- *Corresponding Author:
- I. A. Ghazi
Department of Plant Sciences, School of Life Sciences, University of Hyderabad, Prof. C. R. Rao Road, Gachibowli, Hyderabad-500 046, India
E-mail: irfan@uohyd.ernet.in
| Date of Submission | 03 February 2017 |
| Date of Revision | 18 June 2017 |
| Date of Acceptance | 09 February 2018 |
| Indian J Pharm Sci 2018;80(2):307-317 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Considering the significant potential of many natural products as anticancer agents, the present investigation was undertaken to explore the anticancer potential of Abrus precatorius. Bioassay-guided fractionation led to two active fractions, APH-11 and APM-3 exhibiting IC50 values of 14.64±1.84 and 20.90±3.58 µg/ml, respectively against the human acute monocytic leukemia cell line. In vitro cytotoxicity of APH-11 and APM-3 against the human acute monocytic leukemia cell line was compared with that against peritoneal macrophages and HEK 293 cells. These fractions significantly induced apoptosis, as evaluated by TdT-mediated dUTP nick end labelling assay. Accumulation of cells at sub-G0/G1 phase was demonstrated by cell cycle analysis. Western blot analysis further revealed that the cell death induced by APH-11 and APM-3 fractions occurred through apoptosis involving caspase-3/-7 and PARP cleavage. Liquid chromatography with tandem mass spectrometry study was carried out for the identification of metabolites in these fractions. The overall results provided evidence that fractions from A. precatorius induced cell death of the human acute monocytic leukemia cell line through apoptosis.
Keywords
Medicinal plants, Abrus precatorius, cancer, apoptosis, TUNEL
Cancer is one of the serious health issues, affecting millions of individuals every year across the globe spreading further with continuance and increasing incidence annually. The World Health Organization estimated that deaths from cancer worldwide are projected to rise reaching an estimated 13.1 million casualties in 2030 [1,2]. In spite of considerable advances in imaging and molecular diagnostic tools, the disease still continues to hamper the treatment process of millions of patients globally. The survival has not been improved due to low selectivity, different levels of toxicity in normal tissues and rapid advancement of resistance to chemotherapeutic agents [3]. Natural products have regained prominence and serve as potential starting materials for the discovery of new agents owing to increasing comprehension of their biological roles [4]. Plant-derived molecules comprise an essential source of anticancer agents owing to their structural diversity, drug ability and biological compatibility. A plethora of antineoplastic drugs such as taxoids, camptothecin, vinca alkaloids and podophyllotoxin derivatives have emerged from anticancer screening of ethanopharmacologically important medicinal plants [5]. The role of plant-based drugs like vinblastine, paclitaxel and etoposide in cancer therapy is well-established [6]. Over the years, plant-derived pharmaceuticals have proven to be more potent and less toxic, which is one of the ways to increase the efficacy of anticancer drugs and is also an important approach in the search for novel anticancer compounds [7]. Recent evidence indicates the vital roles of apoptosis in the development of therapeutic agents for treating cancer [8].
The homeostasis in eukaryotic cells is subjected to a delicate balance between survival and death signals originated from extracellular domain [9]. Apoptosis, as a highly related and well-established phenomenon, regulates this homeostasis of tissue systems and cell growth in organisms by eradicating the worn-out, damaged and unwanted cells [10]. This self-suicidal cellular program is crucial for organ development, tissue remodelling and regulation of immune responses [11]. Apoptosis is an organised and well-knit cellular process that takes place in abnormal physiological and pathological conditions. The growth and division of normal cells is under a tight control of various cellular signals. A change in these signals and mechanisms in normal cells because of various factors like mutations make them to evade apoptosis and transform into cancerous cells [12]. Thus, surpassing the process of apoptosis is a key to the development of cancer. Development of approaches that reinstall the apoptotic machinery selectively within tumour cells could be an effective measure of cancer control [13]. A wide variety of natural compounds from medicinal plants appears to possess significant cytotoxic as well as chemopreventive activity via apoptosis. It has been shown that natural products derived from plants promote apoptosis in cancer cells via extrinsic or intrinsic pathway that is blocked in these cells. The most important morphological and physiological alterations that happen to cancer cells during the process of apoptosis include shrinkage of cell membranes, loss of appropriate position of organelles, membrane blebbing, chromatin condensation and fragmentation [14]. Elaborate studies with such compounds with respect to their abilities to induce apoptosis and to understand their mechanism of action may provide valuable information for their possible application in cancer therapy and prevention. However, some traditionally used plants remain to be scientifically validated. Therefore, many investigators are persistently focusing on these plants in quest of novel chemotherapeutics [15].
