- *Corresponding Author:
- S. N. K. R. Gudhanti
K L College of Pharmacy
Koneru Lakshmaiah Education Foundation
Vaddeswaram, Andhra Pradesh 522502, India
E-mail: drgsnkrao@gmail.com
| Date of Received | 09 March 2021 |
| Date of Revision | 04 February 2022 |
| Date of Acceptance | 03 September 2022 |
| Indian J Pharm Sci 2022;84(5):1257-1268 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Natural products have been playing a vital role in protection against different diseases since ancient times. Many medicinal plants are still unexplored about their medicinal values scientifically, but they are still used in traditional medicine. The current study was aimed to explore and evaluate the phytochemical constituents, antioxidant activity against different free radicals with in vitro methods and in vivo methods using albino Wistar rats and hepatoprotective activity against ethanol-induced liver toxicity. The extracts of Aerides odorata show sterols, terpenoids, glycosides, saponins, flavonoids, tannins, phenols, alkaloids, quinones and the absence of amino acids and oils. The hydroalcoholic extract had showed presence of more phenolic and alkaloid contents compared to ethyl acetate and hexane extracts of Aerides odorata. The extracts of Aerides odorata had shown antioxidant potentiality in both in vitro and in vivo studies. The extracts had more inhibition in reducing 2,2-diphenyl-1-picrylhydrazyl and almost the same potentiality in altering antioxidant enzymes levels. The extracts of Aerides odorata had shown concentration-dependent hepatoprotective activity. However, it is moderate as compared with the standard drug Liv 52. The hydroalcoholic extracts had more activity compared to ethyl acetate extract. The activity is more in the reduction of free radicals but is lower in the reduction of hepatoprotection. The compound in Aerides odorata may act synergistically or individually in controlling both activities. Further studies are worthful in the evaluation of different biological activities and the isolation of individual bioactive compounds.
Keywords
Aerides odorata, phytochemicals, free radicals, antioxidant, ethanol, liver toxicity, hepatoprotection.
Natural Products (NPs) have been used in Traditional Medicine (TM) to treat different diseases around the world[1-3]. TMS is the basis for today's Modern Medicine (MM)[1,4-6]. The Medicinal Plants (MPs) contribution to NPs and MM development was crucial[7-9]. Different MPs have been mentioned in different TMs such as Indian Ayurveda, Chinese TM, Unani medicine, Korean TM, Kampo etc.[1,10-12]. The presence of chemical constituents' diversity in different MPs plays a pivotal role in developing new bioactive compounds over the past few decades against different diseases[5,8]. However, the emerging of new diseases challenging human survival and demanding new bioactive moieties to fight against them[5,13]. The applications of new modern technologies in chemical screening, synthesis, pharmacological, pharmacodynamics will help develop new efficient drugs from NPs[14,15].
Many MPs are available around the world and have been using in TM[1,10]. Some have been scientifically proven about their biological activities and isolated bioactive molecules[8,13,16]. However, there were still many MPs have not been reported about their activities[17]. The primary evaluation of biological activities was critical in initial screening about their medicinal use because isolation and development of new bioactive molecules is not an easy process. It has needed more detailed studies as per regulatory guidelines for human usage[14,18]. Aerides odorata (A. odorata) is one such medicinal plant; there were little research works have done on it about its medicinal values. Therefore, the current research was aimed to evaluate its phytochemical constituents, antioxidant and hepatoprotective activities.
A. odorata is a sub-tropical and tropical habitat plant belongs to the Orchidaceae family (fig. 1). It is commonly known as fragrant fox bush orchid, is large to giant shape with very stout drooping branches and produces highly fragrant blossoms[19]. There is literature evidence used in TM, such as in the treatment of inflammation, pneumonia, dyspepsia, fractures, tuberculosis, joint pains and wound healings[19,20].
Materials and Methods
Chemicals and reagents:
The chemicals and reagents used in the current study were analytical grades. The diagnostic kits used in the study were purchased from Span Diagnostics Ltd, Gujarat, India. The standard drugs ascorbic acid, Trolox were from Sigma Aldrich Co. The standard drug for hepatoprotective activity, Liv 52, was purchased from a local medical shop.
Preparation of plant extracts:
The plant material A. odorata (Voucher specimen number:23343) was collected at Araku valley region, Visakhapatnam, and was authenticated by Prof. S. B. Padal, Department of Botany Andhra Pradesh, India. The collected aerial parts were cleaned under running tap water to remove debris and were shade dried. The dried material was made in bristly powder. The powder was used for extraction by maceration process successively using ethyl acetate and hydroalcoholic (70 % ethanol in water v/v) [A. odorata Ethyl Acetate Extracts (AOEAE) and A. odorata Hydroalcoholic Extract (AOHAE)]. The collected solvents evaporated using rotavap, and extracts were stored in a desiccator for further usage.
Phytochemical analysis:
The collected extracts were analyzed to explore extracts' chemical profile using standard phytochemical tests qualitatively[21,22] and quantitatively quantified the alkaloids and phenolics.
Quantification of phenolic and alkaloid contents:
Phenolic content analysis: The phenolic content was analyzed using Folin-Ciocalteau Reagent (FCR) as described in the method by Singleton et al.[23]. The method is the colourimetric method, based on chemical reduction of reagent mixture contains tungsten and molybdenum. To the extract (mg/ml) add FCR (5 ml) and after the 30 min incubation time, colour (blue) of the reaction mixture measured at 760 nm, presence of phenolic content will enhance the absorbance and was calculated against the standard graph of gallic acid and expressed as gallic acid equivalents (mg/g)[22]. The results were showed as mean± Standard Error of Mean (SEM) (n=3).
Alkaloid content analysis: The alkaloid contents of selected plant extracts were quantified by the spectroscopic method as described by Shamsa et al.[24] using Bromocresol Green (BCG) solution. The procedure was to 1 ml plant extracts dissolved in 2 N hydrochloric acid (mg/ml) add 5 ml BCG solution in a separation funnel and 5 ml of phosphate buffer and mixed well. After, the complex formed was extracted (separated) in chloroform (5 ml). The absorbance of chloroform (yellow colour) was measured at 470 nm against the standard graph atropine[22]. The results were showed as mean±SEM (n=3).
