene diacetate |
| 14 | 19.873 | 0.94 | Phenol, 4-methoxy-2,3,6-trimethyl- |
| 15 | 20.359 | 0.54 | Spathulenol |
| 16 | 20.477 | 1.66 | Caryophyllene oxide |
| 17 | 21.198 | 0.95 | Tetracyclo[6.3.2.0(2,5).0(1,8)]tridecan-9-ol, 4,4-dimethyl- |
| 18 | 21.475 | 0.71 | Isoaromadendrene epoxide |
| 19 | 21.668 | 0.59 | Longipinocarveol |
| 20 | 23.295 | 0.20 | 1-Nonadecene |
| 21 | 24.185 | 0.32 | Syringic acid |
| 22 | 24.386 | 0.19 | 3,4,5-Trimethoxyphenylacetic acid |
| 23 | 24.529 | 0.15 | 1,7-Octadiene, 2,5-bis-(cis)-(2,2-dimethyl-3-carboxycyclopropyl)- |
| 24 | 24.68 | 0.17 | Cyclohexanol, 4-(1,1-dimethylethyl)-1-(2-propenyl)- |
| 25 | 25.703 | 0.22 | 4,4,8-Trimethyltricyclo[6.3.1.0(1,5)]dodecane-2,9-diol |
| 26 | 26.768 | 1.03 | Naphthalene, 1,2,3,4,4a,5,6,7-octahydro-4a-methyl- |
| 27 | 27.423 | 1.32 | Hexadecanoic acid |
| 28 | 30.854 | 0.15 | 4-(2,2,6-Trimethyl-bicyclo[4.1.0]hept-1-yl)-butan-2-one |
| 29 | 32.154 | 1.18 | Androstan-17-one, 3-ethyl-3-hydroxy |
| 30 | 32.229 | 0.63 | Retinoic acid |
| 31 | 32.439 | 0.33 | ??? |
| 32 | 32.498 | 0.65 | Norethindrone |
| 33 | 33.093 | 0.17 | 5-Pregnen-3β-ol-20-one, methyl ether |
| 34 | 33.387 | 0.32 | 1,4,7-Androstatrien-3,17-dione |
| 35 | 34.402 | 0.51 | Pregn-5-ene-3,20-dione |
| 36 | 35.878 | 0.35 | 5-Pregnen-3β-ol-20-one, propionate |
| 37 | 36.063 | 0.22 | 16-Methyl-20-oxopregn-5-en-3-yl acetate |
| 38 | 36.44 | 0.66 | 5-(7a-Isopropenyl-4,5-dimethyl-octahydroinden-4-yl)-3-methyl-penta-2,4-dien-1-ol |
| 39 | 36.885 | 0.14 | Bufotalin |
| 40 | 37.539 | 0.38 | Cycloeucalenyl acetate |
| 41 | 38.294 | 0.16 | Naphtho[2,3-b]furan-2-one, 3-[[(benzo[1,3]dioxol-5-ylmethyl)amino] |
| 42 | 42.69 | 0.16 | ??? |
| 43 | 59.249 | 0.15 | 5β-Cholestan-26-oic acid, 3α,7α,12α-trihydroxy- |
aRetention time, brelative percentage obtained from peak area
Table 1: Components of ethanol extract of T. sintenisii
Thymol has been used in traditional medicine for treatment of diseases for many years. Valuable information have been revealed antioxidant, antiinflammatory, antifungal, wound healing, antimutagenic properties [26,27]. Thymol (64.97 %) present in the essential oil of T. sintenisii showed bacteriostatic activity against most of the Grampositive and negative bacteria. The activity of TSEE was found to be 0.5 mg/ml against S. boydii and P. aeruginosa (clinical isolate) strains, whereas ≥2 mg/ml MIC value was identified against other bacterial strains. Extract showed a quite strong activity against C. tropicalis with 0.06 mg/ml (Table 2). Antimicrobial activity level of T. sintenisii ethanol extract was compared in terms of MIC values, it has been found that it had the strongest effect against C. tropicalis, then against S. boydii and P. aeruginosa strains. The antimicrobial effect against other tested strains is found to be weaker. Olasupo et al. revealed antimicrobial effect of thymol [28]. Another study, thymol exhibited antimicrobial activity against S. aureus and Escherichia coli [29]. Thymol inhibits growth of S. aureus, E. coli and Salmonella typhimurium [30]. As mentioned in the literature, thymol exhibited high antibacterial and antifungal activities [25,31].