Abrus precatorius L. (Fabaceae) is a vine, which grows extensively all over the tropical and sub-tropical regions of the world. In the traditional system of medicine, leaves, roots and seeds of this plant have been used for the management of several disorders such as anthelmintic, antidiarrheal, antiemetic and also for inhibition of intestinal motility [16]. We have previously reported that crude extracts of A. precatorius possess significant antiproliferative activities in different human carcinoma cell lines [17]. However, the possible apoptotic mechanism remained unknown. Therefore, this study was intended to investigate the potential cytotoxic and apoptotic effects of A. precatorius on human monocytic leukaemia (THP-1) cell line and to identify phytochemical constituents in active fractions obtained from bioactivity-guided fractionation. This cell line (THP-1) has been quite often used for the screening of natural products for their cytotoxic activities [18,19].
Materials and Methods
Fresh leaves of A. precatorius were obtained from Central Research Institute of Unani Medicine Hyderabad, India. The plant was authenticated, and a voucher specimen (UoH/VS/AP-2) has been preserved for future reference. The crude extracts were prepared as previously described [17] and extracts were designated as APE (ethyl acetate) and APA (methanol extract) and stored at –20° until further use.
Cell lines and culture conditions
The cell lines, THP-1 and human embryonic kidney cells (HEK-293) were obtained from National Centre for Cell Sciences, Pune, India. THP-1 cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 Medium, whereas HEK-293 cells were grown in Dulbecco's modified Eagle's medium. Mouse peritoneal macrophages were harvested from female BALB/c mice following intraperitoneal injection of 3 ml of 4 % thioglycollate medium [20]. The cells (THP- 1, HEK-293 and macrophages) were suspended in recommended media supplemented with 10 % (v/v) heat-inactivated foetal bovine serum, 100 units/ml of penicillin and 0.1 mg/ml of streptomycin sulphate and incubated in a humidified atmosphere of a 5 % CO2 at 37°. Before the experiments, culture medium was used for diluting the test samples to make the final concentration of dimethyl sulphoxide (DMSO) in culture to be ≤0.1 %.
Antiproliferative activity
Antiproliferative activity of plant extracts and their fractions was measured using 3-(4,5-dimethyl-thiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [17]. The MTT assay was undertaken in three stages. In the first stage, 50-200 μg/ml of crude extracts were tested against THP-1 cells. The crude plant extracts that showed >50 % inhibition of proliferation of cells were selected for further investigations in stage II. In the second stage, different fractions obtained from column chromatography of active crude extracts were again tested for their antiproliferative activity. The fractions that displayed >50 % inhibition of growth were selected for IC50 determination at stage III. In the third stage, five concentrations (10, 25, 50, 75 and 100 μg/ml) were prepared from each fraction and further established against THP-1 cells. In order to determine whether the inhibitory effects of crude extracts and fractions were specific for THP-1 cells, the effect of active fractions on the proliferation of peritoneal macrophages and HEK-293 were subsequently monitored.
Selective-index (SI)
To determine the cytotoxic selectivity of the substances tested, the SI was calculated according to the following Eqn.: SI = IC50 non-cancer cells/IC50 cancer cells.