In vitro antioxidant activity:
The In vitro antioxidant activity of selected plant extracts was evaluated on superoxide, hydroxy, 2,2-diphenyl- 1-picrylhydrazyl (DPPH)[22], hydrogen peroxide (H2O2)[25] free radicals and reducing antioxidant power using ferric ion[26]. The extracts were dissolved in Dimethyl Sulphoxide (DMSO) for easy solubility. The experiments results were presented as mean±SEM. The Percentage Inhibition (PI) was calculated as PI=(A0-A1)/A0×100 (A0: Absorbance of control; A1: Absorbance of plant extract or/and Ascorbic acid). The 50 % inhibition of concentrations (IC50 values) were calculated as a graph plotted with a concentration on X-axis and percentage inhibition on Y-axis.
Superoxide free radical scavenging activity:
Superoxide free radical scavenging activity was assessed as per the method described by Mc Cord et al.[27]. This is a spectroscopic method, evaluating the absorbance of light at 560 nm of a solution contain generated superoxide free radicals' riboflavin with Nitroblue Tetrazolium (NBT) reduction of colour with different concentrations of extracts (20-320 µg/100 µl). The ascorbic acid was used as a positive control and the values were measured against the corresponding blank.
Hydroxyl free radical scavenging activity
Hydroxyl scavenging activity of selected plant extracts were carried out by the procedure described by Elizabeth et al.[28]. The method was measuring the absorbance of thiobarbituric acid reactive substances at 532 nm from the reduction of generated hydroxyl radicals through the Fenton reaction mixture (Fe2+/EDTA/H2O2 system).
DPPH free radical scavenging activity:
The DPPH free radical scavenging activity was measured using the procedure Braca et al.[29]. The procedure was measuring the absorbance of alcoholic DPPH (0.004 %) (blue colour to yellow colour) after the addition of 0.1 ml of testing extracts/ascorbic acid at different concentrations.
H2O2 scavenging activity:
H2O2 scavenging activity was measured using the method described by Ruch et al.[30]. The method is measuring the absorbance of the reaction mixture at 230 nm containing 0.1 ml of plant extract, 0.3 ml of 50 mM phosphate buffer and 0.6 ml of 2 mM H2O2 against the blank[25].
Reducing antioxidant power assay:
The reducing antioxidant power of extracts was measured with the spectroscopic method described by Benzie et al.[26] using Trolox as a standard drug. The method is measuring colour complex absorbance at 593 nm for the reduction power of extracts at different concentrations from Fe3+ (colourless) to Fe2+ (blue colour) against the blank.
In vivo antioxidant activity:
The In vivo antioxidant activity was studied using albino Wistar rats of either sex weighing from 200-250 g around 60-90 d aged. During the course of the study, animals were maintained under controlled conditions (12 h light/dark cycle, 24±2°, 40 %-70 % relative humidity) by supplying sufficient food and water. Prior to starting an antioxidant activity on animals, the extracts were tested for their toxicity as per Organization for Economic Cooperation and Development (OECD) guidelines 420 with 1000 and 2000 mg/kg body weight (b.w.) on overnight fasting animals using four groups (n=6 each group) and observed at regular intervals for any changes in animals such as skin, morbidity, aggressiveness, oral secretions, sensitivity, pain, respiratory problems and finally mortality. The animal studies were approved by the institutional ethical committee of Santhiram Medical College and General Hospital (897/PO/RE/S/05/CPCSEA).
After the toxicity study, animals were divided into seven groups (n=6). Group I served as control received distilled water (0.5 ml), groups II to IV received ethyl acetate extract (100-400 µg/ml) and groups V to VII received hydro-alcoholic extract (100-400 µg/ml) of A. odorata for 21 d administered orally using metal oropharyngeal cannula. 24 h after the last dosage, blood was collected from animals using direct cardiac puncture under isoflurane anaesthetic condition to get serum through a centrifuge at 2500 rpm for 15 min to measure lipid peroxidation, estimation of Superoxide Dismutase (SOD) and catalase activity[31,32].
Lipid peroxidation:
Lipid peroxidation was determined as per Draper et al.[33] procedure by measuring the Thiobarbituric Acid Reactive Substances (TBARS) and Malonaldehyde (MDA) in serum[33]. The procedure was, serum was deproteinized by the addition of trichloroacetic acid and thiobarbituric acid and added 0.1 ml of testing extract, and then the mixture was heated for 30 min in a water bath. After cooling, the mixture was centrifuged at 2000 rpm for 10 min, the absorbance of the supernatant (TBARS) at 535 nm spectrophotometrically against blank. The concentration of TBARS was calculated using the molar extinction coefficient of malondialdehyde (1.56×105 mol/l/cm) and results were expressed in nmol/mg of protein.
SOD estimation:
SOD activity was determined by method Sun et al.[34] by xanthine-xanthine oxidase system for production of superoxide flux and NBT for their production. The SOD was measured by the degree of inhibition of enzyme activity after the addition of plant extracts against blank and results expressed as U/ml[34].
Catalase activity:
The catalase activity was carried using spectrophotometrically as per Atawodi method. The method was to measure the absorbance of hydrogen peroxide at 240 nm from a mixture contains serum, potassium phosphate buffer, 30 mM H2O2 and testing extracts against blank after 30 min incubation[35].
Hepatoprotective activity:
The hepatoprotective activity of A. odorata extracts was carried on ethanol-induced liver toxicity as per the method described by Shukla et al.[36]. The animals were divided into nine groups (n=6); Group I is control treated with drug vehicle (2 % v/v tween 80); Group II is toxic-administered with ethanol 3.76 g/kg p.o. twice a day; Group III treated with Liv 52 (25 mg/kg); Groups IV, V, VI treated with AOEAE at 100, 200 and 400 mg/kg b. w. respectively for 21 d after 1 h of ethanol administration and Groups VII, VIII, IX treated with AOHAE at 100, 200 and 400 mg/kg b. w. respectively for 21 d after 1 h of ethanol administration.
On the 22nd d, blood was collected from all the group animals through retro-orbital puncture under isoflurane anaesthetic condition. After collection of blood, immediately serum was separated for estimation of liver profile enzymes such as aspartate Aminotransferase (AST), Alanine Transaminase (ALT), Alkaline Phosphatase (ALP), Total Protein (T.Ptn), and Total Bilirubin (T.Bil) using diagnostic kits on semi- autoanalyzer. Later, the animals were sacrificed and collected liver, stored in 10 % formalin solution for histopathological studies.