| Gram-negative bacteria | Zone of inhibitionz | MIC (mg/ml) | ||
|---|---|---|---|---|
| Ext Conta | Ext Contb | |||
| Klebsiella pneumoniae | 7.3 ± 0.5 | 26.6 ± 2.0 | ˃2 | 0.03 |
| Shigella boydii | 18.6 ± 1.1 | 25.0 ± 1.7 | 0.5 | ˂0.03 |
| Pseudomonas aeruginosa (clinical isolate) | 7.4 ± 0.4 | 25.4 ± 3.1 | 0.5 | ˂0.03 |
| Pseudomonas aeruginosa | 7.6 ± 0.5 | 24.6 ± 1.1 | ˃2 | ˂0.03 |
| Proteus vulgaris | 9.6 ± 1.1 | 28.6 ± 1.1 | 2 | ˂0.03 |
| Acinetobacter baumannii (clinical isolate) | _ | _ | _ | _ |
| Gram-positive bacteria | ||||
| Staphylococcus aureus (clinical isolate) | 7.8 ± 0.9 | 21.9 ± 1.4 | ˃2 | 0.03 |
| Staphylococcus aureus | 8.3 ± 0.5 | 19.6 ± 1.5 | ˃2 | 0.03 |
| Bacillus cereus | 12.3 ± 0.5 | 32.3 ± 0.5 | 1 | ˂0.03 |
| Fungi | Ext Contc | Ext Contd | ||
| Candida tropicalis | 11.8 ± 1.4 | 13.1 ± 0.8 | 0.06 | 0.03 |
Ext: ethanol extract of T. sintenisii, Cont: control, ‘a’ cefoperazone/sulbactam (2:1), ‘b’ piperacillin/tazobactam (8:1), ‘c’ fluconazole disc (25 µg), ‘d’ fluconazole, MIC: minimum inhibitory concentration, ‘z’ values are mean SD (mm, n=3)
Table 2: Antimicrobial activities of T. sintenisii extract
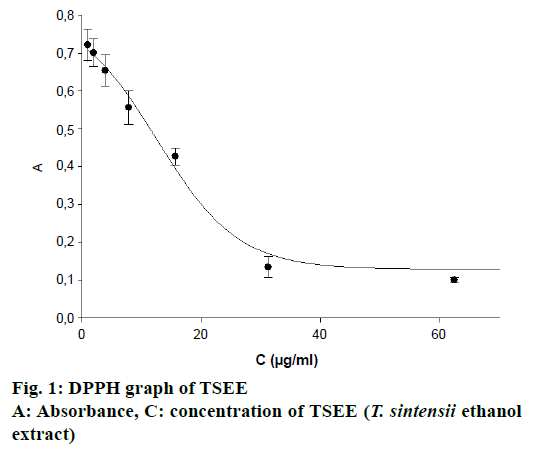
In this study radical scavenging activity of TSEE was investigated and IC50 value was found to be 12.5 μg/ml. IC50 value of the standard ascorbic acid was 5.02 μg/ml (Figure 1). Bozin et al. have reported that the antioxidant activity of Lamiaceae types was high [32]. These authors reported IC50 of DPPH assay as 0.39 μg/ml. Saidi et al. [24] have studied the antioxidant and antimicrobial properties of T. spicata L. and reported that the antioxidant and antimicrobial properties of this plant were remarkable. IC50 value of T. spicata L. was found to be 1.28 μl/ml. In another study, essential oil of T. capitata was reported to exhibit potent antioxidant activity [33]. Strong antioxidant activity observed in this investigation was consistent with the results of other studies.