Fractionation of active crude extracts
The MTT assay for cell viability showed that APE and APA extracts were cytotoxic and thus, warranted further examination. APE extract (20 g) was chromatographed on a sintered glass column with 4 cm internal diameter, 100 cm length and packed with 500 g of silica gel (60-120 mesh size) as a stationary phase prepared as a slurry in hexane. APE extract was adsorbed on silica gel by preparing slurry in methanol and solvent was recovered under reduced pressure with the aid of rotary evaporator. The elution was carried out gradually using a combination of hexane, ethyl acetate and methanol. The following ratios of solvent mixtures were sequentially used in elution process; hexane:ethyl acetate; 100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40:60, 30:70, 20:80 and 1000 ml of different solvent combinations was used in each gradient step. Eluents were collected in portions of 50 ml. Finally, the column was eluted with 100 % methanol. A total of 142 fractions of 50 ml each were collected and all the eluents were pooled together based on the similarity of thin-layer chromatography (TLC) profiles detected on pre-coated TLC silica gel 60 F254 plates using ethyl acetate/methanol and hexane/ ethyl acetate (90:10 respectively; v/v) as a developing solvent. The developed plates were sprayed with vanillin/H2SO4; iodine and 10 % H2SO4 methanol solutions. The excess solvent was evaporated under reduced pressure using a rotary vacuum evaporator to yield a total of 11 fractions designated as APH-1, APH-2, APH-3, APH-4, APH-5, APH-6, APH-7, APH- 8, APH-9, APH-10 and APH-11. Each fraction was weighed and stored at –20° for further analysis.
Similarly, 20 g of APA was also fractionated as described above. The extract was eluted using a combination of chloroform and methanol with an initial ratio of chloroform-methanol, (99:1 v/v), followed by 98:2, 95:5, 90:10, 80:20, 70:30, 60:40, 50:50 and 0:100. A total of 127 fractions of 50 ml each were collected and all the eluted fractions were then monitored individually by TLC and the fractions with same TLC profile were pooled; thereby, 8 major fractions were obtained and designated as APM-1, APM-2, APM-3, APM-4, APM- 5, APM-6, APM-7 and APM-8. Each fraction was then concentrated to dryness under reduced pressure on a rotary evaporator. Some part of the fractions were reconstituted in a DMSO solvent to form stock solutions of 20 mg/ml and stored at –20° until required.
Detection of apoptosis by TUNEL assay
The Apoalert® DNA fragmentation kit (Clontech Laboratories, Inc. Palo Alto, CA) was used for studying apoptosis, which is based on the principle of terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labelling (TUNEL). The assay is based on TUNEL, which catalyses the incorporation of fluorescein-dUTP at the free 3'-hydroxyl ends of fragmented DNA. Briefly, the THP-1 cells (1×106 cells/ ml) were plated in 60 mm sterile dishes and were treated with the APH-11 and APM-3 at the concentration of 50 μg/ml, complete media (as a negative control) and doxorubicin 10 μg/ml (as a positive control) and incubated for 48 h. After the incubation period, the medium was aspirated off and cells were attached on 0.01 % poly-L-lysine-coated slides in coupling jars. The cells were fixed with 4 % methanol-free formaldehyde solution at 4° for 25 min and rinsed again with phosphate-buffered saline (PBS). Subsequently, the cells were permeabilized by immersion in 0.2 % Triton X-100 in PBS for 5 min, washed with PBS, and equilibrated with the equilibration buffer for 10 min. Cells were labelled with the TdT incubation buffer by incubating at 37° for 60 min. The reaction was stopped by immersing the slides in saline sodium citrate for 15 min. Thereafter, the slides were washed with PBS and treated with propidium iodide (PI; 10 mg/ml in PBS) for 15 min in a dark conditions [21]. Observations were carried out using confocal fluorescence microscopy (Carl-Zeiss). Minimum of 10 microscopic fields were perceived for each sample.
Detection of apoptosis by flow cytometry
The effect of plant extracts/fractions on the cell cycle was determined by flow cytometric analysis [22]. The stained cells were incubated in dark at 37° for 30 min and analysed by flow cytometer (FACS calibur, BD Biosciences, USA) with the quantification of population of G0/G1, S and G2/M using CellQuest Pro software (BD Biosciences). During all FACS analysis, 10 000 events for each sample were analysed.
