- *Corresponding Author:
- J. Kim
College of Agriculture & Life Sciences, SARI, Jeju National University, Jeju 63243, Republic of Korea
E-mail: aha2011@jejunu.ac.kr
| Date of Submission | 08 March 2017 |
| Date of Revision | 14 August 2017 |
| Date of Acceptance | 29 March 2018 |
| Indian J Pharm Sci 2018;80(3):489-495 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Although crowberry is a relatively under-utilized wild berry, it holds considerable potential for application in the food and cosmetic industries due to its high phenolic content. In this study, antioxidant, antimicrobial, and antidiabetic activities of crowberry were investigated to shed light on its potential health benefits. Antioxidant activity was evaluated using the 1,1-dephenyl-2-picryl-hydrazyl free radical scavenging, reducing power, and total antioxidant capacity. The crude 70 % ethanol extract, ethyl acetate and butanol fraction of the fruit showed strong antioxidant activity. In addition, the ethyl acetate fraction showed antimicrobial activity against Gram-negative bacteria and significant inhibition of α-glucosidase and α-amylase activities. These findings suggested that the ethyl acetate fraction of the crude 70 % ethanol extract could serve as a useful source of dietary supplements. Furthermore, the analysis of correlation between the phytochemical content and biological activity of crowberry suggested that phenolic compounds could be the major contributors to the antioxidant and antidiabetic activities of the fruit extract. Taken together, these results suggested that crowberry fruits could serve as potential antioxidant and antidiabetic supplements.
Keywords
Antimicrobial activity, antioxidant activity, α-amylase, α-glucosidase, phenolic compounds
Wild and cultivated berry fruits are among the most economically important fresh and processed fruits and are known to be rich source of bioactive compounds, including phenolic acids, tannins, and flavonoids [1]. These compounds offer several health benefits, such as antioxidant and antimicrobial activities, and are useful in medical practice as antiinflammatory, choleretic, and antitumoral agents, among other applications [1-2]. The World Health Organization emphasizes that the antioxidant activity of phenolic compounds, especially those from small colourful fruits, play an important role in preventing many health problems, such as cardiovascular diseases, diabetes, cancer, and obesity [1,3]. Therefore, the level and composition of phenolic compounds in berry fruits are closely correlated with their pharmaceutical properties. Wild berries have been suggested to exhibit a higher level of phenolic content and antioxidant capacity than do domesticated and genetically derived crops [4,5], indicating that the phenolic content in berries is affected by environmental factors, such as the geographic region, storage conditions, ripeness, and climate [6-9]. Wild berries lend themselves to many new applications as functional foods for consumers and as beneficial materials for the food and medical industry [4,8,10].
Crowberry (Empetrum nigrum L.), an evergreen dwarf shrub, is a relatively unused wild berry that widely occurs in the northern hemisphere, Islands of the Northwest Territories, and northern Europe [9,11]. In Korea, one species of crowberry (E. nigrum var. japonicum) grows at altitudes above 4000 ft. on Mt. Halla on Jeju Island [12]. In East Asia, crowberry has long been used in traditional herbal medicine to control inflammatory diseases, such as cystitis, urethritis, and nephritis [12]. In addition, crowberry fruits contain more than 15 different anthocyanins, together with proanthocyanidins and flavonoids, which are antioxidants with health-giving properties [11,13]. These properties indicate the considerable potential of crowberry not only as a functional food, but also as a resource for functional applications in the cosmetic and pharmaceutical industries [11].
Motivated by the aforementioned benefits, this study was attempted to highlight the potential of crowberry as a medicinal plant. The antioxidant, antimicrobial, and antidiabetic activities of 70 % ethanol extract and its fractions of crowberries were evaluated along with determination of the phenolic and flavonoid contents of the fruit. This study is an attempt to generate further interest in the use of crowberry as a natural antioxidant, antimicrobial, and antidiabetic agent in the food, cosmetic, and pharmaceutical industries.