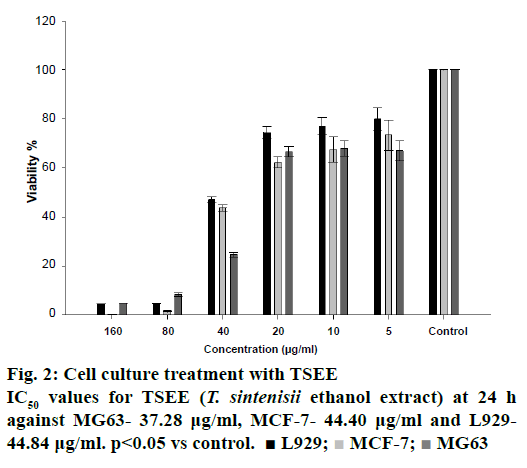
The premier objective is to destroy cancer cells in oncologic research, but to alleviate side effects of anticancer agents it is essential that the newer agents interfere with fewer biochemical pathways. Cytotoxic activity was determined by XTT method after incubating the cells for 24 h with the compound at increased concentrations. The extract was tested on MCF-7 and MG63 cancer cell lines to find out its cytotoxicity activity. Healthy L929 mouse fibroblast cell line was used as control. Extract was found to have cytotoxic effects on both cell lines at 40 μg/ml concentrations and above (Figure 2). IC50 values were found to be against MG63- 37.28 μg/ml, MCF-7- 44.40 μg/ml and L929- 44.84 μg/ml at 24 h (Figure 2). Delgado-Adámez et al. reported that cytotoxic major component of thyme. Yeh et al. demonstrated that thymol decreased cell viability on human prostate cancer cells [35]. Thymol stimulated cytotoxic activity in MCF-7 with IC50 of 2.5 μg/ml [36]. Chang et al. reported that thymol (400 μg/ml) trigger cytotoxic activity in MG63 [37].
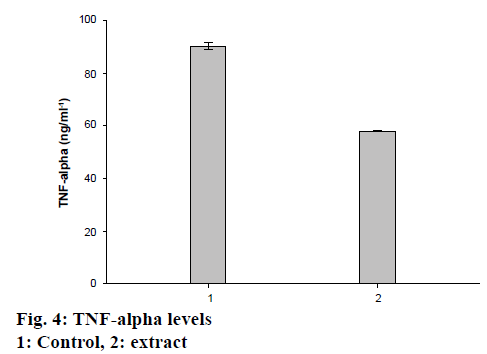
Wound is result of disruption of normal anatomical structure and functional integrity. Recently, many studies reported about wound healing, but these have not been at the desired level yet. TNF-α is a proinflammatory, angiogenic cytokine and growth factor. Therefore, determination of TNF-α level would be an important determinant for wound healing [38]. In the present study, the effect of TSEE was determined and compared to the control, which was found to be at the 7/30 ratio, indicating that the extract possessed at least four times better healing activity compared to untreated (Figure 3). The extract significantly accelerated wound healing process in the treated rats. It has been observed that TNF-α level of the treated group was decreased about 30 % in 7 d (Figure 4).
As a result, in this study it was observed that antimicrobial, antioxidant, cytotoxic activities and wound healing effect of T. sintenisii were quite remarkable. These results indicated that this plant could be used as a natural source for developing newer therapeutic agents. Further studies are needed to better understand the molecular mechanisms underlying these effects of T. sintenisii extract.
Conflict of interest
The authors report that they have no conflicts interest
Financial support and sponsorship
Nil.
References
- Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev 2009;109:3012-43.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils-a review. Food Chem Toxicol 2008;46:446-75.
- Palmeira-de-Oliveira A, Gaspar C, Palmeira-de-Oliveira R, Silva-Dias A, Salgueiro L, Cavaleiro C, et al. The anti-Candida activity of Thymbra capitata essential oil: effect upon pre-formed biofilm. J Ethnopharmacol 2012;140:379-83.
