- *Corresponding Author:
- S. H. Wang
Department of Medical Oncology, The First Affiliated Hospital of The School of Medicine of Xian Jiaotong University, Xian, 710061, Shaanxi Province
E-mail: wsh2003@126.com
| Date of Submission | 26 February 2013 |
| Date of Revision | 13 July 2013 |
| Date of Acceptance | 17 July 2013 |
| Indian J Pharm Sci 2013;75(5):578-584 |
Abstract
Delisheng consists of radix ginseng, radix astragali, venenum bufonis and mylabris. It has been reported that delisheng inhibits the proliferation of adenocarcinoma cells and stimulates their apoptosis. Delisheng can also enhance the body's immunity and induce the redifferentiation of carcinoma cells. Delisheng inhibited the proliferation of HepG2 cells in MTT assay and promoted apoptosis more effectively in contrast to the active components of ginseng extract, Rg3 and gemcitabine. It is possible that Rg3 has an important role in delisheng because they all could regulate the cell cycle, apoptosis and expression of endostatin and VEGFR-2. Delisheng caused the cell cycle to arrest at the S phase, while gemcitabine blocked the cells at the G0/G1 phase in cell cycle analysis. Consequently, the apoptosis rate of the HepG2 cell line can be increased significantly by delisheng in combination with gemcitabine, compared with the single drug. The expression of the procaspase proteins, caspase protein, and dr5 detected by Western blot were increased while bcl-2 and survivin decreased in the delisheng group, compared with controls. The observations suggest that the delisheng induced apoptotic effect might be closely related to the mitochondrial apoptosis pathway, and the death receptor signaling pathway.
Keywords
Apoptosis, Dlisheng, HepG2 cell, proliferation, traditional Chinese medicine
Hepatocellular carcinoma (HCC) is a highly malignant tumour of the digestive system. At present, its incidence is increasing in the world, and it is a major health concern worldwide because of its high morbidity and mortality, and a poor prognosis [1]. Potential curative treatments for HCC include hepatic resection, local ablative therapies, systemic treatment and liver transplantation [2]. Surgery is the most effective treatment, but unfortunately it is feasible in only 10-20% of patients, because a majority of HCC patients present at advanced or unresectable stages of the disease. Even for those eligible for surgical resection, the postoperative recurrence rate can be as high as 50% in 2 years. Chemotherapy could be an option as an alternative to improve the prognosis of HCC. In general, however patients with HCC do not respond well to chemotherapy, and get no survival benefits [3-6]. In addition, for some intermediate and terminal patients, the tolerable doses of chemotherapy are quite low, because of impaired liver function and other complications. As a result, systemic chemotherapy for HCC has been quite ineffective. Moreover, there are very few therapeutic drugs against HCC. Therefore, finding a new method or drug which has better efficiency and lower toxicity will make a significant contribution to the treatment of HCC patients.
Fortunately, traditional Chinese medicines have been shown to have a marked therapeutic effect against many types of cancers such as esophageal cancer [7], lung cancer [8], hepatocellular cancer [9] and colonic carcinoma [10]. They have gained wide acceptance as a safe, palliative and effective treatment in China because of their unique advantages [11,12]. Delisheng (DSL), as a multicomponent antitumour drug, is the first traditional Chinese drug which gains the second kind of new drug certificate in China. DSL is composed of radix ginseng, radix astragali, venenum bufonis and mylabris. It has been reported that DSL has a strong inhibitory effect on the growth of some carcinoma cell lines [13,14]. Furthermore, it can also stimulate immunity, augment the effects of surgery, chemotherapy or radiotherapy, and improve the clinical symptoms and quality of life in patients with late-stage HCC [15,16]. As DSL is attractive as a natural product for medicinal use, increasing attention is being paid to its scientific evaluation and its possible molecular mechanisms.
