- *Corresponding Author:
- C. W. Choi
Department of Biology and Medicinal Science,
Pai Chai University,
Seo-gu, Daejeon 35345,
South Korea
E-mail: choicw@pcu.ac.kr
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “52-61” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In this study, the first purpose was to determine total phenolic, total flavonoid and total terpenoid content of the Acer tegmentosum ethanol extract and four different types of fractions, including chloroform, ethyl acetate, n-butanol and water. Among the samples, Acer tegmentosum ethanol extract and fraction of ethyl acetate showed the highest total phenolic, total flavonoid and total terpenoid content. The second purpose was to evaluate the antioxidant activity of the samples assessed by in vitro methods such as scavenging capacity of 2,2-diphenyl-1-picryl-hydrazyl-hydrate and 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid free radicals. Especially, Acer tegmentosum ethanol extract and fraction of ethyl acetate showed the strongest scavenging capacity with the lowest half maximal scavenging capacity values of these free radicals. The third purpose was to evaluate the anti-inflammatory activity in the lipopolysaccharide-stimulated Ralph and William’s cell line 264.7 macrophages treated with various concentrations of Acer tegmentosum ethanol extract and fraction of ethyl acetate, and no cytotoxic effect on the macrophages was observed at the concentration of 100 μg/ml of this extract. It suggested that this extract suppresses the activation of macrophages to secrete both pro-inflammatory cytokines (interleukin-1 beta, tumour necrosis factor alpha, inducible nitric oxide synthase and interleukin-6) and enzyme (inducible nitric oxide synthase) and anti-inflammatory cytokine (interleukin-10). The fourth purpose was to identify some compounds in a sample responsible for the antioxidant and anti-inflammation activities. In high-performance liquid chromatography chromatograms, the peak retention times detected from Acer tegmentosum ethanol extract and fraction of ethyl acetate in comparison with those from reference standards identified six compounds such as gallic acid, salidroside, (-)-epigallocatechin, (+)-catechin, scopoletin and trans-ferulic acid. It suggested that these compounds are responsible for antioxidant and anti-inflammation activities. Taken together, Acer tegmentosum seems to be one of the most promising species as a therapeutic source against diseases.
Keywords
Acer tegmentosum stem, ethanol extract and its active fractions, anti-inflammatory activity, lipopolysaccharide-stimulated macrophages, high-performance liquid chromatography, 2,2-diphenyl-1- picryl-hydrazyl-hydrate, 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid
When Reactive Oxygen Species (ROS) such as Superoxide (O2-) and Nitric Oxide (NO) are overproduced more than the body’s antioxidant defense system, this unbalance causes oxidative stress[1]. The oxidative stress causes cell death or cellular damage[2,3] and induces inflammation in the pathological processes[1]. Macrophages are critically involved in inflammation and their roles are the release of pro- inflammatory mediators such as NO, Prostaglandin E2 (PGE2), and various cytokines in response to activation signals, including chemical mediators, cytokines and bacterial Lipopolysaccharides (LPS)[4,5]. The secretion of Interleukin (IL)-1, IL-6, Tumor Necrosis Factor alpha (TNF-α), NO (pro-inflammatory cytokines and mediator) and IL-10 (anti-inflammatory cytokine) is the primary response to inflammation in addition to leukocyte recruitment[6]. Adverse effects of current anti- inflammatory drugs lead to identify natural products possessing anti-inflammatory activity for use in pharmaceutical purposes. In this respect, plant extracts containing various phytochemicals have attracted an interest as anti-inflammatory drugs that modulate the immunological response[7,8].
Acer tegmentosum (A. tegmentosum), a member of the Aceraceae family, is a deciduous tree that grows in Korea, Russia and northern China[9]. A. tegmentosum has been used traditionally as a traditional folk medicine for the treatment of liver diseases, eczema, diabetes mellitus in Korea[10,11]. In addition, pharmacological studies revealed that A. tegmentosum has anti-inflammatory, antioxidant and anticancer activities[9,10,12-15]. Besides, A. tegmentosum has reported to contain steroids (beta (β)-sitosterol, β-sitosterol-3-O-β-D-glucoside and epifriedelinol) and phenol compounds such as terpenoid, salidroside, tyrosol, 6'-O-galloylsalidroside, quercetin, isoquercetin, morin-3-O-α-L-lyxoside, fraxin, cleomiscosin A, scopoletin, (+)-catechin, (-)-epicatechin and (-)-epigallocatechin[12,13,16,17].
In this study, the first purpose was to determine total phenolic, total flavonoid and total terpenoid content of the A. tegmentosum Ethanol Extract (AtE) and four different types of fractions, including Chloroform (AtE-C), Ethyl Acetate (AtE-EA), n-Butanol (AtE-B) and Water (AtE-W). The second purpose was to evaluate the antioxidant properties of these samples assessed by in vitro methods such as Scavenging Capacity (SC) of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) and 2,2'-azino-bis(3-ethylbenzothiazoline-6- sulfonic acid (ABTS) free radicals. The third purpose was to determine the effects of AtE-EA on the anti- inflammatory activity in Lipopolysaccharide (LPS)- stimulated Ralph and William’s (RAW) cell line 264.7 macrophages. The fourth purpose was to identify some compounds in samples responsible for the antioxidant and anti-inflammation properties.
Materials and Methods
Materials and preparation of extract and its fractions:
A. tegmentosum was purchased from a well-known trade market for oriental herbal medicines in Daejeon, Korea. A. tegmentosum (50 g) was macerated using a blender and extracted for 24 h with 500 ml of ethanol in a shaking incubator. An aliquot of AtE was filtered using Whatman No. 1 filter paper (GE Healthcare, Buckinghamshire, UK), reduced to 50 ml by a vacuum rotary evaporator (EYELA N-N, Tokyo, Japan) at 60° and lyophilized for 4 d. The dried powder was dissolved in 50 ml of methanol:distilled water (1:1, v/v) and re-extracted successively with equal volumes of chloroform (fraction 1), ethyl acetate (fraction 2), n-butanol (fraction 3), and water (fraction 4) in order. All fractions were evaporated at low temperature under reduced pressure, freeze-dried and powdered for use as samples.