Statistical analysis:
The results of in vivo activity were analyzed by One-Way analysis of variance with Dunnett's multiple comparisons with the control group. The enzyme levels of hepatoprotective activity were presented as mean±SEM and liver protection as the percentage with the below formula. The significance was analyzed with two-way ANOVA followed by Dunnett's multiple comparison test.
Percentage Protection= (Levels in toxic group-Levels in the test group)/ (Levels in toxic group-levels in the control group)×100
Results and Discussion
The phytochemical analysis of A. odorata extracts (AOEAE and AOHAE) showed the presence of different phytochemical constituents and variation between extracts. Both extracts of A. odorata shows the presence of sterols, terpenoids, glycosides, saponins, flavonoids, tannins, phenols, alkaloids, quinones and the absence of amino acids, oils. The AOEAE shows the presence of carbohydrates, but AOHAE gave a negative result (Table 1). The quantification of phenolic and alkaloid contents of both extracts was carried and found AOHAE has more contents than AOEAE (Table 2).
| Name of the phytoconstituent | Name of the extract | |
|---|---|---|
| Ethyl acetate | Hydroalcoholic | |
| Sterols | + | + |
| Terpenoids | + | + |
| Glycosides | + | + |
| Saponins | + | + |
| Flavonoids | + | + |
| Tannins | + | + |
| Carbohydrates | - | + |
| Alkaloids | + | + |
| Amino acids | - | - |
| Oils | - | - |
| Quinones | + | + |
| Phenols | + | + |
Note: +: Present; -:Absent
Table 1: Phytochemical Analysis of Aerides odorata Extracts
| Name of the Component (mg/g) | Name of the Extract | |
|---|---|---|
| Ethyl acetate | Hydroalcoholic | |
| Total phenols | 17.05±0.25 | 20.79±0.33 |
| Total Alkaloids | 16.15±0.15 | 23.57±0.44 |
Table 2: Phenolic and Alkaloid Contents of Aerides odorata Extracts
The extracts of A. odorata evaluated for their antioxidant potentiality using standard in vitro methods on different free radicals such as superoxide, hydroxyl, DPPH, H2O2, reducing power assay and with in vivo method on rats by estimation of antioxidant enzymes' levels such as SOD, catalase and malonaldehyde compound indicate the oxidative damage in the body through lipid peroxidation. The extracts have shown concentration-dependent Percentage Inhibition (PI) and enhancement in antioxidant enzymes' levels.
Both the extracts of A. odorata possess variation in inhibition of different free radicals, and AOHAE had shown better potentiality compared to AOEAE. The extracts showed better PI inhibition at maximum concentration, i.e., 320 µg, and as concentration increases, PI was increased. The PI of AOEAE and AOHAE at 320 µg on superoxide free radical was 59.67±0.88 and 76.00±1.15 with IC50 values 220.94 µg and 135.63 µg (Table 3 and fig. 2). The PI of both extracts on hydroxyl free radical was 55.00±1.73 and 79.00±2.08 at 320 µg with IC50 values 274.9 µg and 131.25 µg (Table 3 and fig. 3).
| Name of the extract/compound |
IC50 Value (µg) | ||||
|---|---|---|---|---|---|
| Name of the free radical | |||||
| Superoxide | Hydroxyl | DPPH | H2O2 | Reducing power | |
| AOEAE | 220.94 | 274.9 | 140.73 | 141.46 | 175.00 |
| AOHAE | 135.63 | 131.25 | 104.27 | 133.44 | 188.13 |
| Ascorbic acid | 79.48 | 73.36 | 73.65 | 85.31 | N/AP |
| Trolox | N/AP | N/AP | N/AP | N/AP | 72.19 |
Note: AOEAE: Aerides odorata Ethyl Acetate Extract; AOHAE: Aerides odorata Hydro-Alcoholic Extract; N/AP: Not Applicable.
Table 3: IC50 Values of Aerides odorata Extracts on Different Free Radicals
The PI on DPPH free radical of AOEAE and AOHAE at 320 µg was 69.67±1.20 and 83.67±0.88 with IC50 values 140.73 µg and 104.27 µg (Table 3 and fig. 4). The PI on H2O2 generated free radicals of AOEAE and AOHAE at 320 µg was 76.33±0.88 and 73.00±1.15 with IC50 values 141.46 µg and 133.44 µg (fig. 5). The PI of AOEAE and AOHAE at 320 µg on reduction assay was 68.33±1.45 and 65.33±1.76 with IC50 values 175.00 µg and 188.13 µg (fig. 6).
The free radicals are generated in the body as by-products of different metabolisms, and they will react with other stable molecules to stabilize themselves. In this process, they are causing oxidative stress by reacting with different cellular components such as lipids, proteins, nucleotides and other macromolecules[28,37,38]. During oxidative stress, the levels of the oxidative enzyme will increase, and antioxidant enzymes levels will decrease[39,40], and the cellular component damage will occur through lipid peroxidation, i.e., increased levels of TBARS levels indicated by MDA[41,42]. The antioxidant activity of A. odorata extracts results showed that good inhibition of free radicals in in vitro study against tested free radicals and in in vivo study results supports the in vitro results with their capability in ameliorating the levels of the antioxidant enzyme, i.e., rise in catalase and SOD levels and decrease the cellular component damages in the body (Tables 3 and 4). The catalase and SOD are different enzymes involve in the decomposition of free radicals such as catalase reduces the hydrogen peroxide, and other free radicals' production in aerobic and anaerobic metabolisms. The increased levels of SOD reduce the superoxide and its conversion to hydroxyl and oxygen-free radicals. The MDA is an endogenous compound present in the body indicates the toxicity level due to lipid peroxidation in a healthy individual. In the current study, the MDA level was reduced as the dose was increased and indicated that, extracts of A. odorata reduced the lipid peroxidation and its' toxicity.