In this study, we confirmed that DSL inhibits the proliferation of the HepG2 cell line. We then compared the role of DSL, and the active components of ginseng extract, Rg3 and gemcitabine (GEM), in cell cycle, apoptosis and expression of VEGFR-2 and endostatin (ES). We demonstrated that both Rg3 and DSL have effects on these processes but the latter is more powerful, and cannot be substituted by Rg3. DSL blocked the cells in the S phase, while GEM arrested the cells in the G0/G1 phase. Consequently, DSL combined with GEM could strengthen its capability against cancer. Moreover, molecular analysis of the apoptosis pathway revealed that the expression of dr5, pro-caspase and caspase proteins increased, while the level of bcl-2 and survivin decreased. These observations indicated that DSL induces apoptosis most likely via both mitochondrial and death receptor pathways.
Materials and Methods
The human hepatoma cell line HepG2 was cultured in RPMI 1640 with 10% fetal bovine serum and 2 mM L-glutamine (Gibco/BRL). The effects of DSL (Zhengbang Pharmaceutical Company, Beijing, P.R. China), Rg3 (Fusheng Nature Pharmaceutical Company, Dalian, P.R. China) and GEM (Haosen Pharmaceutical Company, Jiangsu, P.R. China) were evaluated according to previously described method [10]. The IC50 was calculated using the SPSS 13.0 software to standardise drug administration in the following experiments. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma, St Louise, MO, USA. Mouse monoclonal antibodies against human ES, VEGFR-2, caspase-3, caspase-8, caspase-9, dr5, bcl-2, and surviving were purchased from Santa Cruz Biotechnology Inc., Texas, USA.
MTT cell viability assay
Cells were seeded in 96 well plates at 1000 cells per well in 200 μl of medium the day before the experiment. DSL, Rg3 and GEM were added to the cells at the indicated concentrations (DSL: 3.125, 6.25, 12.5, 25, 50 and 100 μg/ml; Rg3: 5, 10, 20, 40, 80 and 160 μg/ml; GEM: 0.158, 0.316, 0.625, 1.25, 2.5 and 5 μg/ml). Cell growth was assayed at 24, 48 and 72 h after drug treatment, by aspiring half of the medium (100 μl) and adding an equal volume of fresh medium containing MTT (1 mg/ml). Cells were incubated further at 37°, 5% CO2 for 4 h, and 150 μl of dimethyl sulphoxide (DMSO, Sigma, USA) was added to each well, followed by mixing at room temperature for 10 min. The absorbance of the supernatant was measured at 570 nm using a Multifunction microplate reader (POLARstarOPTIMA, BMG, Germany). The inhibition of cell viability was calculated by the formula, %inhibition= [1-(A570 nm/A'570 nm)]×100, where A570 nm is from the treated cells and A'570 nm is from the untreated cells. Each experiment was repeated at least three times.
Cell cycle analysis
Cells were treated by drugs (DSL 25 μg/ml, Rg3 80 μg/ml and GEM 2.5 μg/ml) for 24 h. After 24 h intervention by drugs, treated and untreated cells (1×106) were collected and washed with PBS, then fixed in 75% alcohol for 30 min at room temperature. The cells were washed three times with cold PBS, and resuspended in 1 ml PBS containing 40 μg propidium iodide (PI, Sigma) and 100 μg RNase A (Sigma), and incubated at 37° for 30 min. Samples were analysed for DNA content using a FACScaliburTM instrument (BD Immunocytometry Systems, San Jose, CA). Each experiment was repeated at least three times.
Apoptosis
Following treatment by drugs (DSL 25 μg/ml, Rg3 80 μg/ml and GEM 2.5 μg/ml) for 24 h, apoptotic cells in the population were detected using the AnnexinV-FITC apoptosis Detection KIT I (Pharmingen, San Diego, CA), according to the manufacturer’s instructions. Briefly, cells were washed twice with cold cell staining buffer, and then cells were resuspended in Annexin V binding buffer at a concentration of 1×106 cells/ml. Annexin V-FITC (5 μl) and 7-AAD viability staining solution (5 μl) were added in 100 μl cell suspension in a 5 ml test tube. Cells were gently vortexed and incubated for 15 min at room temperature (25°) in the dark. 400 μl of Annexin V binding buffer was added to each tube, and cells were analysed by flow cytometry with proper machine settings.