Determination of total phenolic content:
Total phenolic content in each sample was determined by the Folin-Ciocalteu method[18], with a minor modification. To prepare the calibration curve, 100 μl aliquot of gallic acid in methanol (0.024, 0.075, 0.105 and 0.3 mg/ml) was mixed with 500 μl of 10 % Folin- Ciocalteau’s phenol reagent and 400 μl of 1 M sodium bicarbonate. After 30 min of treatment in the dark, absorbance was read at 750 nm and the calibration curve (y=6.6286 x–0.0263, Correlation Coefficient (r2)=0.9936) was drawn. Each sample (100 μl of 1 mg/ ml in methanol) was mixed with the same reagents as described above. After 30 min, absorbance was measured against the blank prepared using methanol. The results were expressed as mg of Gallic Acid Equivalent (GAE)/g of dry powder. All determinations were performed in triplicate.
Determination of total flavonoid content:
Total flavonoid content in each sample was determined by Chang et al.[19], with slight modifications. Each sample (20 μl of 1 mg/ml in methanol) was mixed with 20 μl of 10 % (w/v) aluminum nitrate, 4 μl of 1 M potassium acetate, 60 μl of methanol and 96 μl of distilled water. The mixture was kept at 27° for 30 min and then its absorbance at 415 nm was read using an Ultraviolet–Visible (UV-VIS) spectrophotometer (Libra S22, Biochrom Ltd., Cambridge, UK). A calibration curve (y=3.0718+0.0337, r2=0.9992) was prepared by measuring the absorbance of quercetin in methanol (0-100 μg/ml) under the same conditions. The results were expressed as mg of Quercetin Equivalent (QE)/g of dry powder. All determinations were performed in triplicate.
Determination of total terpenoid content:
Total terpenoid content in each sample was determined by linalool as a standard reagent according to the Ghorai et al.[20], with slight modifications. To each sample (200 μl of 1 mg/ml in methanol), chloroform (1.2 ml) was added and vortexed. After incubation at room temperature for 3 min, concentrated sulfuric acid (100 μl) was added into the mixture and that was further incubated at 27° for 1.5 h in the dark. When a reddish-brown precipitate was formed, the precipitate was dissolved with 1.0 ml of methanol (95 %, v/v) completely. The resulting mixture was transferred to a cuvette and read its absorbance at 538 nm against methanol as a blank. The results were expressed as mg of Linalool Equivalent (LE)/g of dry powder using the regression equation of the Linalool standard curve (y=1.5115 x+0.00441, r2=0.9944). All determinations were repeated at least three times.
Assessment of radical scavenging activity (DPPH and ABTS assays):
DPPH• and ABTS•+ are free radicals for use in assessing radical SC or antioxidant activity. Each sample was thoroughly dissolved in methanol (1000 μg/ml), further diluted to 500, 200, 100, 50, 20 and 10 μg/ml, and performed DPPH radical scavenging assay according to the method of Choi et al.[21], with a minor modification. The diluted sample (20 μl) mixed with 180 μl of 0.2 mM DPPH (Sigma-Aldrich, St. Louis, USA) was dissolved in methanol and the mixture was kept to react at room temperature for 30 min in the dark. Negative control was DPPH solution in methanol without a sample, while positive control was ascorbic acid dissolved in methanol (1000 μg/ml). The absorbance was measured against a blank at 490 nm. The percent SC was converted using the following equation: % SC=(Absorbance of negative control−Absorbance of a sample)×100/Absorbance of negative control. The Half Maximal Scavenging Capacity (SC50) value was measured by linear regression of the plots and defined as the concentration of a sample required to reduce 50 % of the DPPH free radicals. The improved ABTS method[22] was used in this study, with a minor modification. ABTS radicals were generated by reacting with 100 μl of each sample (1000, 500, 200, 100, 50, 20 and 10 μg/ml) and then mixed with 900 μl of ABTS•+ (Sigma-Aldrich, St. Louis, USA) stock solution (7 mM ABTS and 2.5 mM potassium persulfate (1:1, v/v)) after incubation at room temperature for 5 min in the dark. The absorbance of the reactive mixture was immediately recorded at 734 nm by using a spectrophotometer. Ascorbic acid (10 μg/ml) was used as a positive control. All assays were repeated at least three times and the values were plotted using the average of three determinations. Besides, the free radical SC of each sample was expressed as an SC50 value.
Cell culture and cell viability determination by using 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl Tetrazolium Bromide (MTT) assay:
RAW 264.7 macrophage cells were procured from the Korean Cell Line Bank (KCLB, Seoul, Korea) and cultured at 37° under 5 % Carbon Dioxide (CO2) atmosphere in Dulbecco’s Modified Eagle Medium (DMEM), (GIBCO Invitrogen Corporation, Grand Island, NY, USA) containing 10 % Fetal Bovine Serum (FBS), 100 U/ml penicillin, 100 mg/ml streptomycin and 1.176 g/l sodium bicarbonate. Cytotoxicity was determined by MTT assay (Thermo Fisher Scientific, Waltham, MA, USA) that measured the metabolic activity of cell cultures with a color reaction catalyzed by mitochondrial succinate dehydrogenase. The cells were plated at a density of 1×104 cells/well in a 96-well plate, incubated at 37° for 24 h in a CO2 incubator and treated with 100 µl of various concentrations (1.56, 3.13, 6.25, 12.5, 25, 50, 100 and 200 µg/ml) of AtE- EA. The cells were incubated for 48 h and washed with 1× Phosphate Buffered Saline (PBS). The medium was replaced with a fresh medium containing MTT solution (0.5 mg/ml in PBS) and incubated at 37° for 4 h. After decanting the supernatant, 100 µl of Dimethyl Sulfoxide (DMSO) was added to each well to dissolve the formazan produced. The optical density of the cells was measured using a microplate reader at 595 nm. The percent of viable cells was measured relative to the untreated control considered to have 100 % viability.