| Name of the extract/compound |
Name of the enzyme | ||
|---|---|---|---|
| Catalase (μmol/mg protein) | MDA (nmol/mg protein) | SOD (unit/ml) | |
| Control | 20.83±0.95 | 0.103±0.03 | 11.05±0.28 |
| AOEAE 100 mg/Kg | 23.67±0.56* | 0.098±0.00ns | 14.00±0.26ns |
| AOEAE 200 mg/Kg | 40.00±1.06* | 0.090±0.00ns | 17.87±0.31ns |
| AOEAE 400 mg/Kg | 76.50±0.89** | 0.078±0.001* | 23.23±0.37* |
| AOHAE 100 mg/Kg | 26.67±0.80* | 0.095±0.001* | 16.90±0.30ns |
| AOHAE 200 mg/Kg | 46.67±0.42** | 0.084±0.001** | 22.03±0.22ns |
| AOHAE 400 mg/Kg | 85.33±1.56** | 0.067±0.001** | 30.30±0.45* |
Note: ns=Non significant; *: p<0.05; **: p<0.01 Vs. control group
Table 4: Enzymatic Levels in Different Groups Due To the Effect of Aerides odorata Extracts at Different Doses
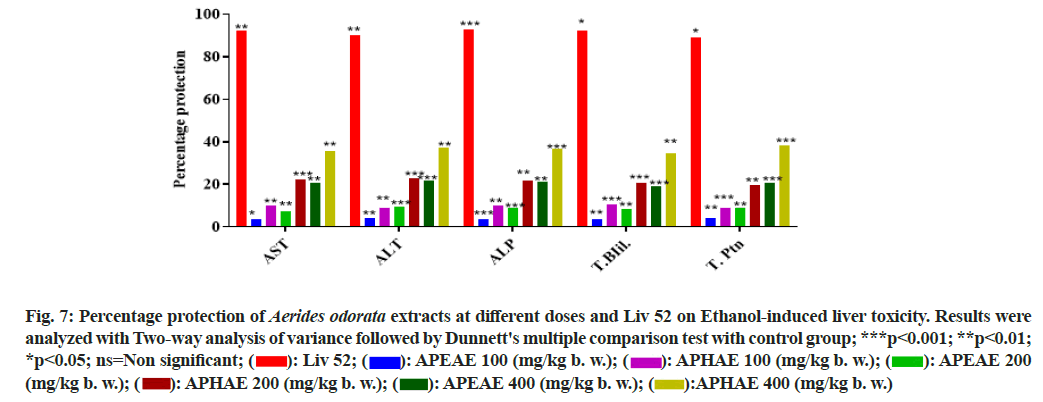
The extracts of A. odorata had no signs of mortality and physio-psychological changes at tested doses 1000 mg/kg b. w. and upper dose 2000 mg/kg b. w. on tested animals under observation of 14 d. Then, the extracts of A. odorata were used for in vivo studies at different concentrations. The hepatoprotective activity of A. odorata was evaluated using an ethanol-induced liver toxicity model[36]. Nowadays, liver diseases are one of the major causes of the worlds' mortality rate[43]. Livery diseases are very common in people who are consuming alcohol[45,46], some liver diseases are due to infections and some are due to inadequate or chronic usage of medicines' side effects[47]. Both extracts of A. odorata had shown hepatoprotective activity along with the standard drug Liv 52. The group I served as control and the liver biomarker enzymes levels (AST, ALT, ALP, T.Pth and T.Bil) were found normal, group II as negative control and the liver biomarker enzyme levels were abnormal as it treated with the alcohol. Group III was treated with both alcohol and standard drug Liv 52 and found it restored the changed enzyme levels to almost normal with Percentage Protection (PP) 92.23 % on AST, 90.38 % on ALT, 92.63 % on ALP, 92.31 % on T.Ptn and 89.35 % on T.Bil. The groups IV, V, VI and groups VII, VIII, IX were treated with ethanol and different concentrations of AOEAE and AOHAE. Both the extracts had shown concentration-dependent protection on altered liver enzyme levels. The extract showed higher protection at 400 mg/kg b.w. Among both extracts, AOHAE had shown better activity and is less compared with standard drug Liv 52. The PP of AOHAE at 400 mg/kg b.w was 35.63 % on AST, 37.13 % on ALT, 36.59 % on ALP, 34.57 % on T.Ptn and 38.46 % on T.Bil (Table 5 and fig. 7).
| Name of the Drug | Name of Enzymes | ||||
|---|---|---|---|---|---|
| AST (U/L) |
ALT (U/L) |
ALP (U/L) |
T. Bil (mg/dl) | T. Ptn (gm/dl) |
|
| GROUP-I Control (Drug Vehicle) |
85.00±1.03 | 46.33±1.28 | 129.00±0.82 | 0.24±0.01 | 6.97±0.07 |
| GROUP-II Ethanol |
327.33±2.16 | 159.00±0.63 | 483.83±2.47 | 2.17±0.06 | 4.15±0.05 |
| GROUP-III (Liv 52 25 mg) |
103.83±0.65 | 57.17±0.60 | 155.17±1.42 | 0.39±0.04 | 6.67±0.08 |
| GROUP-IV AOEAE (100 mg) |
318.67±0.88 | 154.00±1.06 | 470.67±0.99 | 2.10±0.04 | 4.27±0.05 |
| GROUP-V AOEAE (200 mg) |
309.67±1.17 | 148.33±0.76 | 452.17±1.76 | 2.00±0.04 | 4.40±0.05 |
| GROUP-IX AOEAE (400 mg) |
277.00±3.04 | 134.67±0.95 | 407.83±2.56 | 1.80±0.05 | 4.73±0.04 |
| GROUP-VII AOHAE (100 mg) |
303.67±1.20 | 148.67±0.84 | 448.33±1.52 | 1.97±0.06 | 4.40±0.07 |
| GROUP-VIII AOHAE (200 mg) | 272.83±1.87 | 133.33±0.84 | 406.67±2.42 | 1.77±0.06 | 4.70±0.04 |
| GROUP-IX AOHAE (400 mg) |
241.0±1.51 | 117.17±1.05 | 354.00±1.93 | 1.50±0.04 | 5.23±0.06 |
Note: The results were expressed as mean±SEM
Table 5: Liver Biomarker Enzymes Levels in Different Groups in Hepatoprotective Activity of Aerides odorata Extracts
Fig. 7: Percentage protection of Aerides odorata extracts at different doses and Liv 52 on Ethanol-induced liver toxicity. Results were analyzed with Two-way analysis of variance followed by Dunnett's multiple comparison test with control group; ***p<0.001; **p<0.01; *p<0.05; ns=Non significant;  APEAE 100 (mg/kg b. w.);
APEAE 100 (mg/kg b. w.);  APEAE 200 (mg/kg b. w.);
APEAE 200 (mg/kg b. w.);  APHAE 400 (mg/kg b. w.)