Immunohistochemical staining
The immunohistochemical staining (IHC) was performed as per the reported procedure [17]. Briefly, a layer of appropriate size glass coverslips were placed in 24-well plates, and 1×104 cells were seeded in the wells. After treatment by drugs (DSL 25 μg/ml, Rg 3 80 μg/ml and GEM 2.5 μg/ml) for 24 h, the cells were fixed with 4% polyoxymethylene. Following quenching endogenous peroxidase with 3% H2O2 in methanol for 15 min, the cells were incubated for 30 min using block buffer (3% bovine serum albumin (BSA) and 0.1% Triton-100). The cells were then incubated overnight at 4° with mouse monoclonal antibodies against human ES, VEGFR-2, caspase-3, caspase-8, caspase-9, dr5, bcl-2 and survivin in 1:200 dilution. The next day, the cells were incubated with secondary antibody for 2 h at room temperature. The bound secondary antibody was visualised by the activity of the horseradish peroxidase conjugate using 3,3-diaminobenzidine tetrahydrochloride (Sigma, UK) as a substrate. The cells were counter-stained with hematoxylin. For semiquantitative evaluation of the IHC by Image-Pro Puls (IPP) 5.1 image analysis software, 20 random visual fields were examined using the LeicaQ550cw image analysis system (Germany).
Western blotting
After treatment by drugs (DSL 25 μg/ml, Rg3 80 μg/ ml, GEM 2.5 μg/ml) for 24 h, the cells were lysed in the RIPA buffer (50 mM Tris–Cl, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 0.5% NP-40, 1 mg/ml BSA, 0.1 mM PMSF). The untreated cells were used as controls. Protein concentrations in the cell extracts were determined using the BCA Protein Assay reagents (Pierce Biotechnology, Rockford, USA), according to the manufacturer’s instructions. Samples were separated by SDS-10% polyacrylmide gel electrophoresis, and were electroblotted onto polyvinylidene difluoride membranes. Membranes were probed with monoclonal mouse antibodies against human pro-caspase-3, pro-caspase-8, pro-caspase-9, bcl-2, survivin and β-actin protein at appropriate dilutions, followed by incubation with horseradish peroxidase-conjugated secondary antirabbit or antimouse IgG antibody (Sigma, USA). Blots were developed using an enhanced chemiluminescence system (Roche, Basel, Switzerland).
Statistical analysis
Data was reported as means±SD. The paired t-test was used for statistical analysis and P<0.05 was considered statistically significant.
Results
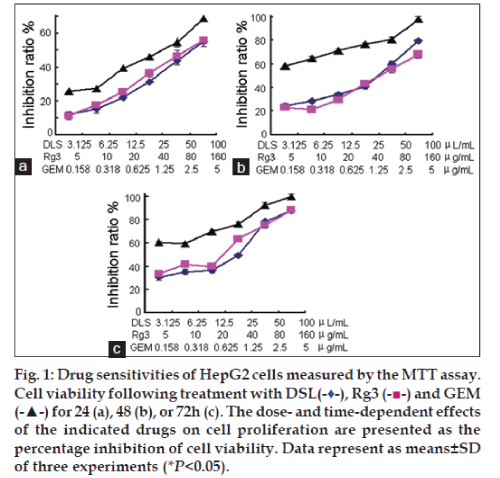
DSL blocked HepG2 cells in S phase
The sensitivity of HepG2 cell lines to DSL, Rg3 and GEM was investigated using the MTT assay (fig. 1) after treatment for 24 h (fig. 1a), 48 h (fig. 1b) and 72 h (fig. 1c). When drug concentrations and the time of incubation were increased, cell inhibition ratios in all groups increased. The IC50 values for DSL, Rg3 and GEM were approximately 25, 80 and 2.5 μg/ml, respectively. DSL and Rg3 both cause the cell cycle to arrest at the S phase. The data in Table 1 show that the percentage of cells arrested in the S phase is higher when they are treated with DSL, compared to that when treated with Rg3 (P<0.05). By contrast, there were more cells in the G0/G1 phase after GEM treatment. Consequently, GEM blocked HepG2 cell proliferation at the G0/G1 phase.