Measurement of NO production:
RAW 264.7 macrophage cells (1×106 cells/well) were cultured in 6-well plates, incubated at 37° for 24 h in a CO2 incubator, stimulated the cells in the presence of LPS (final concentration of 1 µg/ml) for 30 min and then exposed with 1 ml of AtE-EA (6.12, 12.5 and 25 µg/ml). After incubating the plate for an additional 24 h, cell supernatants were collected by centrifugation, filtered using 0.22 µm filter and dispensed 100 µl into a new plate. NO inhibition was determined by Nitric Oxide Plus Detection Kit, according to the manufacturer’s protocol (iNtRON Biotechnology, Seongnam-si, Gyeonggi-do, Korea). The plate was incubated at room temperature for 10 min after adding N1 buffer (50 µl) and N2 buffer (50 µl), respectively. The mixture was measured at 560 nm.
Quantitative analysis of cytokine messenger Ribonucleic Acid (mRNA) by quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR):
RAW 264.7 macrophages (1×106 cells/well) were cultured in 6-well plates for 24 h at 37° in a CO2 incubator, stimulated the cells in the presence or absence of LPS (final concentration of 1 µg/ml) for 30 min and then exposed with 1 ml of AtE-EA (25 and 50 µg/ml). After different incubation times for each cytokine, total RNA was isolated by WizolTM reagent (Wizbio Solutions Inc., Seongnam-si, Gyeonggi-do, Korea) according to the manufacturer’s instructions. mRNA levels of TNF-α, IL-1β, IL-6, IL-10 and inducible Nitric Oxide Synthase (iNOS) were quantified by RT-qPCR amplification. Primers of target genes are described in Table 1. RT reaction was performed in a reaction mixture (20 μl) containing 300 ng of total RNA, 50 mM Tris(hydroxymethyl)aminomethane Hydrochloride (Tris-HCl) (pH 8.3), 75 mM Potassium chloride (KCl), 8 mM (MgCl2), 10 mM Dithiothreitol (DTT), 0.1 % Nonidet P-40 (NP-40), 40 mM Deoxynucleoside Triphosphate (dNTP), 2 pM of respective primer, 20 U of Ribonuclease (RNase) inhibitor and 200 U Prime Script Reverse Transcriptase (Takara Bio Inc., Shiga, Japan). The thermal cycler was programmed for 1 RT cycle at 50° for 30 min and 70° for 15 min. Complementary Deoxyribonucleic acid (cDNA) was amplified in a 20 μl reaction mixture containing 10 μl 2x SYBR® Premix ExTaq™ II (TliRNase H Plus, Takara Bio, Shiga, Japan), 0.4 μl Rhodamine-X (ROX) reference dye II, 0.4 μl of 10 pM of forward and reverse primer each and 2 μl of cDNA (1 ng), using Stratagene Mx3005P cycler (1 cycle: 95° for 10 min and 40 cycles: Denaturation at 95° for 30 s, primer annealing at 60° for 30 s and extension at 72° for 30 s). Each gene is amplified in triplicate and cDNA concentration differences normalized to Glyceraldehyde 3?Phosphate Dehydrogenase (GAPDH).
| Gene | Polarity | Sequences 5’ to 3’ |
|---|---|---|
| IL-1β | + | ATGGCAACTGTTCCTGAACTCAACT |
| | AGTAGCCCTTCATCTTTTGGGG | |
| IL-6 | + | ATGAAGTTCCTCTCTGCAAGAGACT |
| | GTCTCCTCTCCGGACTTGTGA | |
| TNF-α | + | ATGAGCACAGAAAGCATGATCCG |
| | GCTGAGACATAGGCACCGC | |
| iNOS | + | ATGAACCCCAAGAGTTTGACCAGA |
| | GGAGCCATAATACTGGTTGATGAAC | |
| IL-10 | + | ATGCCTGGCTCAGCACTGCTA |
| | CTGGGAAGTGGGTGCAGTTATTG | |
| GAPDH | + | ATGGTGAAGGTCGGTGTGAACG |
| | CAATGAAGGGGTCGTTGATGGC |
Table 1: Primer Sequences of the Targeted Genes In RT-qPCR
High-Performance Liquid Chromatography (HPLC) analysis:
A lyophilized fraction of AtE-EA was reconstituted in 70 % aqueous methanol (5 mg/ml) and passed through a 0.2 μm nylon filter. Four standards were gallic acid, salidroside, (-)-epigallocatechin, (+)-catechin, scopoletin and trans-ferulic acid (1 mg/ml each). Chromatographic separations were performed on a Shiseido Capcell Pak UG 120 cartridge (4.6 mm×250 nm, 5 μm particle size, Shiseido, Japan). Samples (10 μl) were injected into HPLC instrument (Waters 2690, Waters Corp., USA). The mobile phase for AtE-EA consisted of 0.5 % phosphoric acid in water (solvent A) and 0.5 % phosphoric acid in acetonitrile (solvent B). The preparative system was run for 60 min of total running time at a constant flow rate of 1 ml/min at ambient temperature and then the spectrum was monitored at 225 nm. The identification of each compound was based on a combination of retention time and spectral matching.
Statistical analyses:
Determinations of all samples were performed in triplicate for all assays. All statistical analyses were conducted by one-way Analysis of Variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) software (version 14.0). Data expressed as mean±Standard Deviation (SD). Differences were considered statistically significant at p<0.05, p<0.01 or p<0.001. Pearson’s correlation coefficients between antioxidant activity and total phenolic, total flavonoid, or total terpenoid content (p<0.05) were determined by using web-based software.