Western blot analysis
The identification of proteins involved in apoptosis was performed using sodium dodecyl sulphatepolyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting experiments as described previously [23]. The protein concentration in supernatants was determined by the Pierce® BCA protein assay kit (Thermo Scientific, USA) according to manufacturer’s instructions. A microplate reader (TECAN, Japan) was used to measure the absorbance at λ 595 nm and the concentration of the protein was calculated according to the bovine serum albumin standard curve of 0 to 1 mg/ml range. For western blot analysis, equal amount of total protein was mixed with SDS sample buffer, incubated at 100° for 5 min and separated by SDS-PAGE. After electrophoresis, protein was blotted on a nitrocellulose membrane and blocked for 3 h in blocking solution at room temperature. Each membrane was incubated with appropriate primary antibodies at 4° overnight and washed with Tris-buffered saline with Tween 20 (TBST). The blots were incubated with Horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 90 min, washed three times with TBST, and then followed by visualization with enhanced chemiluminescence kit (ECL Western blotting detection kit, GE Healthcare, USA).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The active fractions were analysed by LC-MS/MS analysis using both positive and negative electrospray ionization-tandem mass spectrometry (ESI-MS/ MS) modes. An electrospray interface with excellent sensitivity, fragmentation and linearity were optimized to characterize the fragment ions of analysed fractions. Agilent 1200 series coupled with DAD-UV detector that was equipped with Agilent Technologies 6520 with Accurate-Mass Q-TOF mode was used to perform mass spectrometry and Zorbax XDB-C18 column rapid resolution (1.8 μm, 4.6×50 mm). The flow rate was maintained at 0.2 ml/min, and the injection volume was 0.2 μl/sample. Analyses was performed using binary gradients of Milli-Q water: mobile phases (A) 5 mM ammonium formate in 0.1 % (v/v) formic acid and (B) 100 % (v/v) HPLC-grade acetonitrile with the following elution profile; from 0 min: 35 % (B) in (A); 1 min: 35 % (B) in (A); 25 min: 90 % (B) in (A); 29 min: 98 % (B) in (A); 40 min: 35 % (B) in (A). ESI parameters were both negative and positive ion mode; the mass range was 100-1700 m/z with spray voltage 4 