| Cell cycle | G0/G1 | S | G2/M |
|---|---|---|---|
| Control | 53.4±2.27 | 26.92±1.64 | 19.68±1.86 |
| DSL | 26.9±1.32 | 57.78±1.68* | 15.31±1.28 |
| Rg3 | 34.1±2.03 | 48.7±1.57* | 17.2±2.54 |
| GEM | 74.31±2.08* | 13.91±2.18 | 11.78±1.52 |
Percentage of cells in the different phases of the cell cycle. Each experiment was repeated three time (*P<0.05)
Table 1: The effects of drug on cell cycle progression
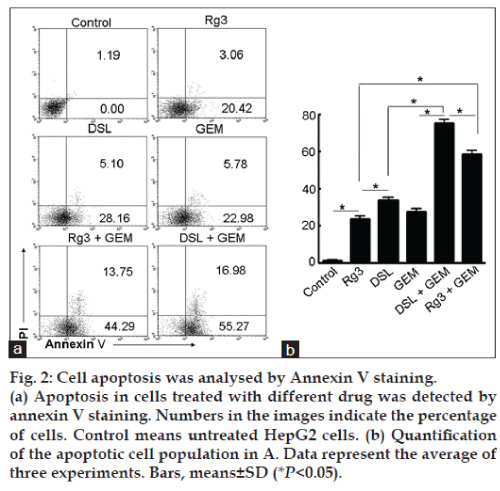
DSL induced the apoptosis of HepG2 cells
DSL, Rg3 and GEM induced cell apoptosis compared with controls (fig. 2). The average percent of apoptotic cells treated with DSL, GEM, and Rg3 were 33.26, 28.76, and 23.48%, respectively (P<0.05) (fig. 2a). Thus, the rate of apoptosis is higher in the DSL group than in the Rg3 and GEM groups. It is important to note that the percent apoptotic apoptotic cells increased to 58.04% in the presence of both Rg3 and GEM. The percentage of apoptotic cells increased to 72.25% when the cells were treated with a combination of DSL and GEM (fig. 2a). The analysis of the percentage of apoptotic cells induced by different condition (fig. 2b) confirmed that DSL combined with GEM enhanced the antitumour activity of DSL.
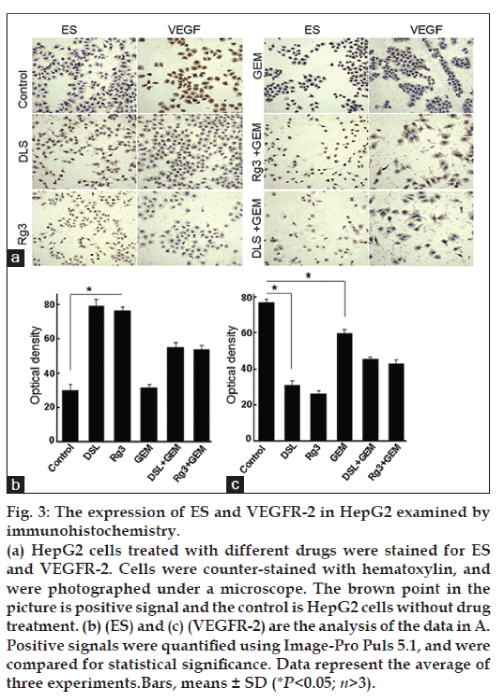
Expression of ES and VEGFR-2 in HepG2 by immunohistochemistry
IHC analysis demonstrated that vascular endothelial growth factor receptor (VEGFR-2) was down-regulated in DSL group and Rg3 group compared to control, while ES levels were higher in DSL- and Rg3-treated cells (fig. 3). The expression of VEGFR-2 decreased in the GEM group, although the expression of ES was not significantly different between the GEM group and control. Positive staining for VEGFR-2 was observed in both, cell membrane and cytoplasm. ES was detected predominantly in the cytoplasm (fig. 3a). Further analysis showed that the level of VEGFR-2 was lower (fig. 3c) while the level of ES was higher (fig. 3b) in the DSL group than that in the Rg3 and GEM groups. When the drugs were combined, however, the expression of VEGFR-2 and ES did not change significantly in comparison with monotherapy.