Results and Discussion
In this study, total phenolic, total flavonoid and total terpenoid content varied among samples (Table 2). The highest value of each content was observed in AtE-EA (445.34 mg GAE/g, 36 mg QE/g and 14.71 mg LE/g), while the lowest value of each content was observed in AtE-W (78.49 mg GAE/g, 6.36 mg QE/g, 7.21 mg LE/g). Each sample showed a significant (p<0.001) dose-dependent increase of DPPH radical SC (Table 3). The DPPH radical SC of samples at 50 μg/ml tended to decrease in the following order: AtE-EA (88.32 %)>AtE-B (82.8 %)>AtE (57.66 %)>AtE-C (25.12 %)>AtE-W (15.04 %). The SC values of samples are competitive when compared to the SC value (88.19 %) of ascorbic acid at the given concentration (1000 μg/ml). In Table 4, AtE-EA showed the strongest SC with the lowest SC50 value (9.98 μg/ml), while AtE-W showed the weakest SC with the highest SC50 value (134.1 μg/ml). The efficiency of samples to scavenge ABTS radicals was increased significantly (p<0.001) with the increasing concentration of each sample (Table 3). The ABTS radical SC of samples at 10 μg/ml decreased in the following order: AtE-EA (91.90 %)>AtE-B (55.89 %)>AtE (31.72 %)>AtE-C (17.27 %)>AtE-W (13.19 %). Compared to the SC value (100 %) of ascorbic acid at the given concentration (10 μg/ml), the SC value of AtE-EA is competitive. As indicated in Table 4, AtE-EA showed the strongest SC with the lowest SC50 value (5.29 μg/ml), while AtE-W showed the weakest SC with the highest SC50 value (52.71 μg/ml). Several plant extracts containing phenolic acids and flavonoids have revealed strong antioxidant properties, such as SC of DPPH and ABTS free radicals[23]. Likewise, A. tegmentosum showed strong antioxidant activity based on DPPH and ABTS assays. Pearson’s correlation analysis indicated that the DPPH radical SC50 shows a strong negative correlation with total phenolic content (r2=0.6917, p<0.05) and total terpenoid content (r2=0.7021, p<0.05) content and a moderate negative correlation with total flavonoid content (r2=0.5491, p<0.05) of AtE and its four fractions. In addition, Pearson’s correlation analysis indicated that the ABTS radical SC50 showed a strong negative correlation with total phenolic content (r2=0.7043, p<0.05), total flavonoid content (r2=0.5708, p<0.05) and total terpenoid content (r2=0.7271, p<0.05) of the samples.
| Extraction/Fraction | Total phenolic content (mg GAE/g±SD) | Total flavonoid content (mg QE/g±SD) | Total terpenoid content (mg LE/g±SD) |
|---|---|---|---|
| AtE | 167.8±1.44 | 13.85±1.17 | 9.64±1.91 |
| AtE-C | 82.57±3.16 | 16.13±1.51 | 9.2±1.32 |
| AtE-EA | 445.34±1.11 | 36.07±1.34 | 14.71±0.38 |
| AtE-B | 283.16±2.95 | 19.79±1.47 | 11.4±0.38 |
| AtE-W | 78.49±1.96 | 6.36±2.55 | 7.21±0.66 |
Note: The data are means of three replicates (n=3), mean±SD. AtE: Ethanol extract and fractions of (-C: Chloroform; -EA: Ethyl acetate; -B: Butanol; -W: Water fraction)
Table 2: Total Phenolics, Flavonoids and Terpenoids in Ethanol Extract and Four Fractions from A. Tegumentosum Stem
| Assay | Extract/Fractions | Scavenging activity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Concentration (μg/ml) | ||||||||
| 10 | 20 | 50 | 100 | 200 | 500 | 1000 | ||
| DPPH | AtE | 13.85±0.7*** | 26.47±0.61*** | 57.66±0.83*** | 85.5±0.67*** | 88.54±0.42*** | 89.54±1.78*** | 88.61±0.52*** |
| AtE-C | 5.57±0.59** | 11.58±0.33*** | 25.12±0.52*** | 40.58±0.87*** | 61.18±3.57*** | 82.41±1.65*** | 86.09±3.61*** | |
| AtE-EA | 44.77±2.49*** | 74.09±2.08*** | 88.32±1.07*** | 88.58±1.26*** | 89.01±0.42*** | 88.2±1.26*** | 87.62±1.46*** | |
| AtE-B | 25.95±2.05*** | 47.38±1.49*** | 82.8±0.95*** | 86.74±0.49*** | 87.05±0.59*** | 87.33±0.36*** | 88.3±0.4*** | |