APHAE 400 (mg/kg b. w.)
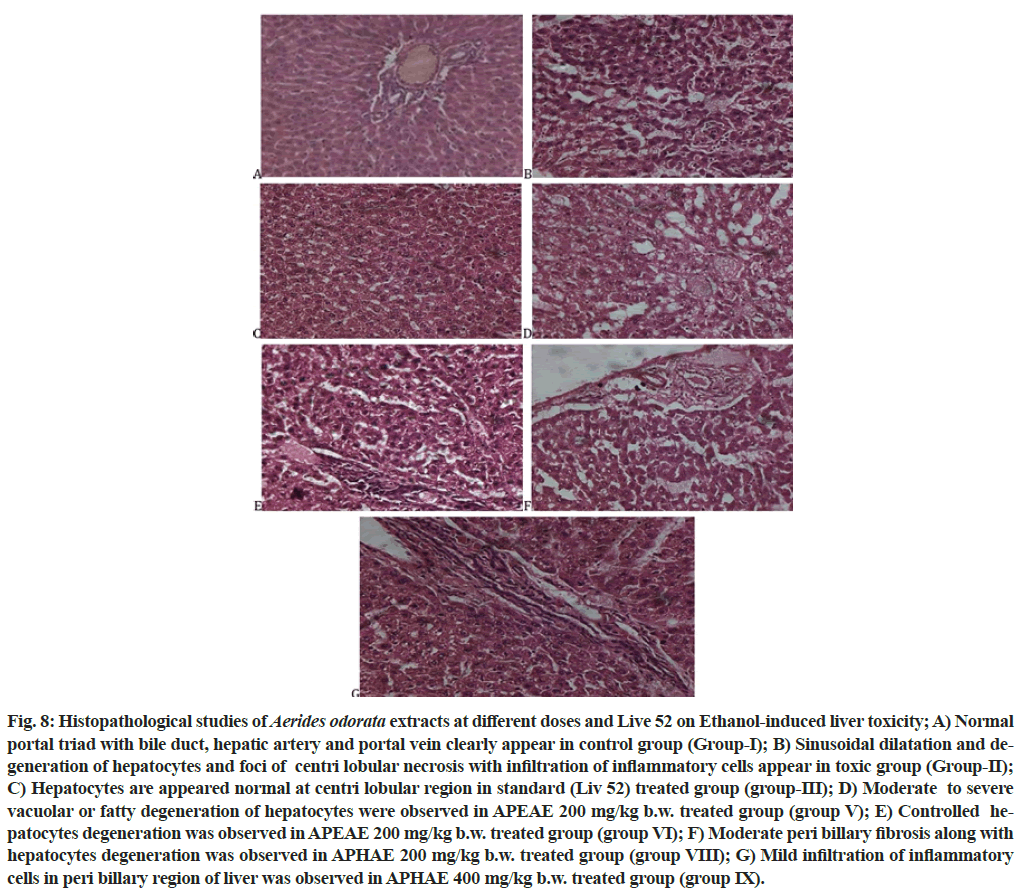
The histopathological studies (fig. 8) were clearly reveals that, the extracts of A. odorata possess the moderate to significant hepatoprotectective activity as standard drug Liv 52 at different dosess. The two extracts of A. odorata had showed different pathological and physiological conditions on treated animals on ethanol-induced liver toxicity.
Fig. 8: Histopathological studies of Aerides odorata extracts at different doses and Live 52 on Ethanol-induced liver toxicity; A) Normal portal triad with bile duct, hepatic artery and portal vein clearly appear in control group (Group-I); B) Sinusoidal dilatation and degeneration of hepatocytes and foci of centri lobular necrosis with infiltration of inflammatory cells appear in toxic group (Group-II); C) Hepatocytes are appeared normal at centri lobular region in standard (Liv 52) treated group (group-III); D) Moderate to severe vacuolar or fatty degeneration of hepatocytes were observed in APEAE 200 mg/kg b.w. treated group (group V); E) Controlled hepatocytes degeneration was observed in APEAE 200 mg/kg b.w. treated group (group VI); F) Moderate peri billary fibrosis along with hepatocytes degeneration was observed in APHAE 200 mg/kg b.w. treated group (group VIII); G) Mild infiltration of inflammatory cells in peri billary region of liver was observed in APHAE 400 mg/kg b.w. treated group (group IX).
The current study results reveal the presence of different bioactive phytochemicals in A. odorata, hydro-alcoholic extract possesses more phenolic and alkaloid contents and extracts had antioxidant and hepatoprotective potentiality as standard drugs ascorbic acid and Liv 52. As earlier said, there is a need to search for new bioactive compounds with scientific evidence; it needs basic research, which is very important to provide preliminary information. A. odorata is one of the MPs which is not explored in its basic research and it possesses different medicinal value and there are few scientific reports. The current study provides useful knowledge on its biological potentiality.
There were previous reports on different MPs about their biological activities and isolated bioactive compounds against various diseases including antioxidant and hepatoprotective. As the presence of phenolics, alkaloids and other compounds in the A. odorata, the same molecules are explored in different MPs about their antioxidant and hepatoprotective activities[48-50]. The potentiality of this plant may be because of such molecules are present in it. The phytochemical compounds in A. odorata may be effective as single compounds or work synergistically against free radicals and hepatic injury due to ethanol. The plant extracts of A. odorata had showed moderate potentiality in biological activity and may be these extracts are not be useful as complete herbal drugs. But, the identification of individaul molecules may act as potential bioactive compounds. The ethanol consumption damages the liver gradually as initially with fatty liver, middle stage as liver inflammation, and finally as alcoholic cirrhosis. At these stages, there are reports about the role of increased levels of free radical in the body also damages the hepatic tissue structure and enhances the lipid peroxidation which plays a vital role in liver damage[46,51]. However, the extracts of A. odorata had shown potentiality in control of free radicals and from ethanol-induced hepatotoxicity. The standard drug Liv 52 is a herbal drug that is widely using against liver protection and reported about its capacity in reduction of oxidative stress and have enhancing compounds in control against livers ethanol-induced damage[52]. The main components of Liv 52 herbal drug were chicory and caper bus, these components were already proven about their potentiality against oxidants and protect the liver damage by decreasing MDA levels[52,53]. As these components of Liv 52 possess phytochemical componets flavonoids[53]. The outcome of the current study supports that may be the presence of similar or new compounds in A. odorata as in Liv 52 were fight against free radicals and hepatotoxicity synergistically or individually.