Materials and Methods
Plant materials and extraction
Fresh crowberry fruits were collected from Mt. Halla (in Jeju) at an altitude of about 4000 feet in August 2012. The voucher specimen (JEJUA-6) was deposited in Majors in Plant Resource and Environment at Jeju National University. The crowberry fruits were air dried at room temperature and the dried material was ground into a fine powder. The dried material was soaked with 70 % ethyl alcohol for 24 h and sonicated at 55° in an ultrasonic bath (Power Sonic 520, Hwashin Co., Korea). Subsequently, the extract was filtered and evaporated under reduced pressure and lyophilized to produce dried powder extract. The crude 70 % ethyl alcohol extract was suspended in water and sequentially partitioned with hexane, ethyl acetate, and n-butanol. The remaining aqueous extract was used as the aqueous fraction. Each fraction was evaporated using a vacuum evaporator and 1000 μg each of the 70 % ethyl alcohol extract and its fractions were redissolved in 1 ml 70 % ethyl alcohol or hexane (for hexane fraction) for further analysis.
Determination of total phenolic and flavonoid contents
Total phenolic content (TPC) was determined using the Folin-Ciocalteu assay as described by Hyun et al. [14]. Each tested sample (100 μl) and 2 N Folin-Ciocalteu reagent (50 μl) was mixed thoroughly. After 5 min, 0.3 ml of 20 % Na2CO3 was added to the mixture and incubated for 15 min. The resultant blue colour was read at an absorbance of 725 nm (UV-1800, Shimadzu, Tokyo, Japan). The TPC in the dried samples was calculated using a gallic acid standard calibration curve and expressed as milligram gallic acid equivalent (GAE) per gram of extract.
To analyze the total flavonoid content (TFC) of each sample, 0.2 ml of 70 % ethyl alcohol extract and its fractions were mixed with 0.1 ml of 10 % aluminium nitrate (w/v), 0.1 ml of 1 M potassium acetate, and 4.6 ml of 80 % ethanol. The reaction mixtures were kept at room temperature for 40 min, and then absorbance was measured against a blank at 415 nm. Quercetin was used for the standard calibration curve, and the TFC was expressed as milligrams of quercetin equivalent (QE) per gram of samples.
Analysis of free radical scavenging activity
The free radical scavenging activity of each sample was evaluated with the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay [14] based on the measurement of the reducing ability of antioxidants toward the DPPH radical. Different concentrations of 70 % ethyl alcohol extract and its fractions were added to 4 ml of the methanol DPPH solution (0.15 M DPPH). The mixture was incubated for 30 min at room temperature in the dark, after which absorbance was measured at 517 nm against a blank. The ability of the samples to scavenge the DPPH radical (three replicates per treatment) was calculated using the following Eqn., percent inhibition = [(absorbance value of control–absorbance value of sample)/absorbance value of control)]×100. The IC50 (50 % reduction of DPPH radicals) was calculated from a graph of radical scavenging activity versus extract concentration.
Determination of reducing power
The reducing power of 70 % EtOH extract and its fractions was estimated following the method of Hyun et al. [14]. Different concentrations of each sample (100, 200, and 300 μg/ml), sodium phosphate buffer (0.5 ml, 0.2 M, pH 6.6), and potassium ferricyanide (0.5 ml, 10 mg/ml) were mixed and then kept at 50° for 20 min. After the addition of 2.5 ml of 10 % trichloroacetic acid, the mixture was centrifuged at 6500 rpm for 10 min. The upper layer (0.5 ml) was mixed with distilled water (0.5 ml) and ferric chloride (0.1 ml, 0.1 %), and then absorbance was measured at 700 nm using a UV/Vis spectrophotometer. The increased absorbance of the reaction mixture suggests high reducing power.
Analysis of total antioxidant capacity
The samples were assayed by the ammonium molybdate reduction method to determine total antioxidant capacity [15]. Different concentrations of each sample (100, 200, and 300 μg/ml) were mixed with 3 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The reaction mixture was incubated in a water bath at 95° for 90 min. After the mixture was cooled to room temperature, absorbance was measured at 695 nm. Ascorbic acid and butylated hydroxytoluene (BHT) were used for comparison.