| AtE-W | 2.5±0.72 | 5.93±0.49*** | 15.04±0.84*** | 30.29±1.18*** | 53.8±1.89*** | 85.14±0.31*** | 84.82±0.88*** | |
| Ascorbic acid | 88.19±0.74*** | |||||||
| ABTS | AtE | 31.72±0.91*** | 57.94±0.39*** | 96.76±0.29*** | 100.38±0.32*** | 100.29±0.37*** | 100.13±0.46*** | 100.76±2.07*** |
| AtE-C | 17.27±0.62*** | 30.72±0.39*** | 52.89±0.21*** | 70.76±1.13*** | 87.03±1.25*** | 99.08±0.9*** | 99.07±2.01*** | |
| AtE-EA | 91.9±1.01*** | 99.79±0.16*** | 100.04±0.16*** | 100.7±0.99*** | 100.21±0.21*** | 100.17±0.62*** | 99.84±3.37*** | |
| AtE-B | 55.89±0.77*** | 90.02±0.26*** | 100.04±0.25*** | 99.96±0.5*** | 99.92±0.5*** | 100.54±0.76*** | 100.5±0.67*** | |
| AtE-W | 13.19±2.48*** | 21.56±2.47*** | 42.54±2.11*** | 70.21±1.46*** | 97.21±0.17*** | 99.92±0.09*** | 98.37±1.17*** | |
| Ascorbic acid | 100.04±0.01*** | |||||||
Note: Positive control-1000 μg/ml (DPPH assay) and 10 μg/ml (ABTS assay) of ascorbic acid; ***p<0.001 and p<0.01 indicate statistically significant difference from a negative control (methanol for DPPH and distilled water for ABTS assays). The data shown represent the mean values of triplicate assays±S.D; AtE: Ethanol extract and fractions of (-C: Chloroform; -EA: Ethyl acetate; -B: Butanol; -W: Water fraction)
Table 3: DPPH and ABTS Radical Sc of Ethanol Extract and Four Fractions from A. tegmentosum Stem
| Extraction/Fraction | SC50 (µg/ml) | |
|---|---|---|
| DPPH scavenging capacity | ABTS scavenging capacity | |
| AtE | 31.81±0.01 | 15.74±0.01 |
| AtE-C | 98.25±0.01 | 41.62±0.01 |
| AtE-EA | 9.98±0.01 | 5.29±0.07 |
| AtE-B | 16.95±0.01 | 9.23±0.002 |
| AtE-W | 134.1±0.02 | 52.71±0.02 |
Note: The data are means of three replicates (n=3), mean±SD. AtE: Ethanol extract and fractions of (-C: Chloroform; -EA: Ethyl acetate; -B: Butanol; -W: Water fraction)
Table 4: SC50 of DPPH and ABTS Radicals of Ethanol Extract and Four Fractions from A. tegmentosum Stem
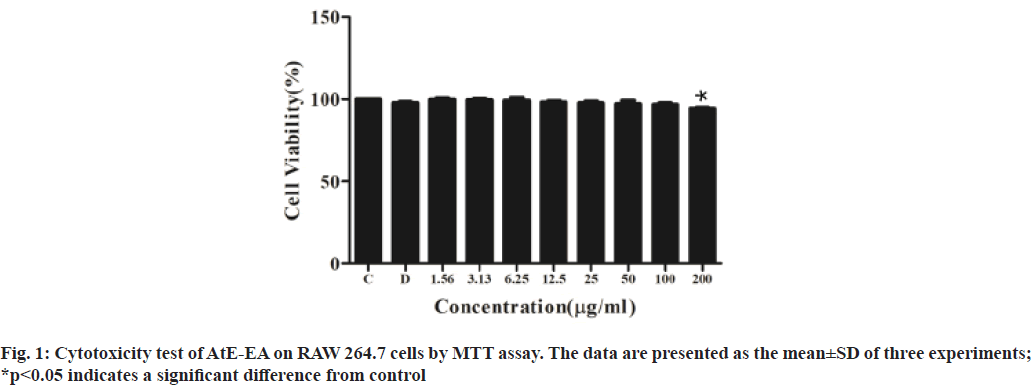
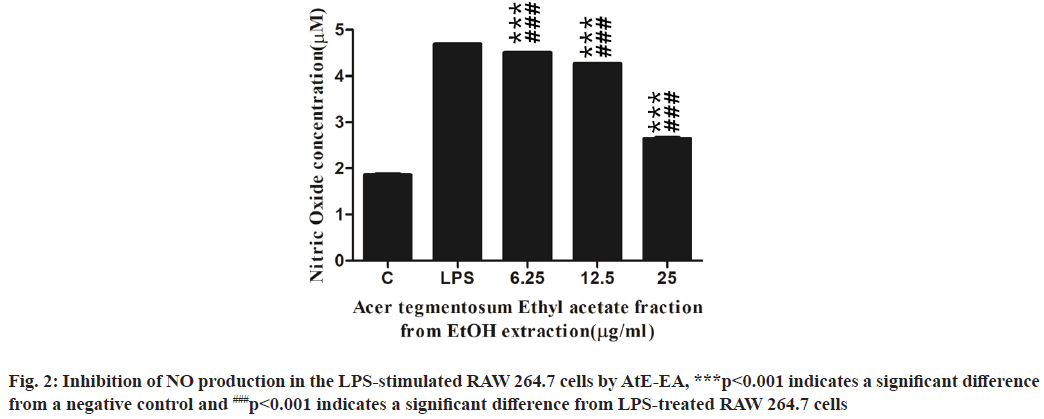
Based on antioxidant results, AtE-EA was selected for further experiments. As shown in fig. 1, no cytotoxic effect to RAW 264.7 cells observed at all up to the 100 µg/ml concentration of AtE-EA. Even the 200 µg/ml concentration presented cell viability close to that of the untreated control. The NO accumulation in the culture medium was determined to investigate the inhibitory effects of AtE-EA at various concentrations on the NO production in the LPS-stimulated macrophage cells. The NO production in the LPS-stimulated macrophages dramatically increased about 2.5 times more than that in the negative control (fig. 2). However, AtE-EA significantly suppressed the LPS-induced NO in a dose-dependent manner (p<0.001). The NO production significantly reduced to 3.9, 9.1 and 43.6 % at 6.25, 12.5 and 25 µg/ml of concentrations of AtE- EA, respectively.
In a previous study, NO implicates the inflammatory process through the action of iNOS expressed predominantly by macrophages recognizing bacterial LPS[24]. However, excessive NO production by macrophages appears to be associated with harmful effects on healthy cells[25]. Therefore, the inhibition of iNOS activation and NO production may provide therapeutic benefits against various types of inflammation[25.26]. Salidroside isolated from AtE-W did not show any inhibitory effect on NO production, while (+)-catechin showed the inhibition of NO production at high concentration[12]. In this study, AtE-EA also significantly reduced the LPS-stimulated NO production in a dose-dependent manner. This observation correlates with the finding in previous research, in that AtE showed a dose-dependent inhibition of the NO production in the LPS-stimulated macrophages[10].