KV with scan rate 1.4; helium gas temperature was 325° with a flow rate of 8 ml/min; nebulizer pressure was maintained at 35 psi. MS/MS data were acquired in negative ionization mode to obtain m/z of the fragment ions. The Dictionary of Natural Products on DVD software (CRC Press, Taylor and Francis Group), MassHunter software (Agilent), MassBank were used to analyse the chromatography profiling data.
Calculations and statistical analysis
The antiproliferative and cell viability data of this study were based on three replicates with mean ± SD. Analysis of variance (ANOVA), followed by Student’s t- test and Bonferroni post-test were used to determine the statistical significance. P-value less than 0.05 was considered statistically significant. IC50 value (the concentration of extracts/fractions required to inhibit 50 % growth of cells) was calculated for different samples. Statistical tests as well as mean and SD calculations and graphical representation of results were performed using GraphPad Prism v5 software.
Results and Discussion
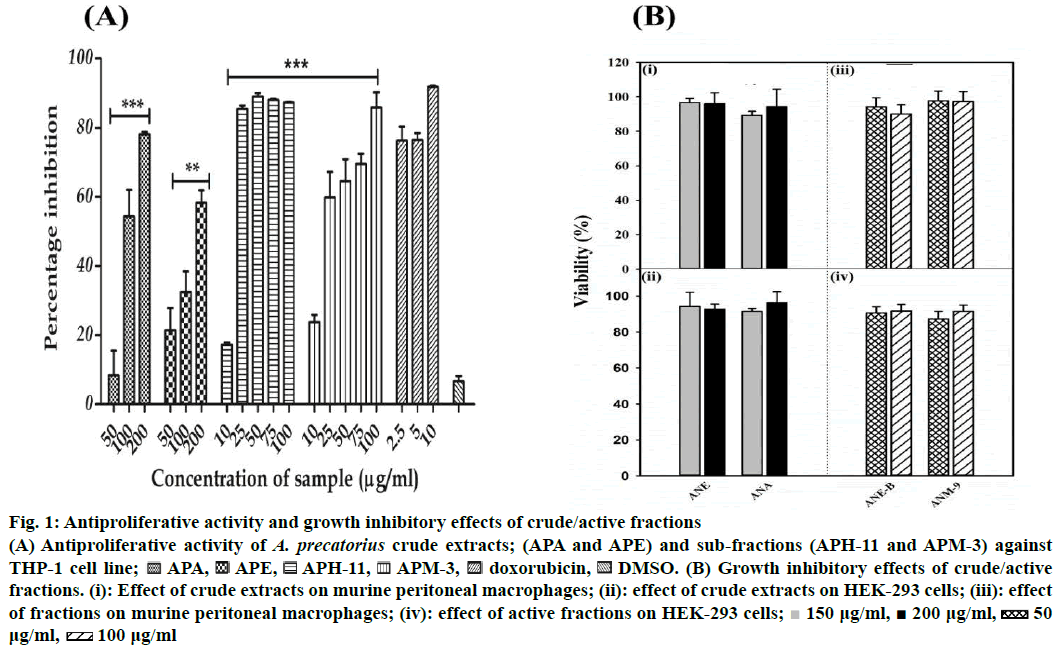
The preliminary study undertaken to investigate the antiproliferative nature of extracts of A. precatorius showed that the extracts are cytotoxic to THP-1 cells in a concentration-dependent manner. The antiproliferative activity of A. precatorius crude extracts (APE and APA) and active fractions with 50 % cytotoxicity (IC50) values are presented in Table 1 and Figure 1A. As shown in Figure 1A, APE and APA exhibited a significant antiproliferative activity in a concentration-dependent manner against THP-1 cells. APA showed the maximum inhibition of cell growth (78.11 ± 0.66 %) with 200 μg/ml of extracts (p<0.05) with an IC50 value of 298.68 ± 11.17 μg/ml. The APE also possessed significant cytotoxic activity and inhibited the cell growth (58.30 ± 6.16 %) at 200 μg/ml concentration (p<0.05) with IC50 values of 116.84 ± 7.09 μg/ml. Furthermore, sub-fractions of these extracts showed significant cytotoxicity to THP-1 cells, with different IC50 values, when compared to parent extracts. The fraction; APH- 11, obtained from bioassay-guided fractionation of APE presented superior cytotoxic effects compared to original extract (APE), with an IC50 value of 14.64 ± 1.84 μg/ml and 87.32 ± 0.17 % suppression at the highest concentration of 100 μg/ml. Similarly, the APM-3 obtained from APA presented