The current study aimed to evaluate the antioxidant and hepatoprotective potency of A. odorata against different free radicals and ethanol-induced liver toxicity and found the extracts of A. odorata possess effective antioxidant activity and moderate hepatoprotective activities compared to standard drugs. The results provide scientific evidence to traditional medical usage of A. odorata and the presence of different bioactive compounds in it. Further studies are worthful in the investigation of the mechanism of action and isolation of pure bioactive compounds from it and are undergoing in our lab about its other biological activities and bioactive compounds isolation.
Acknowledgements:
The author, U. Praveen Kumar, would like express thanks to Santhiram Medical College and General Hospital for providing necessary facilities to complete In vivo studies.
Conflict of interest:
The authors have none to provide.
References
- Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules 2016;21(5):559.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Harwansh RK, Bahadur S, Banerjee S, Kar A, Chanda J, et al. Development of Ayurveda tradition to trend. J Ethnopharmacol 2017;197:10-24.
[Crossref] [Google Scholar] [PubMed]
- Jansen C, Baker JD, Kodaira E, Ang L, Bacani AJ, Aldan JT, et al. Medicine in motion: Opportunities, challenges and data analytics-based solutions for traditional medicine integration into western medical practice. J Ethnopharmacol 2021;267:113477.
[Crossref] [Google Scholar] [PubMed]
- Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019;9(11):258.
[Crossref] [Google Scholar] [PubMed]
- Chen J, Luo X, Qiu H, Mackey V, Sun L, Ouyang X. Drug discovery and drug marketing with the critical roles of modern administration. Am J Transl Res 2018;10(12):4302.
[Google Scholar] [PubMed]
- Hesari M, Mohammadi P, Khademi F, Shackebaei D, Momtaz S, Moasefi N, et al. Current advances in the use of nanophytomedicine therapies for human cardiovascular diseases. Int J Nanomed 2021;16:3293.
[Crossref] [Google Scholar] [PubMed]
- Shaito A, Thuan DT, Phu HT, Nguyen TH, Hasan H, Halabi S, et al. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front Pharmacol 2020;11:422.
[Crossref] [Google Scholar] [PubMed]
- Taghipour YD, Hajialyani M, Naseri R, Hesari M, Mohammadi P, Stefanucci A, et al. Nanoformulations of natural products for management of metabolic syndrome. Int J Nanomed 2019;14:5303-21.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee PK, Banerjee S, Biswas S, Das B, Kar A, Katiyar CK. Withania somnifera (L.) Dunal-Modern perspectives of an ancient Rasayana from Ayurveda. J Ethnopharmacol 202;264:113157.
[Crossref] [Google Scholar] [PubMed]
- Benzie IF, Wachtel-Galor S, editors. Herbal medicine: biomolecular and clinical aspects. 2nd ed. Boca Raton: CRC Press; 2011.
- Song P, Xia J, Rezeng C, Tong L, Tang W. Traditional, complementary, and alternative medicine: Focusing on research into traditional Tibetan medicine in China. BioSci Trends 2016;10(3):163-70.
[Crossref] [Google Scholar] [PubMed]
- Sen T, Samanta SK. Medicinal plants, human health and biodiversity: A broad review. Adv Biochem Eng Biotechnol 2015;147:59-110.
[Crossref] [Google Scholar] [PubMed]
- Akhondzadeh S. The importance of clinical trials in drug development. Avicenna J Med Biotechnol 2016;8(4):151.
- Chin YW, Balunas MJ, Chai HB, Kinghorn AD. Drug discovery from natural sources. AAPS J 2006;8(2):E239-53.
- Sharma SB, Gupta R. Drug development from natural resource: A systematic approach. Mini Rev Med Chem 2015;15(1):52-7.
[Crossref] [Google Scholar] [PubMed]
- Lautie E, Russo O, Ducrot P, Boutin JA. Unraveling plant natural chemical diversity for drug discovery purposes. Front Pharmacol 2020;11:397.
- Allkin B. Useful plants-Medicines: At least 28,187 plant species are currently recorded as being of medicinal use. London (UK): Royal Botanic Gardens; 2017.
- Sofowora A, Ogunbodede E, Onayade A. The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med 2013;10(5):210-29.
[Crossref] [Google Scholar] [PubMed]
- Huda MK, Wilcock CC, Rahman MA. The ethnobotanical information on indigenous orchids of Bangladesh. Hamdard Med (Pakistan) 2006;49(3):138-43.
- Hossain MM. Traditional therapeutic uses of some orchids of Bangladesh. Med Aromat Plant Sci Biotechnol 2009;42(1):101-6.
- Trease G, Evans SM. Pharmacognosy. 16th ed. Bailer Tindal. London: Elsevier Publisher; 2009. p. 23-67.
- Rao BG, Rao YV, Rao TM. Hepatoprotective and antioxidant capacity of Melochia corchorifolia extracts. Asian Pac J Trop Med 2013;6(7):537-43.
[Crossref] [Google Scholar] [PubMed]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 1965;16(3):144-58.
- Shamsa F, Monsef H, Ghamooshi R, Verdian-rizi M. Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci 2008;32:17-20.
- Bhatti MZ, Ali A, Ahmad A, Saeed A, Malik SA. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res Notes 2015;8(1):1-8.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 1996;239(1):70-6.
[Crossref] [Google Scholar] [PubMed]
- McCord JM, Fridovich I. Superoxide dismutase: An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244(22):6049-55.
[Crossref] [Google Scholar] [PubMed]
- Kunchandy E, Rao MN. Oxygen radical scavenging activity of curcumin. Int J Pharm 1990;58(3):237-40.
- Braca A, Fico G, Morelli I, De Simone F, Tomè F, De Tommasi N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J Ethnopharmacol 2003;86(1):63-7.