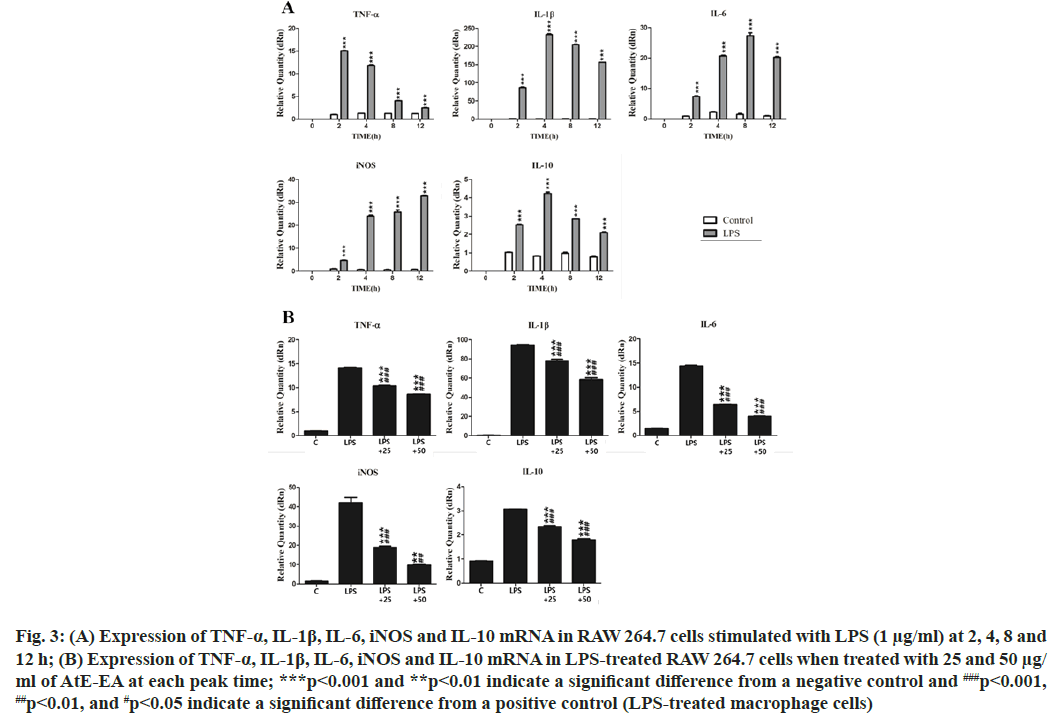
In this study, expression levels of TNF-α, IL-1β, IL- 6, iNOS and IL-10 mRNA were measured by RT- qPCR to assess the effect of AtE-EA on the LPS- stimulated macrophages. The peak levels of TNF-α, IL-1β, IL-6, iNOS and IL-10 mRNA in the LPS- stimulated macrophages were observed at 2, 4, 8 and 12 h, respectively (fig. 3A). The peak mRNA levels of TNF-α,IL-1β, IL-6, iNOS and IL-10 at each given time significantly reduced in the LPS-stimulated macrophages treated with 25 µg/ml and 50 µg/ml of AtE-EA, respectively (fig. 3B). Numerous inflammatory mediators are usually divided into two main categories: Pro-inflammatory and anti-inflammatory mediators. TNF-α, IL-1β and iNOS are pro-inflammatory cytokines and enzyme, while IL-10 is a potent anti-inflammatory cytokine. IL-1α and IL-6 act as pro-inflammatory cytokines with some anti-inflammatory effects[27]. Upon stimulation by LPS, activated macrophages secrete pro- inflammatory cytokines that induce early inflammatory reactions[28]. Previously, ethanol extract from A. tegmentosum suppressed the TNF-α production in the LPS-stimulated macrophages[10]. Besides, a butanol fraction of methanol extract from A. tegmentosum showed the reduced mRNA expression of TNF-α, iNOS and IL-6 through the down-regulation of Nuclear Factor kappa B (NF-κB) activity[29]. In this study, AtE- EA effectively suppressed mRNA levels of TNF-α, IL- 1β, IL-6, IL-10 and iNOS. It is not clear why AtE-EA reduced the mRNA level of IL-10.
Fig. 3: (A) Expression of TNF-α, IL-1β, IL-6, iNOS and IL-10 mRNA in RAW 264.7 cells stimulated with LPS (1 μg/ml) at 2, 4, 8 and 12 h; (B) Expression of TNF-α, IL-1β, IL-6, iNOS and IL-10 mRNA in LPS-treated RAW 264.7 cells when treated with 25 and 50 μg/ ml of AtE-EA at each peak time; ***p<0.001 and **p<0.01 indicate a significant difference from a negative control and ###p<0.001, ##p<0.01, and #p<0.05 indicate a significant difference from a positive control (LPS-treated macrophage cells)
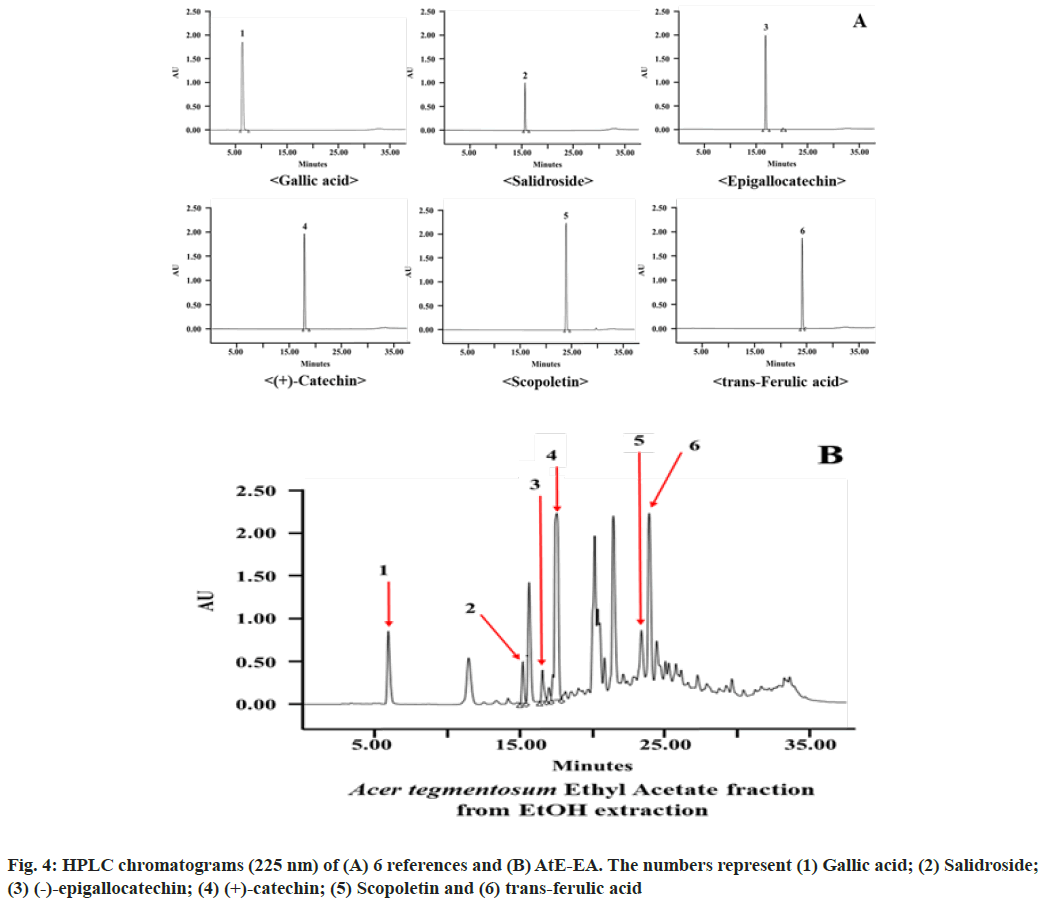
Acetonitrile-water containing 0.5 % phosphoric acid system as the mobile phase demonstrated good separation of gallic acid, salidroside, (-)-epigallocatechin, (+)-catechin, scopoletin and trans-ferulic acid at the retention time of 6.4, 9.7, 15.3, 22.3, 24.4 and 24.9 min, respectively (fig. 4A). In HPLC chromatograms, the peak retention times detected from AtE-EA in comparison with those from reference standards identified six compounds among ten peaks (fig. 4B). Major peaks identified in gallic acid, (+)-catechin and trans-ferulic acid, while minor peaks identified in salidroside, (-)-epigallocatechin and scopoletin. Gallic acid and various catechins show many biological and pharmacological properties such as antioxidant, anti-inflammatory, antimicrobial, antiviral, anti-allergenic and anti-cancer activities[30-33]. Similarly, trans-ferulic acid, salidroside and scopoletin (6-methoxy-7-hydroxycoumarin) isolated from several plant species show versatile pharmacological properties such as antioxidant, anti-inflammatory, antitumor, antimicrobial, antiviral and antifungal activities[34-40]. Therefore, HPLC analysis identified six compounds from AtE-EA such as gallic acid, salidroside, (-)-epigallocatechin, (+)-catechin, scopoletin and trans- ferulic acid. It suggested that the presence of those compounds in AtE-EA is responsible for the antioxidant and anti-inflammation activities.