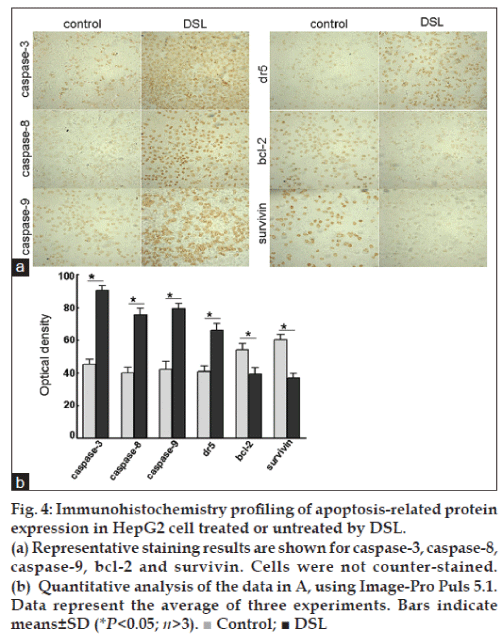
Immunohistochemistry profiling of apoptosis-related protein expression in HepG2 cell treated or untreated by DSL
Intense staining for caspase-3, caspase-8 and caspase-9 was observed in cells treated with DSL, compared to the untreated control cells. In parallel, the expression of bcl-2 was lower than in control cells. Importantly, dr5 was expressed at a high level in the DSL treated cells group, while survivin was down-regulated (fig. 4). Caspase-3, caspase-8, caspase-9, and bcl-2 proteins were expressed both in the cell membrane and in the cytoplasm, but the levels in the cytoplasm were higher. Survivin protein was detected in cytoplasm, while dr5 was located mainly in the cell membrane (fig. 4a).
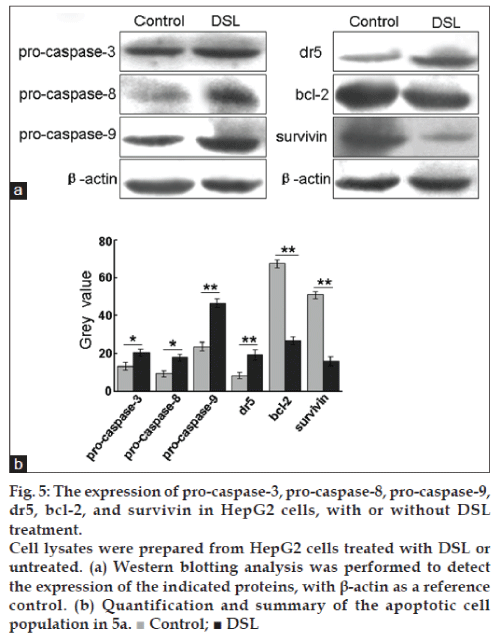
The expression of pro-caspase-3, pro-caspase-8, pro-caspase-9, dr5, bcl-2 and survivin in HepG2 cells, with or without DSL treatment
We also examined by western blot the zymogens of caspase, dr5, bcl-2 and survivin. DSL treatment induced the expression of procaspase-3, procaspase-8, procaspase-9 and dr5 and caused a decrease in the expression of survivin and bcl-2 (fig. 5).
Discussion
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world in terms of incidence, accounting for approximately 630 thousand new cases per year; in addition, HCC is the third most common cause of cancer death. The standard treatment in the early stages of the disease, such as surgical resection, local ablation and liver transplantation, are able to cure a proportion of patients, but most cases of HCC present in advanced stages, precluding the use of such treatments with curative intent. In these advanced stages, systemic treatments are commonly used. Unfortunately, chemotherapy with conventional cytotoxic agents, such as doxorubicin, cisplatin, and capecitabine, is ineffective and does not seem to modify the natural history of disease. Traditional Chinese medicines have been used clinically for more than 5000 years in China and Asia. There is abundant evidence that traditional Chinese medicine has a marked effect on the treatment of several diseases, including tumours [11,12]. DSL is a common Chinese medicinal compound, whose composition includes radix ginseng, radix astragali, venenum bufonis, and mylabris. Analysis of many clinical reports has shown that DSL can be used in patients who are unwilling to or cannot accept surgery, chemotherapy or radiotherapy [13,14]. Moreover, DSL can reinforce the immunity of patients, and augment the effects of surgery, chemotherapy or radiotherapy. The mechanisms of action of DSL, however, are still unclear.