[Crossref] [Google Scholar] [PubMed]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989;10(6):1003-8.
[Crossref] [Google Scholar] [PubMed]
- Onoja SO, Omeh YN, Ezeja MI, Chukwu MN. Evaluation of the in vitro and in vivo antioxidant potentials of Aframomum melegueta methanolic seed extract. J Trop Med 2014;2014:159343.
[Crossref] [Google Scholar] [PubMed]
- Malomo SO, Ore A, Yakubu MT. In vitro and in vivo antioxidant activities of the aqueous extract of Celosia argentea leaves. Indian J Pharmacol 2011;43(3):278-85.
[Crossref] [Google Scholar] [PubMed]
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid Peroxidation. Methods Enzymol. 1990;186:421-31.
- Sun YI, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988;34(3):497-500.
- Atawodi SE. Evaluation of the hypoglycemic, hypolipidemic and antioxidant effects of methanolic extract of" ata-ofa" polyherbal tea (APolyherbal) in alloxan-induced diabetic rats. Drug Invention Today 2011;3(11):270-6.
- Shukla V, Tiwari RK, Agarwal DP. Hepatoprotective role of Picroliv isolated from Picrohiza kurroa on alcohol induced liver necrosis. Adv Pharmacol Toxicol 2001;2:9-16.
- Rasheed N, Ahmad A, Al-Sheeha M, Alghasham A, Palit G. Neuroprotective and anti-stress effect of A68930 in acute and chronic unpredictable stress model in rats. Neurosci Lett 2011;504(2):151-5.
[Crossref] [Google Scholar] [PubMed]
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 1995;41(12):1819-28.
[Crossref] [Google Scholar] [PubMed]
- Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front Pharmacol 2018;9:1162.
- Hahm JR, Jo MH, Ullah R, Kim MW, Kim MO. Metabolic stress alters antioxidant systems, suppresses the adiponectin receptor 1 and induces Alzheimer’s like pathology in mice brain. Cells 2020;9(1):249.
[Crossref] [Google Scholar] [PubMed]
- Incani A, Marras L, Serreli G, Ingianni A, Pompei R, Deiana M, et al. Human Herpesvirus 8 infection may contribute to oxidative stress in diabetes type 2 patients. BMC Res Notes 2020;13(1):1-6.
- Feriyani F, Maulanza H, Lubis RR, Balqis U, Darmawi D. Effects of binahong (Anredera cordifolia (Tenore) Steenis) extracts on the levels of malondialdehyde (MDA) in cataract goat lenses. Scientific World J 2021;2021:6617292.
[Crossref] [Google Scholar] [PubMed]
- Suh JI. Drug-induced liver injury. Yeungnam Univ J Med 2020;37(1):2-12.
[Crossref] [Google Scholar] [PubMed]
- Hirschfield GM, Thain C, Walmsley M, Brownlee A, Jones DE. Liver disease in the UK. Lancet 2015;385:503.
- Eashwar VA, Umadevi R, Gopalakrishnan S. Alcohol consumption in India–An epidemiological review. J Family Med Primary Care 2020;9(1):49-55.
- Sharma P, Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: Spectrum and diagnosis. Transl Gastroenterol Hepatol 2020;5:19.
[Crossref] [Google Scholar] [PubMed]
- Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterol 2020;158(7):1851-64.
[Crossref] [Google Scholar] [PubMed]
- Saha P, Talukdar AD, Nath R, Sarker SD, Nahar L, Sahu J, et al. Role of natural phenolics in hepatoprotection: A mechanistic review and analysis of regulatory network of associated genes. Front Pharmacol 2019;10:509.
[Crossref] [Google Scholar] [PubMed]
- Addolorato G, Abenavoli L, Dallio M, Federico A, Germani G, Gitto S, et al. Alcohol associated liver disease 2020: A clinical practice guideline by the Italian Association for the study of the liver (AISF). Dig Liver Dis 2020;52(4):374-91.
[Crossref] [Google Scholar] [PubMed]
- Jan NU, Ahmad B, Ali S, Adhikari A, Ali A, Jahan A, et al. Steroidal alkaloids as an emerging therapeutic alternative for investigation of their immunosuppressive and hepatoprotective potential. Front Pharmacol 2017;8:114.
[Crossref] [Google Scholar] [PubMed]
- Osna NA, Donohue Jr TM, Kharbanda KK. Alcoholic liver disease: Pathogenesis and current management. Alcohol Res 2017;38(2):147-161.
[Google Scholar] [PubMed]
- Stickel F, Hellerbrand C. Herbs to treat liver diseases: More than placebo? Clin Liver Dis 2015;6(6):136-8.
- Zelber-Sagi S, Ivancovsky-Wajcman D, Fliss-Isakov N, Hahn M, Webb M, Shibolet O, et al. Serum malondialdehyde is associated with non-alcoholic fatty liver and related liver damage differentially in men and women. Antioxidants 2020;9(7):578.