In conclusion, A. tegmentosum showed antioxidant activity based on DPPH and ABTS assays. In addition, A. tegmentosum showed anti-inflammatory activity because AtE-EA effectively inhibited the NO production and suppressed the macrophages to express mRNA of pro-inflammatory and anti-inflammatory mediators in the LPS-stimulated RAW 264.7 cells.
Acknowledgements:
This research was financially supported by the Ministry of Small and Medium-sized Enterprises (SMEs) and Startups (MSS), Korea, under the “Regional Specialized Industry Development Plus Program (R&D, S2894086)” supervised by the Korea Institute for Advancement of Technology (KIAT).
Conflict of interests:
The authors declare that they have no conflict of interests.
References
- Chatterjee S. Chapter two-oxidative stress, inflammation and disease. In: Dziubla T, Butterfield D, editors. Oxidative stress and biomaterials. Amsterdam: Elsevier; 2016. p. 35-8.
- Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules 2015;5(2):545-89.
[Crossref] [Google Scholar] [PubMed]
- De Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol 2017;996:15-23.
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 2005;4(3):281-6.
- Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest 2012;122(3):787-95.
[Crossref] [Google Scholar] [PubMed]
- Lam D, Harris D, Qin Z. Inflammatory mediator profiling reveals immune properties of chemotactic gradients and macrophage mediator production inhibition during thioglycollate elicited peritoneal inflammation. Mediators Inflamm 2013;2013:1-9.
[Crossref] [Google Scholar] [PubMed]
- Arulselvan P, Fard MT, Tan WS, Gothai S, Fakurazi S, Norhaizan ME, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev 2016;2016.
[Crossref] [Google Scholar] [PubMed]
- Nunes CD, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, et al. Plants as sources of anti-inflammatory agents. Molecules 2020;25(16):3726.
[Crossref] [Google Scholar] [PubMed]
- Rhim TJ. Anticytotoxic and radical scavenging activities of Acer tegmentosum Maxim stem extracts. J Environ Sci Int 2015;24(11):1315-29.
- Yu T, Lee J, Lee YG, Byeon SE, Kim MH, Sohn EH, et al. In vitro and in vivo anti-inflammatory effects of ethanol extract from Acer tegmentosum. J Ethnopharmacol 2010;128(1):139-47.
[Crossref] [Google Scholar] [PubMed]
- Park HS, Jo E, Han JH, Jung SH, Lee DH, Park I, et al. Hepatoprotective effects of an Acer tegmentosum Maxim extract through antioxidant activity and the regulation of autophagy. J Ethnopharmacol 2019;239:111912.
[Crossref] [Google Scholar] [PubMed]
- Song NY, Lee KJ, Ma JY. Isolation and identification of phenol compounds from Acer tegmentosum and their anti-inflammatory activity. Korean J Pharmacogn 2014;45(2):93-100.
- Lee KJ, Song NY, Oh YC, Cho WK, Ma JY. Isolation and bioactivity analysis of ethyl acetate extract from Acer tegmentosum using in vitro assay and on-line screening HPLC-ABTS+ system. J Anal Methods Chem 2014;2014.