Determination of antimicrobial activity
The test strains used for the analysis of antimicrobial activity were five Gram-positive bacteria, Bacillus atrophaeus (KACC 14742), Kocuria rhizophila (KACC 14744), Micrococcus luteus (KACC 14819), Staphylococcus epidermidis (KACC 14822), and Bacillus subtilis subsp. Spizizenii (KACC 14741) and four Gram-negative bacteria, Klebsiella pneumonia (KACC 14816), Enterobacter cloacae (KACC11958), Salmonella enterica subsp. enterica (KACC 10769), and Pseudomonas aeruginosa (KACC 10186). All the strains were obtained from the Korean Agricultural Culture Collection (KACC) in South Korea. Antimicrobial activity was analysed by critical dilution as previously described [16]. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of 70 % ethyl alcohol extract and its fractions that inhibited the visible growth of a microorganism after overnight incubation. Experiments were carried out in triplicate for each sample with each microorganism.
α-Glucosidase and α-amylase inhibition assay
The inhibition of α-glucosidase activity was performed according to a previously described method [16]. Various concentrations of each sample (50 μl), 50 μl of α-glucosidase (0.5 U/ml), and 50 μl of 0.2 M potassium phosphate buffer (pH 6.8) were mixed and incubated at 37° for 15 min. A total of 3 mM of p-nitrophenyl glucopyranoside was added to the mixture and incubated at 37° for 10 min. The reaction was terminated by the addition of 2.0 ml Na2CO3 solution. The absorbance was measured at 405 nm using a UV/Vis spectrophotometer. The half maximal inhibitory concentration (IC50) value was calculated by linear regression analysis of activity under the assay conditions. All the assays were carried out in triplicate.
For the α-amylase assay, a starch hydrolysis assay was performed with the agar plates prepared amended 1 % starch with 1.5 % agar as described by Kim et al. [17]. Mixtures (10 μl porcine pancreatic α-amylase with or without samples) were applied to filter paper disks (sterile Whatman No.1, 8 mm) placed on agar plates. After incubation at 37° for 72 h, α-amylase activity was analysed by starch staining with iodine solution (5 mM I2 in 3 % KI). Inhibitory activity was determined on the basis of the diameter of the hydrolysed areas around the wells.
Statistical analysis
All experiments were conducted in three independent replicates, and data were expressed in terms of mean and standard deviation. Statistical analyses were performed by ANOVA. Duncan’s test was used to determine the significance of differences between the groups. The correlation analysis between phenolics, flavonoids and biological activities were determined by Pearson’s correlation analysis using SPSS version 18 software, as described by Sarepoua et al. [18].
Results and Discussion
Polyphenolic compounds are widely distributed in the plant kingdom and are commonly found in raw materials and plant-derived food products. One of the best properties of polyphenolic compounds is their antioxidant capacity, which is attributed to their ability to donate hydrogen and form stable radical intermediates [19,20]. Such compounds have therefore been suggested as active compounds that can protect the human body from oxidative stress-related diseases, such as inflammation, cardiovascular diseases, cancers, atherosclerosis, coronary heart disease, and aging-related disorders [21,22]. TPC and TFC in the crude 70 % ethyl alcohol extract of the crowberry fruits were 697±8 mg GAE/g extract and 20.3±0.9 mg QE/g extract, respectively (Table 1). In addition, the highest level of TPC was analysed from the n-butanol fraction, which contained 1110±57 mg GAE/g extract, and then from the crude 70 % ethyl alcohol extract, ethyl acetate fraction (579±22 mg GAE/g extract), aqueous fraction (308±6 mg GAE/g extract), and hexane fraction (70±2 mg GAE/g extract). The ethyl acetate fraction contained the highest flavonoid content (76.9±5.3 mg QE/g extract), whereas the aqueous fraction contained no detectable amounts of flavonoids (Table 1). This result suggested that solvent polarity is important for the extraction of phenolic and flavonoid compounds from crowberry fruits.