[Crossref] [Google Scholar] [PubMed]

 Ascorbic acid;
Ascorbic acid;  Ethyl acetate extract;
Ethyl acetate extract;  Hydro-Alcholic extract
Hydro-Alcholic extract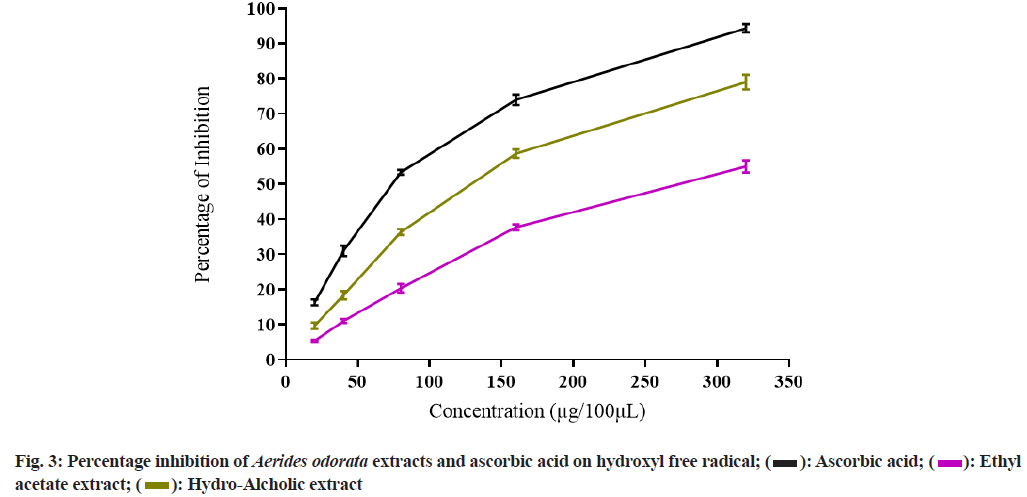
 Ethyl acetate extract;
Ethyl acetate extract;  Hydro-Alcholic extract
Hydro-Alcholic extract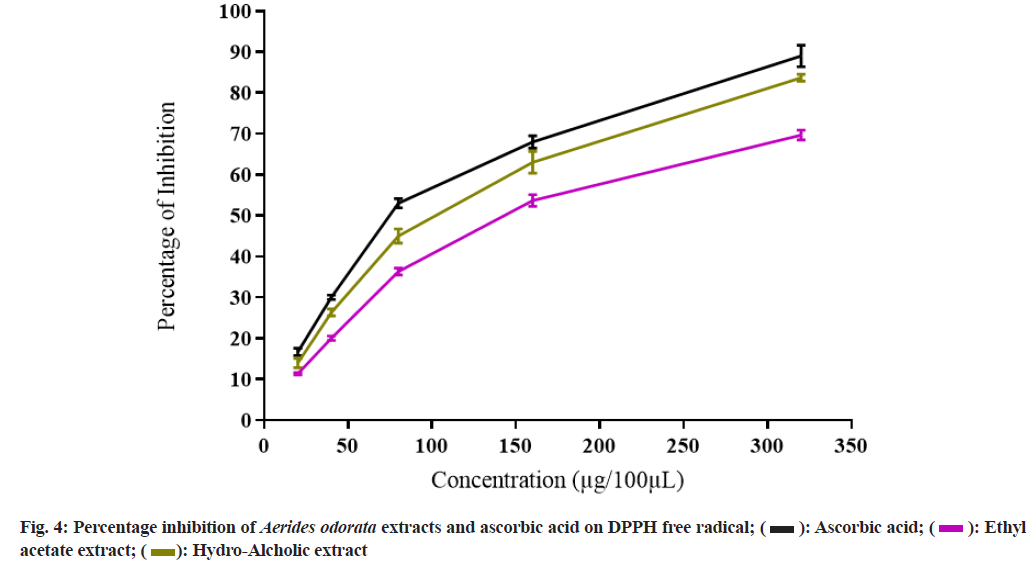
 Ethyl acetate extract;
Ethyl acetate extract;  Hydro-Alcholic extract
Hydro-Alcholic extract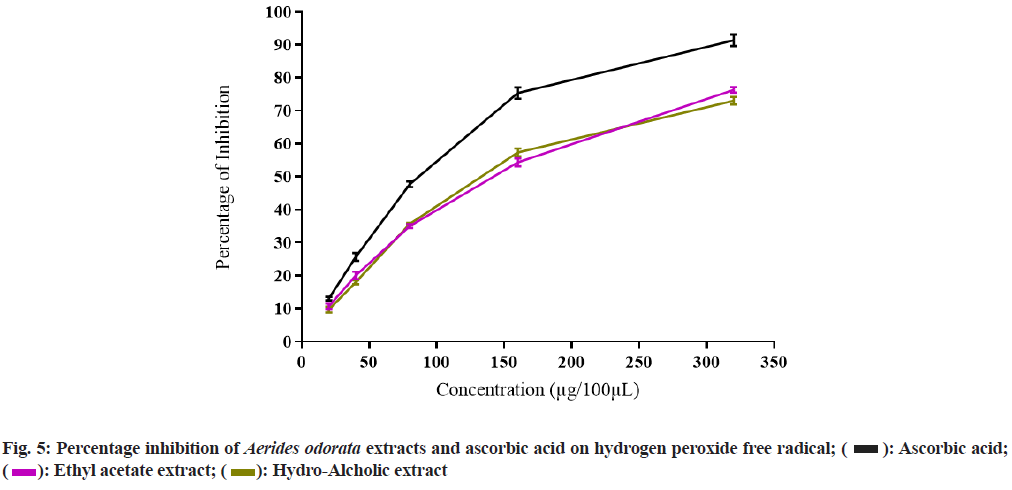
 Ascorbic acid;
Ascorbic acid;  Hydro-Alcholic extract
Hydro-Alcholic extract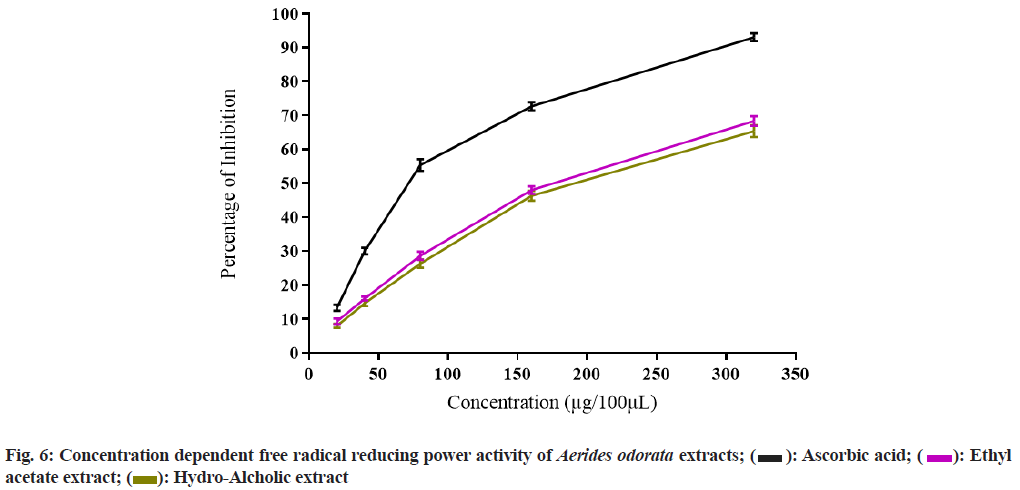
 Ethyl acetate extract;
Ethyl acetate extract;  Hydro-Alcholic extract
Hydro-Alcholic extract