[Crossref] [Google Scholar] [PubMed]
- Kim EC, Kim SH, Piao SJ, Kim TJ, Bae K, Kim HS, et al. Antiangiogenic activity of Acer tegmentosum Maxim water extract in vitro and in vivo. J Korean Med Sci 2015;30(7):979-87.
[Crossref] [Google Scholar] [PubMed]
- Eo HJ, Park GH, Kim DS, Kang Y, Park Y. Antioxidant and anticancer activities of leaves extracts from Acer tegmentosum. Korean J Plant Res 2020; 33:551-7.
- Park SJ, Shin EH, Kim DH, Rha YA. Nutrition components and physicochemical properties of Acer termentosum Maxim leaf. Culin Sci Hosp Res 2016;22(8):27-38.
- Lee J, Hwang IH, Jang TS, Na M. Isolation and quantification of phenolic compounds in Acer tegmentosum by high performance liquid chromatography. Bull Korean Chem Soc 2017;38(3):392-6.
- Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoco 2007;2(4):875-7.
[Crossref] [Google Scholar] [PubMed]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 2002;10(3):178-82.
[Crossref] [Google Scholar] [PubMed]
- Ghorai N, Chakraborty S, Gucchait S, Saha SK, Biswas S. Estimation of total terpenoids concentration in plant tissues using a monoterpene, linalool as standard reagent. Protoco Exch 2012;2012:5.
- Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, et al. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 2002;163(6):1161-8.
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 2004;74(17):2157-84.
[Crossref] [Google Scholar] [PubMed]
- Pisoschi AM, Pop A, Cimpeanu C, Predoi G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev 2016;2016.
[Crossref] [Google Scholar] [PubMed]
- Hwang JS, Kwon MY, Kim KH, Lee Y, Lyoo IK, Kim JE, et al. Lipopolysaccharide (LPS)-stimulated iNOS induction is increased by glucosamine under normal glucose conditions but is inhibited by glucosamine under high glucose conditions in macrophage cells. J Biol Chem 2017;292(5):1724-36.
[Crossref] [Google Scholar] [PubMed]
- Lind M, Hayes A, Caprnda M, Petrovic D, Rodrigo L, Kruzliak P, et al. Inducible nitric oxide synthase: Good or bad? Biomed Pharmacother 2017;93:370-5.
- Kim MJ, Kim JG, Sydara KM, Lee SW, Jung SK. Croton hirtus L 'Hér extract prevents inflammation in RAW264. 7 macrophages via inhibition of NF-κB signaling pathway. J Microbiol Biotechnol 2020;30:490-6.
[Crossref] [Google Scholar] [PubMed]
- Azab A, Nassar A, Azab AN. Anti-inflammatory activity of natural products. Molecules 2016;21(10):1321.
[Crossref] [Google Scholar] [PubMed]
- Lu G, Zhang R, Geng S, Peng L, Jayaraman P, Chen C, et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat Commun 2015;6(1):1-4.
[Crossref] [Google Scholar] [PubMed]
- Won SJ, Park HJ, Lee KT. Inhibition of LPS induced iNOS, COX-2 and cytokine expression by salidroside through the NF-kB inactivation in RAW 264.7 cells. Korean J Pharmacogn 2008; 39:110–7.
- El-Toumy SA, Salib JY, El-Kashak WA, Marty C, Bedoux G, Bourgougnon N. Antiviral effect of polyphenol rich plant extracts on herpes simplex virus type 1. Food Sci Hum Wellness 2018;7(1):91-101.
- Wang Q, Leong WF, Elias RJ, Tikekar RV. UV-C irradiated gallic acid exhibits enhanced antimicrobial activity via generation of reactive oxidative species and quinone. Food Chem 2019;287:303-12.
[Crossref] [Google Scholar] [PubMed]
- Nouri A, Heibati F, Heidarian E. Gallic acid exerts anti-inflammatory, anti-oxidative stress, and nephroprotective effects against paraquat-induced renal injury in male rats. Naunyn Schmiedebergs Arch Pharmacol 2021;394(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Bae J, Kim N, Shin Y, Kim SY, Kim YJ. Activity of catechins and their applications. Biomed Dermatol 2020;4(1):1-10.
- Paiva LB, Goldbeck R, Santos WD, Squina FM. Ferulic acid and derivatives: molecules with potential application in the pharmaceutical field. Braz J Pharm Sci 2013;49:395-411.
- Rezaeiroshan A, Saeedi M, Morteza-Semnani K, Akbari J, Hedayatizadeh-Omran A, Goli H, et al. Vesicular formation of trans-ferulic acid: An efficient approach to improve the radical scavenging and antimicrobial properties. J Pharm Innov 2021:1-10.
- Sharma N, Mishra KP, Ganju L. Salidroside exhibits anti-dengue virus activity by upregulating host innate immune factors. Arch Virol 2016;161(12):3331-44.
[Crossref] [Google Scholar] [PubMed]
- Liu J, Cai J, Fan P, Zhang N, Cao Y. The abilities of salidroside on ameliorating inflammation, skewing the imbalanced nucleotide oligomerization domain-like receptor family pyrin domain containing 3/autophagy, and maintaining intestinal barrier are profitable in colitis. Front Pharmacol 2019;10:1385.
[Crossref] [Google Scholar] [PubMed]
- Wang YS, Zhou SS, Shen CY, Jiang JG. Isolation and identification of four antioxidants from Rhodiola crenulata and evaluation of their UV photoprotection capacity in vitro. J Funct Foods 2020;66:103825.
- Mogana R, Teng-Jin K, Wiart C. Anti-inflammatory, anticholinesterase and antioxidant potential of scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth). Evid Based Complement Alternat Med 2013;2013.
- Ikanovic T, Sehercehajic E, Saric B, Tomic N, Hadziselimovic R. In silico analysis of scopoletin interaction with potential SARS-CoV-2 target. In: Karabegovi? I, editor. New technologies, development and application IV. NT 2021. Lecture notes in networks and systems 233. Cham: Springer; 2021. p. 897-903.