| Extract and fractions | Total phenol (mg GAE/g)a |
Total flavonoid (mg QE/g)b | IC50 (μg/ml)c |
|---|---|---|---|
| 70 % EtOH extract | 697±8b | 20.3±0.9b | 12.81±0.76c |
| Hexane fraction | 70±2e | 13.6±1.3c | >50f |
| EtOAc fraction | 579±22c | 76.9±5.3a | 6.46±0.12a |
| BuOH fraction | 1110±57a | 18.3±0.0b | 8.82±0.30b |
| Aqueous fraction | 308±6d | - | 21.43±0.56d |
| Butylated hydroxy toluene | 43.74±1.78e |
aTotal phenolic content was analysed as the gallic acid equivalent (GAE) mg/g of the extract; values are the average of triplicates. bTotal flavonoid content was analysed as the quercetin equivalent (QE) mg/g of the extract; values are the average of triplicates. cIC50: Amount required for a 50 % reduction of DPPH free radicals after 30 min
Table 1: Total phenolic content and total flavonoid content in crowberry 70 % ethanol crude extract and its fractions
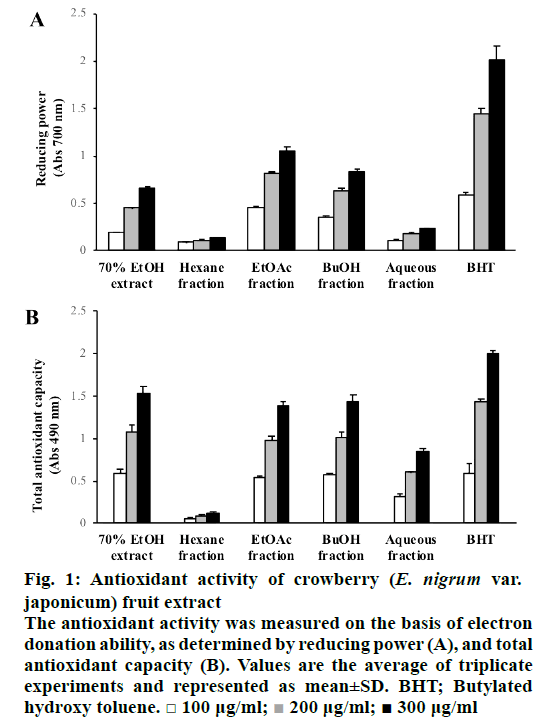
The antioxidant activity of the crowberry fruits were examined by assays on DPPH free radical scavenging, reducing power, and total antioxidant capacity. The DPPH assay (Table 1) indicated that the highest scavenging effect was found in the ethyl acetate fraction (IC50 6.46±0.12 μg/ml), followed by the n-butanol fraction (IC50 8.82±0.30), crude 70 % ethyl alcohol extract (IC50 12.81±0.76), aqueous fraction (IC50 21.43±0.56), and hexane fraction. In addition, the crude 70 % ethyl alcohol extract and its fractions exhibited a dose-dependent increase in antioxidant activity (Figure 1). The hexane fraction revealed the lowest antioxidant activity, and the ethyl acetate fraction exhibited a relatively higher reducing power and total antioxidant capacity than did the other samples (Figure 1A and B). Notably, the ethyl acetate and n-butanol fractions and the crude 70 % EtOH extract exhibited better scavenging ability than did BHT (IC50 = 43.74±1.78; Table 1), but exhibited lower reducing power and total antioxidant capacity than did BHT (Figure 1). This result suggested that the antioxidant activity of crowberry fruit extract is due to its proton-donating abilities. In addition, the fraction for which a non-polar solvent was used exhibited weak antioxidant activity, indicating that solvents with different polarities significantly affect antioxidant activity [23]. The ethyl acetate extract of crowberry showed protective effects against ultraviolet Binduced cell death and γ-ray-induced apoptosis via the inhibition of oxidative stress [18,24]. These findings suggested that crowberry fruits could be very useful in the development of antioxidant supplements for the prevention of oxidative stress-related diseases.