We have found that DSL can inhibit cell proliferation in a dose- and time-dependent fashion. Although DSL has potential advantages, its irritation of veins has hampered its application in clinical practice. Panaxoside, is one of the active components of ginseng extract and has caused widespread concern in the last two decades. Recent studies have shown that ginsenosides (Rg3, RH2) are the main antitumour agents in ginseng extract [18-22]. Some studies have demonstrated that Rg3 could not only induce tumour cells differentiation, but also inhibit tumour growth and promote apoptosis of tumour cells [23,24]. Therefore, we estimated the role of DSL and Rg3 in inhibiting the growth of carcinoma cells in vitro. To our surprise, both Rg3 and DSL regulate the cell cycle and apoptosis. The percentage of cells treated by DSL or Rg3 in the S phase is significantly higher than that of control, which suggests that both DSL and Rg3 induce the HepG2 cells to start their cell cycle, but block them in the S phase. The data, however, showed that the rate of apoptosis in the DSL group is 33.26%, but that in the Rg3 group is 23.48%, demonstrating DSL is more powerful in inducing apoptosis. Cui et al. [14] reported that DSL could increase the expression of ES in HepG2. We also found that the expression of ES is upregulated and the expression of VEGFR-2 is downregulated in HepG2 cells treated with DSL or Rg3, compared to controls. These observations confirm that Rg3 has some antitumour effect. It is possible that Rg3 is one of major active constituents of DSL, but DSL is a more powerful activator because of synergism with other components in the complex mixture.
DSL can increase the body’s immunity, augment the effects of surgery, chemotherapy or radiotherapy, and improve the clinical symptoms and the quality of life of patients. Thus, DSL is usually combined with various chemotherapy drugs. GEM is a deoxycytidine analogue, with cycle-specific drug features. Experiment with pancreatic carcinoma and lung cancer confirmed that GEM depresses cell growth [25-27]. According to our study, GEM could inhibit the proliferation and induce the apoptosis of HepG2 cells. Interestingly, DSL causes cells to be arrested in the S phase, while cell populations of G0/ G1 phase prevail following treatment with GEM. The target of GEM is known to be the cell populations in the DNA synthesis stage [28]. Thus, we thought that if the DSL is combined with GEM, the resulting antiproliferative effect would be stronger; this was indeed observed in our experiments. The percentage of cell apoptosis is 33.26% when the cells are treated with DSL alone, while the rate increases to 72.25% when the cells are treated with DSL combined with GEM. This combination, however, did not affect the expression of VEGFR-2 and ES in HepG2 cells, most likely because GEM does not regulated the expression of VEGFR-2 and ES in these cells. These observations indicate that the combination of DSL and GEM may be of significant value. Nevertheless, the DSL/GEM combination may not be useful in suppressing angiogenesis.
Consistent with our study, a number of investigations have shown that DSL can induce apoptosis. The molecular mechanisms of apoptosis induced by DSL are not clear. Classic apoptosis pathways include the intrinsic mitochondrial pathway, and the extrinsic death receptor-mediated pathway. The extrinsic pathway is triggered by the activation of death receptors in response to an extracellular signal, which initiates the chain activation of the caspases [29]. Firstly, the dimerisation of caspases-8 and -10 were promoted by increasing the local concentration of procaspase monomers [30], followed by the cleavages of the zymogens of caspases-3, -6, or -7. The caspase cascade can also be activated by the so called mitochondria-dependent pathway [31]. In response to the apoptotic stimuli, the permeability of the mitochondrial membrane increases, and the mitochondria release a series of molecules, including cytochrome C. In the cytosol, the association of cytochrome C with the adaptor protein Apaf-1 and several procaspase-9 molecules gives rise to the formation of the apoptosome. Caspase-9 is thus able to recruit and activate caspase-3 [32], which is an effector of both pathways.