better cytotoxic effects compared to parent extract (APA) with the IC50 value of 20.90 ± 3.58 μg/ml with percent inhibition of 85.84 ± 7.52 (Figure 1A, Table 1). Furthermore, the safety of these fractions was again monitored by assessing their cytotoxic effects towards normal peritoneal macrophages and HEK-293 cells. The results prominently showed that these fractions were non-toxic and had a slight inhibitory effect on cell proliferation on these normal cell models (Figure 1B). The APE and APM presented the best selectivity index against the THP-1 cell line (8.56 and 3.49, respectively).
| Sample | Percentage Inhibition | IC50 Value |
|---|---|---|
| APA (200 μg/ml) | 78.11 ± 0.66 | 298.68 ± 11.17* |
| APE (200 μg/ml) | 58.30 ± 6.16 | 116.84 ± 7.09* |
| APH-11 (100 μg/ml) | 87.32 ± 0.17 | 14.64 ± 1.84 |
| APM-3 (100 μg/ml) | 85.84 ± 7.52 | 20.90 ± 3.58 |
| Doxorubicin (10 μg/ml) | 91.80 ± 0.58 | 1.63 ± 0.58 |
| DMSO | 6.66 ± 2.60 | -- |
Table 1: Antiproliferative Activity of Samples on THP-1 Cells
Figure 1: Antiproliferative activity and growth inhibitory effects of crude/active fractions
(A) Antiproliferative activity of A. precatorius crude extracts; (APA and APE) and sub-fractions (APH-11 and APM-3) against THP-1 cell line;  APA,
APA, APE,
APE, APH-11,
APH-11, APM-3,
APM-3, doxorubicin,
doxorubicin, DMSO. (B) Growth inhibitory effects of crude/active fractions. (i): Effect of crude extracts on murine peritoneal macrophages; (ii): effect of crude extracts on HEK-293 cells; (iii): effect of fractions on murine peritoneal macrophages; (iv): effect of active fractions on HEK-293 cells;
DMSO. (B) Growth inhibitory effects of crude/active fractions. (i): Effect of crude extracts on murine peritoneal macrophages; (ii): effect of crude extracts on HEK-293 cells; (iii): effect of fractions on murine peritoneal macrophages; (iv): effect of active fractions on HEK-293 cells;  150 μg/ml,
150 μg/ml,  200 μg/ml,
200 μg/ml,  50 μg/ml,
50 μg/ml,  100 μg/ml
100 μg/ml
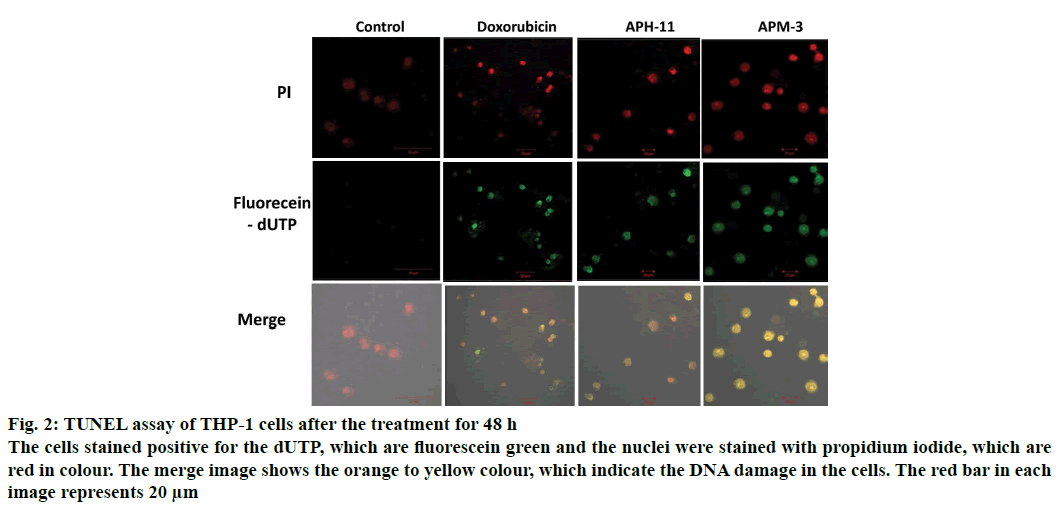
The pro-apoptotic efficacy of APH-11 and APM-3 were further verified and substantiated by TUNEL assay. Apoptosis in a cell usually results in the introduction of nicks in genome and these nicks can be visualised using TUNEL assay by a fluorescent microscope. At the concentration 50 μg/ml of APH-11 and APM-3, there was a positive increase in the TUNEL stain (green fluorescein-dUTP) that indicates the accumulating DNA damage (Figure 2). No significant DNA damage was observed in untreated cells. These results demonstrate that APH-11 and APM-3 efficiently induced apoptosis in THP-1 cells by producing nicks in the genome.