Figure 1: Antioxidant activity of crowberry (E. nigrum var.
japonicum) fruit extract
The antioxidant activity was measured on the basis of electron
donation ability, as determined by reducing power (A), and total
antioxidant capacity (B). Values are the average of triplicate
experiments and represented as mean±SD. BHT; Butylated
hydroxy toluene.  100 μg/ml;
100 μg/ml;  200 μg/ml;
200 μg/ml;  300 μg/ml
300 μg/ml
The antimicrobial activity of the crude 70 % ethyl alcohol extract and its fractions from the crowberry fruits were evaluated in vitro against five Grampositive bacteria and four Gram-negative bacteria. The results are expressed as MIC values (Table 2). Among the fractions, the ethyl acetate fraction exhibited good antimicrobial activity against B. atrophaeus (MIC 125 μg/ml), K. rhizophila (MIC 125 μg/ml), M. luteus (MIC 63 μg/ml), and S. epidermidis (MIC 125 μg/ml). The crude 70 % ethyl alcohol extract showed significant antimicrobial activity against B. subtilis (MIC 250 μg/ml) compared with its fractions, whereas the hexane and aqueous fractions exerted no effect on all the tested bacteria (Table 2). In addition, the extract of crowberry fruit showed greater activity against Gram-positive bacteria than Gram-negative bacteria (Table 2). This activity may be attributed to the structural differences in the bacterial cell walls of Grampositive and Gram-negative bacteria. Gram-negative bacteria possess a cell wall located between the inner and outer membranes of a cell, whereas Gram-positive bacteria have a cell wall that constitutes the outer layer of the cell [25,26]. Therefore, Gram-negative bacteria are resistant to the extract of crowberry fruit.
| Minimum inhibitory concentration (MIC) (μg/ml)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Extract and fractions | B.a.b | K.r.b | M.l.b | St.e.b | B.s.b | K.p.b | E.c.b | S.e.b | P.a.b |
| 70% EtOH extract | 500 | 250 | 250 | 500 | 250 | >1000 | 1000 | 1000 | 1000 |
| Hexane fraction | >1000 | 1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| EtOAc fraction | 125 | 125 | 63 | 125 | 1000 | 1000 | 1000 | 250 | 500 |
| BuoH fraction | 500 | 250 | 500 | 500 | 500 | 1000 | 1000 | 1000 | 1000 |
| Aqueous fraction | >1000 | >1000 | 500 | 1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Tetracycline | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
aMIC values against bacteria and yeast were determined by the serial two-fold dilution method. bB.a.: Bacillus atrophaeus KACC 14742, K.r.: Kocuria rhizophila KACC 14744, M.l.: Micrococcus luteus KACC 14819, St.e.: Staphylococcus epidermidis KACC 14822, B.s.: Bacillus subtilis subsp. Spizizenii KACC 14741, K.p. Klebsiella pneumonia KACC 14816, E.c.: Enterobacter cloacae KACC11958, S.e.: Salmonella enterica subsp. enterica KACC 10769, P.a.: Pseudomonas aeruginosa KACC 10186
Table 2: Antimicrobial activities of crowberry fruit extract and its fractions.
Epidemiological studies have suggested that edible berries are beneficial in reducing the risk of type-2 diabetes [27,28]. To identify the potential of crowberry fruit as an antidiabetic supplement, crude 70 % ethyl alcohol extract and its fractions were tested for α-glucosidase and α-amylase inhibitory activities. As shown in Table 3, the ethyl acetate fraction exhibited a higher inhibitory effect (IC50 0.5±0 μg/ml) than did the other fractions, and the hexane fraction showed the lowest inhibitory effect (IC50>100 μg/ml). Additionally, starch degradation by pancreatic α-amylase was inhibited by 200 μg of the ethyl acetate and n-butanol fractions (Table 3), indicating that the significant inhibition of α-glucosidase and α-amylase activities by the ethyl acetate fraction (versus the other fractions) is due to the presence of polyphenolic compounds, which are known to be soluble in polar solvents [29].