We analysed the critical molecules in the two apoptosis pathways, namely caspase-3, caspase-8, caspase-9, pro-caspase-3, pro-caspase-8, pro-caspase-9, and dr5. It is surprising that DSL not only regulates the expression of proteins involved in the extrinsic pathway (death receptor dr5 and caspase-8), but also a protein in the intrinsic pathway (caspase-9). Based on these observations, we propose that the apoptosis of HepG2 cells induced by DSL is regulated by both apoptosis pathways, but not by either of them alone. The expression of caspase-3 and zymogens of caspase, including pro-caspase-3, pro-caspase-8 and pro-caspase-9, were increased substantially over the control, confirming our presumption. Several studies have shown that the intrinsic and extrinsic pathways are not mutually exclusive [33]. It is reported the regulation of the mitochondrial apoptotic pathway is governed by the bcl-2 protein family [34,35]. Survivin inhibits apoptosis by blocking the activation of effector caspases in both the extrinsic and intrinsic pathways of apoptosis. The high expression of survivin in HCC is significantly higher than that in adjacent cirrhosis tissues and normal tissues [36]. DSL reduced the levels of bcl-2 and survivin in HepG2 cells. Thus, DSL might effect apoptosis by the regulating the expression of apoptosis proteins, including bcl-2 and survivin.
In summary, DSL inhibited the proliferation and promoted the apoptosis of HepG2 cells, partly through the action of Rg3. Our results indicate that a better therapeutic effect could be obtained by the combination of DLS and GEM. They also illuminate the mechanisms of apoptosis induced by DSL; this may help us in the development of new approaches to the therapy of HCC.
Acknowledgements
We thank Professor Hua Han for his guidance on the experimental design and performance of the project. This work was greatly supported by the National Natural Science Foundation (No. 81001090 and No. 81101471).
References
- Chan SL, Mok T, Ma BB. Management of hepatocellular carcinoma: Beyond sorafenib. CurrOncol Rep 2012;14:257-66.
- Papoulas M, Theocharis S. Primary liver tumors: Origin and target therapy. Expert OpinTher Targets 2009;13:957-65.
- Shire AM, Roberts LR. Prevention of hepatocellular carcinoma: Progress and challenges. Minerva GastroenterolDietol 2012;58:49-64.
- Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): An update. SeminOncol 2007;34 (Suppl 2):S12-20.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17.
- Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet 2009;373:614-6.
- Wei X, Chen Z, Yang X, Wu T. Chinese herbal medicines for esophageal cancer. Cochrane Database Syst Rev 2009;7:CD004520.
- McCulloch M, Broffman M, van der Laan M, Hubbard A, Kushi L, Kramer A, et al. Lung cancer survival with herbal medicine and vitamins in a whole-systems approach: Ten-year follow-up data analyzed with marginal structural models and propensity score methods. Integr Cancer Ther 2011;10:260-79.
- Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese Medicines in the treatment of hepatocellular cancers: A systematic review and meta-analysis. J ExpClin Cancer Res 2009;12:112.
- Hong KJ, Dunn DM, Shen CL, Pence BC. Effects of Ganodermalucidum on apoptotic and antiinflammatory function in HT-29 human colonic carcinoma cells.Phytother Res 2004;18:768-70.
- Cheng JT. Review: Drug therapy in Chinese traditional medicine. J ClinPharmacol 2000;40:445-50.
- Pandolfi M, Zilletti L. Herbal medicine, Chaplin, and “The Kid”. Eur J Intern Med 2012;23:330-2.
- Abdel-Hamid NM, Nazmy MH, Mahmoud AW, Fawzy MA, Youssof M. A survey on herbal management of hepatocellular carcinoma. World J Hepatol 2011;27:175-83.
- Cui J, Nan KJ, Tian T, Guo YH, Zhao N, Wang L. Chinese medicinal compound delisheng has satisfactory antitumor activity, and is associated with up-regulation of endostatin in human hepatocellular carcinoma cell line HepG2 in three-dimensional culture. World J Gastroenterol 2007;13:5432-9.