- Saeedi M, Morteza-Semnani K, Mahdavi MR, Rahimi F. Antimicrobial studies on extracts of four species of Stachys. Indian J Pharm Sci 2008;70:403-6.
- Ali IB, Guetat A, Boussaid M. Variation of volatiles in Tunisian populations of Thymbra capitata (L.) Cav. (Lamiaceae). Chem Biodivers 2012;9:1272-85.
- Erken S. Morphological and anatomical studies on Thymbra sintenisii Bornm. and Aznav. (Labiatae). Turk J Bot 2005;29:389-97.
- Dandlen SA, Lima AS, Mendes MD, Miguel MG, Faleiro ML, Sousa MJ, et al. Antioxidant activity of six Portuguese thyme species essential oils. Flavour Fragr J 2010;25:150-5.
- Figueiredo AC, Barroso JG, Pedro LG, Salgueiro L, Miguel MG, Faleiro ML. Portuguese Thymbra and Thymus species volatiles: chemical composition and biological activities. Curr Pharm Des 2008;14:3120-40.
- Kiliç T. Analysis of essential oil composition of Thymbra spicata var. spicata: antifungal, antibacterial and antimycobacterial activities. Z Naturforsch C 2006;61(5-6):324-8.
- Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol 2003;80:223-30.
- Abay G, Altun M, Karakoç ÖC, Gül F, Demirtas I. Insecticidal activity of fatty acid-rich Turkish bryophyte extracts against Sitophilus granarius (Coleoptera: Curculionidae). Comb Chem High Throughput Screen 2013;16:806-16.
- Selim SA, Adam ME, Hassan SM, Albalawi AR. Chemical composition, antimicrobial and antibiofilm activity of the essential oil and methanol extract of the Mediterranean cypress (Cupressus sempervirens L.). BMC Complement Altern Med 2014;14:179.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. CLSI document M100-S24. Wayne, PA: CLSI; 2014.
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qıan M. Free radical scavenging properties of wheat extracts. J Agric Food Chem 2002;50:1619-24.
- Gopinath D, Ahmed MR, Gomathi K, Chitra K, Sehgal PK, Jayakumar R. Dermal wound healing processes with circumin incorporated collagen films. Biomaterials 2004;25:1911-7.
- Wen X, Han XR, Wang YJ, Fan SH, Zhang ZF, Wu DM, et al. Effects of S100A12 gene silencing on serum levels of anti-inflammatory/pro-inflammatory cytokines in septic rats through the ERK signalling pathway. J Cell Biochem 2018;119(5):4038-49.
- Murthy S, Gautam MK, Goel S, Purohit V, Sharma H, Goel RK. Evaluation of in vivo wound healing activity of Bacopa monniera on different wound model in rats. Biomed Res Int 2013;29:972028.
- Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res 2007;27(5A):3293-9.
- Davis PH. Thymbra L. In: Davis PH. Flora of Turkey and the East Aegean Islands. Edinburgh: Edinburgh University Press; 1982. p. 382-4.
- World Health Organization. Antimicrobial resistance: global report on surveillance. Available from: http://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf;jsessionid=058559D2031C213F8B238EB050317342?sequence=1.
- Thomas S, Mani B. Chemical composition, antibacterial and antioxidant properties of essential oil from the rhizomes of Hedychium forrestii var. palaniense Sanoj and M. Sabu. Indian J Pharm Sci 2016;78:452-7.
- Faleiro L, Miguel G, Gomes S, Costa L, Venâncio F, Teixeira A, et al. Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J Agric Food Chem 2005;53:8162-8.
- Giweli A, Džamić AM, Soković M, Ristić MS, Marin PD. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 2012;17:4836-50.
- Saidi M, Ghafourian S, Zarin-Abaadi M, Movahedi K, Sadeghifard N. In vitro antimicrobial and antioxidant activity of black thyme (Thymbra spicata L.) essential oils. Roum Arch Microbiol Immunol 2012;71:61-9.