| Extract and fractions | α-Glucosidase activity | α-Amylase activity (mm) | |
|---|---|---|---|
| IC50 (μg/ml)a | 100 μg/disc | 200 μg/disc | |
| 70% EtOH extract | 3.3±0.1b | 14b | 9c |
| Hexane fraction | >100d | 10a | 10c |
| EtOAc fraction | 0.5±0a | 9a | 0a |
| BuOH fraction | 3.2±0.2b | 15b | 5b |
| Aqueous fraction | 3.7±0c | 15b | 12d |
aIC50, 50 % inhibition of α-glucosidase activity under assay conditions
Table 3: Inhibitory effect of crowberry (E. nigrum var. japonicum) fruit extract on α-glucosidase and α-amylase activities
Plant-derived phenolic and flavonoid compounds have drawn considerable attention because of their medicinal properties [30-32]. A widely accepted finding, therefore, is that the medicinal properties of plant extracts are related to TPC and TFC levels [14,33]. To analyse the correlation between the phytochemical content and biological activity of crowberry fruit, the Pearson correlation coefficient of TPC and TFC was employed. Table 4 showed that phenolic content exhibited good correlation with free radical scavenging activity, reducing power, total antioxidant capacity, and antidiabetic activity (inverse correlation with α-glucosidase inhibitory activity). Conversely, the correlation of TFC with total antioxidant capacity and antidiabetic activity was nonsignificant. These findings indicate that phenolic and flavonoid compounds are major contributors to the antioxidant activity of crowberry fruit extract and support the importance of phenolic compounds in the antidiabetic properties of plant extracts [34].
| Correlation R2 | ||
|---|---|---|
| Phenolic | Flavonoids | |
| Free radical scavenging activity | 0.889*** | 0.740*** |
| Reducing power | 0.868*** | 0.741*** |
| Total antioxidant capacity | 0.657 | 0.367 |
| Antidiabetic activity | –0.639 | –0.286 |
***Significance at p<0.001. The crowberry 70 % ethanol crude extract and its fractions were used in the correlation
Table 4: Correlations between the biological activity and total phenolic and flavonoid contents of crowberry fruit
In this study, the antioxidant, antimicrobial, and antidiabetic activities of crude 70 % ethyl alcohol extract and its fractions were analysed to understand the biological activity of crowberry. The overall findings suggested that the ethyl acetate and n-butanol fractions were useful sources of natural antioxidant, antimicrobial, and antidiabetic agents. The analysis of correlation between the phytochemical content and biological activity of crowberry fruit showed that the presence of notable biological activities of the ethyl acetate and n-butanol fractions and the crude 70 % ethyl alcohol extract could be due to the presence of phenolic compounds. This finding indicated that phenolic compounds appear to be the major contributors to the biological activity of crowberry fruit extract. Further investigations are needed to systematically analyse the pharmaceutical value of crowberry fruits by functional assays.
Financial support and sponsorship
This research was supported by the 2017 scientific promotion program funded by Jeju National University.
Conflict of interest
The authors declare that this paper content has no conflict of interests.
References
- Paredes-López O, Cervantes-Ceja ML, Vigna-Pérez M, Hernández-Pérez T. Berries: improving human health and healthy aging, and promoting quality life-a review. Plant Foods Hum Nutr 2010;65:299-308.
- Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics-a Finnish perspective. Mol Nutr Food Res 2007;51:684-91.
- Stapleton AP, James EM, Goodwill GA, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology 2008;15:79-89.
- Burns Kraft TF, Dey M, Rogers RB, Ribnicky DM, Gipp DM, Cefalu WT, et al. Phytochemical composition and metabolic performance-enhancing activity of dietary berries traditionally used by Native North Americans. J Agric Food Chem 2008;56:654-60.
- Mikulic-Petkovsek M, Schmitzer V, Slatnar A, Stampar F, Veberic R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J Food Sci 2012;77:C1064-70.
- Häkkinena SH, Törrönen AR. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: influence of cultivar, cultivation site and technique. Food Res Int 2000;33:517-24.
- Hyun TK, Lee S, Rim Y, Kumar R, Lee SY, Lee CH, et al. De-novo RNA sequencing and metabolite profiling to identify genes involved in anthocyanin biosynthesis in Korean black raspberry (Rubus coreanus Miquel). PLoS One 2014;9:e88292.
- Kellogg J, Wang J, Flint C, Ribnicky D, Kuhn P, De Mejia EG, et al. Alaskan wild berry resources and human health under the cloud of climate change. J Agric Food Chem 2010;58:3884-900.
- Väisänen M, Martz F, Kaarlejärvi E, Julkunen-Tiitto R, Stark S. Phenolic responses of mountain crowberry (Empetrum nigrum ssp. hermaphroditum) to global climate change are compound specific and depend on grazing by reindeer (Rangifer tarandus). J Chem Ecol 2013;39:1390-99.