- Song X, Bao S, Wu L, Hu S. Ginseng stem-leaf saponins (GSLS) and mineral oil act synergistically to enhance the immune responses to vaccination against foot-and-mouth disease in mice. Vaccine 2009;27:51-5.
- See DM, Broumand N, Sahl L, Tilles JG. In vitro effects of echinacea and ginseng on natural killer and antibody-dependent cell cytotoxicity in healthy subjects and chronic fatigue syndrome or acquired immunodeficiency syndrome patients. Immunopharmacology 1997;35:229-35.
- Nayak MT, Singh A, Desai RS, Vanaki SS. Immunohistochemical analysis of vimentin in oral submucous fibrosis. J Cancer Epidemiol2013;2013:549041.
- Ochiai T, Ikoma H, Murayama Y, Shiozaki A, Komatsu S, Kuriu Y, et al. Factors resulting in 5-year disease-free survival after resection of hepatocellular carcinoma. Anticancer Res 2012;32:1417-22.
- Leung TW, Johnson PJ. Systemic therapy for hepatocellular carcinoma.SeminOncol 2001;28:514-20.
- Wang BS, Zhang LS, Song DM, Zhang JH, Liu YM. Effect of gensenoside Rg3 on apoptosis of Hep-2 and expression of HIF-1alha in human laryngeal cancer cell line under anoxic conditions.Zhong Yao Cai 2009;32:102-6.
- Kim HS, Lee EH, Ko SR, Choi KJ, Park JH, Im DS. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Arch Pharm Res 2004;27:429-35.
- Kim SM, Lee SY, Yuk DY, Moon DC, Choi SS, Kim Y, et al. Inhibition of NF-kappaB by ginsenoside Rg3 enhances the susceptibility of colon cancer cells to docetaxel. Arch Pharm Res 2009;32:755-65.
- Chen ZJ, Cheng J, Huang YP, Han SL, Liu NX, Zhu GB, et al. Effect of adjuvant chemotherapy of ginsenoside Rg3 combined with mitomycin C and tegafur in advanced gastric cancer. Zhonghua Wei Chang WaiKeZaZhi 2007;10:64-6.
- Xu TM, Cui MH, Xin Y, Gu LP, Jiang X, Su MM, et al. Inhibitory effect of ginsenoside Rg3 on ovarian cancer metastasis. Chin Med J (Engl) 2008;121:1394-7.
- Furuiye M, Ishiwata N, Jin Y, Miyashita Y, Takano S, Yoshizawa M, et al. Randomized phase II study of carboplatin/paclitaxel followed by gemcitabine versus carboplatin/gemcitabine followed by docetaxel in patients with advanced nonsmall cell lung cancer. Gan To Kagaku Ryoho 2008;35:1133-8.
- Angelova AL, Aprahamian M, Grekova SP, Hajri A, Leuchs B, Giese NA, et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin Cancer Res 2009;15:511-9.
- Chen J, Wu SY, Ou-Yang ZG, Zhen YS. Synergy of gemcitabine and lidamycin associated with NF-kappaBdownregulation in pancreatic carcinoma cells. ActaPharmacol Sin 2008;29:614-9.
- Mose S, Class R, Weber HW, Rahn A, Brady LW, Böttcher HD. Radiation enhancement by gemcitabine-mediated cell cycle modulations. Am J ClinOncol 2003;26:60-9.
- Dempsey PW, Doyle SE, He JQ, Cheng Q. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev 2003;14:193-209.
- MacKenzie SH, Clark AC. Targeting cell death in tumors by activating caspases. Curr Cancer Drug Targets 2008;8:98-109.
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis 2000;21:485-95.
- Russo A, Terrasi M, Agnese V, Santini D, Bazan V. Apoptosis: A relevant tool for anticancer therapy. Ann Oncol 2006;17:i115-23.
- Fulda S, Debatin KM. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006;25:4798-811.
- Reed JC. Proapoptoticmultidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ 2006;13:1378-86.
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324-37.
- Akhtar M, Gallagher L, Rohan S. Survivin: Role in diagnosis, prognosis, and treatment of bladder cancer. AdvAnatPathol 2006;13:122-6.