- Khoury M, Stien D, Eparvier V, Ouaini N, El Beyrouthy M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid Based Complement Alternat Med 2016;2547169.
- Pivetta TP, Simões S, Araújo MM, Carvalho T, Arruda C, Marcato PD. Development of nanoparticles from natural lipids for topical delivery of thymol: Investigation of its anti-inflammatory properties. Colloids Surf B Biointerfaces 2018;164:281-90.
- Shahbazi Y. The antibacterial effect of Ziziphora clinopodioides essential oil and nisin against Salmonella typhimurium and Staphylococcus aureus in doogh, a yoghurt-based Iranian drink. Vet Res Forum 2016;7:213-9.
- Olasupo N, Fitzgerald D, Gasson M, Narbad A. Activity of natural antimicrobial compounds against Escherichia coli and Salmonella enterica serovar. typhimurium. Lett Appl Microbiol 2003;37:448-51.
- Trombetta D, Castelli F, Sarpietro MG, Venuti, V, Cristani M, Daniele C, et al. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Chemother 2005;49:2474-78.
- Nagoor Meeran MF, Javed H, Al Taee H, Azimullah S, Ojha SK. Pharmacological Properties and Molecular Mechanisms of Thymol: Prospects for Its Therapeutic Potential and Pharmaceutical Development. Front Pharmacol 2017;26:8:380.
- Marchese A, Orhan IE, Daglia M, Barbieri R, Di Lorenzo A, Nabavi SF. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem 2016;210:402-14.
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem 2006;54:1822-8.
- Miguel MG, Gago C, Antunes MD, Megías C, Cortés-Giraldo I, Vioque J, et al. Antioxidant and antiproliferative activities of the essential oils from Thymbra capitata and Thymus species grown in Portugal. Evid Based Complement Alternat Med 2015;851721.
- Delgado-Adámez J, Garrido M, Bote ME, Fuentes-Pérez MC, Espino J, Martín-Vertedor D. Chemical composition and bioactivity of essential oils from flower and fruit of Thymbra capitata and Thymus species. J Food Sci Technol 2017;54:1857-65.
- Yeh JH, Chou CT, Chen IS, Lu T, Lin KL, Yu CC, et al. Effect of thymol on Ca²⁺ homeostasis andviability in PC3 human prostate cancer cells. Chin J Physiol 2017;28:60:32-40.
- Melo JO, Fachin AL, Rizo WF, Jesus HC, Arrigoni-Blank MF, Alves PB, et al. Cytotoxic effects of essential oils from three Lippia gracilis Schauer genotypes on HeLa, B16, and MCF-7 cells and normal human fibroblasts. Genet Mol Res 2014;13:2691-F7.
- Chang HT, Hsu SS, Chou CT, Cheng JS, Wang JL, Lin KL, et al. Effect of thymol on Ca2+ homeostasis and viability in MG63 human osteosarcoma cells. Pharmacology 2011;88:201-12.
- Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res 2010;89:219-29.
 L929;
L929;  MCF-7;
MCF-7;  MG63
MG63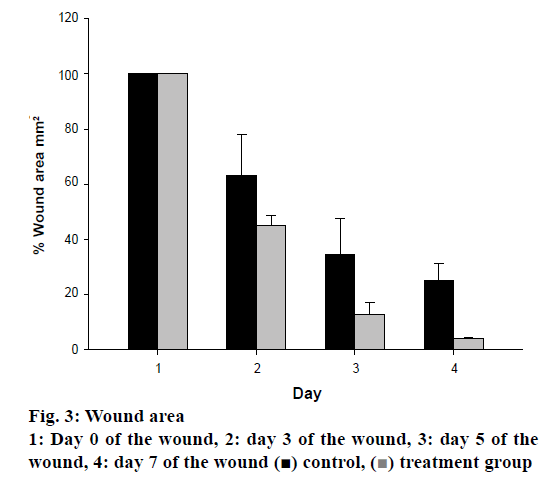
 ) control, (
) control, ( ) treatment group
) treatment group