- Lila MA. The nature-versus-nurture debate on bioactive phytochemicals: The genome versus terroir. J Sci Food Agric 2006;86:2510-15
- Koskela AK, Anttonen MJ, Soininen TH, Saviranta NM, Auriola S, Julkunen-Tiitto R, et al. Variation in the anthocyanin concentration of wild populations of crowberries (Empetrum nigrum L. subsp. hermaphroditum). J Agric Food Chem 2010;58:12286-91.
- Park SY, Lee ES, Han SH, Lee HY, Lee S. Antioxidative effects of two native berry species, Empetrum nigrum var. japonicum K. Koch and Rubus buergeri Miq, from the Jeju Island of Korea. J Food Biochem 2012;36:675-82.
- Laaksonen O, Sandell M, Järvinen R, Kallio H. Orosensory contributing compounds in crowberry (Empetrum nigrum) press-byproducts. Food Chem 2011;124:1514-24.
- Hyun TK, Kim MO, Lee H, Kim Y, Kim E, Kim JS. Evaluation of anti-oxidant and anti-cancer properties of Dendropanax morbifera Léveille. Food Chem 2013;141:1947-55.
- Aliyu AB, Ibrahim MA, Ibrahim H, Musa AM, Lawal AY, Oshanimi JA, et al. Free radical scavenging and total antioxidant capacity of methanol extract of Ethulia conyzoides growing in Nigeria. Rom Biotechnol Lett 2012;17:7458-65.
- Hyun TK, Kim HC, Kim JS. Evaluation of the pharmaceutical properties of Thymus quinquecostatus Celak. Ind Crop Prod 2014;52:611-6.
- Kim KC, Lee IK, Kang KA, Kim BJ, Kim D, Moon JY, et al. Empetrum nigrum var. japonicum extract suppresses γ-ray radiation-induced cell damage via inhibition of oxidative stress. Am J Chin Med 2011;39:161-70.
- Sarepoua E, Tangwongchai R, Suriharn B, and Lertrat K. (2013) Relationships between phytochemicals and antioxidant activity in corn silk. Int Food Res J 20: 2073-79.
- Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 2005;45:287-306.
- Zhao Y, Du SK, Wang H, Cai M. In vitro antioxidant activity of extracts from common legumes. Food Chem 2014;152:462-66.
- Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol 2012;2012:936486.
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables- the millennium's health. Int J Food Sci Tech 2001;36:703-25.
- Xu BJ, Chang SK. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci 2007;72:S159-66.
- Kim KC, Kim D, Kim SC, Jung E, Park D, Hyun JW. Empetrum nigrum var. japonicum extract suppresses ultraviolet B-induced cell damage via absorption of radiation and inhibition of oxidative stress. Evid Based Complement Alternat Med 2013;2013:983609.
- Beveridge TJ. Structures of Gram-negative cell walls and their derived membrane vesicles. J Bacteriol 1999;181:4725-33.
- Silici S, Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol 2005;99:69-73.
- Knekt P, Kumpulainen J, Järvinen R, Rissanen H, Heliövaara M, Reunanen A, et al. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76:560-68.
- Fan J, Johnson MH, Lila MA, Yousef G, de Mejia EG. Berry and citrus phenolic compounds inhibit dipeptidyl peptidase IV: Implications in diabetes management. Evid Based Complement Alternat Med 2013;2013:479505.
- Akhter F, Hashim A, Khan MS, Ahmad S, Iqbal D, Srivastava AK, et al. Antioxidant, α-amylase inhibitory and oxidative DNA damage protective property of Boerhaavia diffusa (Linn.) root. S Afr J Bot 2013;88:265-72.
- Es-Safi NE, Ghidouche S, Ducrot PH. Flavonoids: hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 2007;12:2228-58.
- Wahle KW, Brown I, Rotondo D, Heys SD. Plant phenolics in the prevention and treatment of cancer. Adv Exp Med Biol 2010;698:36-51.
- Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol 2012;86:345-91.
- Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CY, et al. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int J Mol Sci 2012;13:16544-53.
- Elisashvili V. Submerged cultivation of medicinal mushrooms: bioprocesses and products (review). Int J Med Mushrooms 2012;14:211-39.