Figure 2: TUNEL assay of THP-1 cells after the treatment for 48 h
The cells stained positive for the dUTP, which are fluorescein green and the nuclei were stained with propidium iodide, which are red in colour. The merge image shows the orange to yellow colour, which indicate the DNA damage in the cells. The red bar in each image represents 20 μm
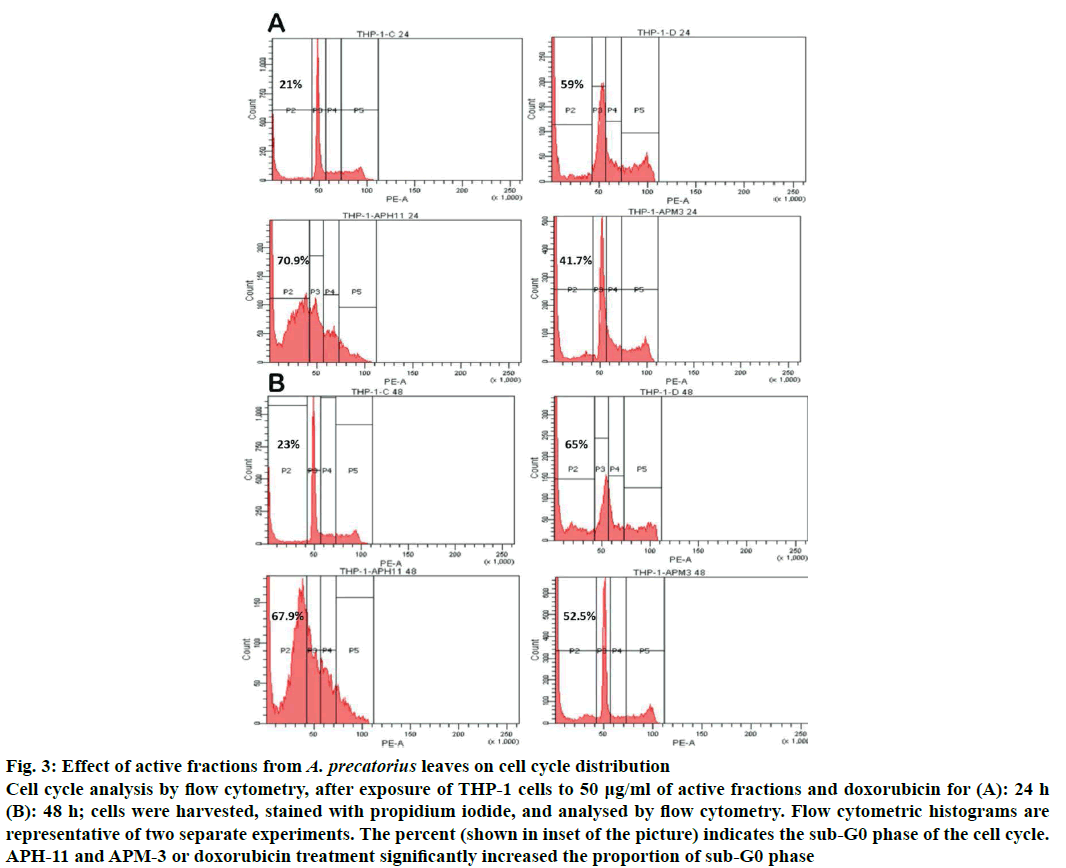
Apoptosis and changes in cell cycle were analysed by flow cytometry using propidium iodide staining. Cell cycle was examined using flow cytometry after exposure to 50 μg/ml APH-11 and APM-3 for 24 and 48 h. The relative DNA content in treated and untreated THP-1 was represented as G0/G1, S and G2/M phases within the interphase of cell cycle. The results clearly establish the alteration of cell cycle profile in THP-1 cells treated with active fractions. The results showed that treatment with 50 μg/ml fractions caused a significant increase in sub-G0 population (apoptotic cells) with time compared to control group (APH-11; 70.9 % for 24 h and 67.9 % for 48 h; APM-3; 41.7 % for 24 h and 52.5 % for 48 h). APH-11 treatment exhibited growth arrest in S-phase as there was no change in G1 and G2/M percentage cells at 24 and 48 h but APM-3 exhibited growth arrest at G1 phase due to which there is a decrease in S and G2/M cells at the time point of 24 and 48 h. These results suggested that APH-11 and APM-3 can induce alterations in cell cycle pattern, which may lead to apoptosis in cells (Figure 3a and b).
Figure 3: Effect of active fractions from A. precatorius leaves on cell cycle distribution
Cell cycle analysis by flow cytometry, after exposure of THP-1 cells to 50 μg/ml of active fractions and doxorubicin for (A): 24 h (B): 48 h; cells were harvested, stained with propidium iodide, and analysed by flow cytometry. Flow cytometric histograms are representative of two separate experiments. The percent (shown in inset of the picture) indicates the sub-G0 phase of the cell cycle. APH-11 and APM-3 or doxorubicin treatment significantly increased the proportion of sub-G0 phase
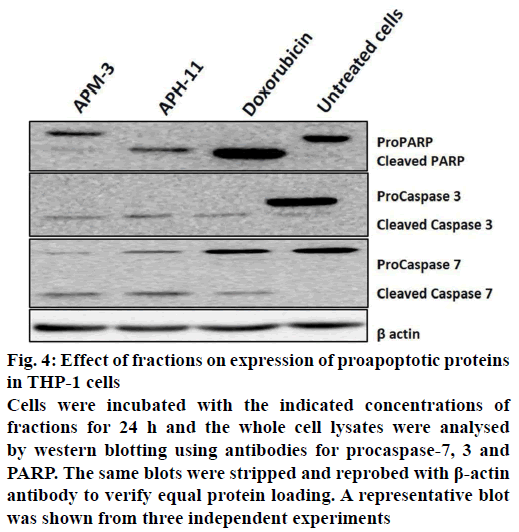
To explain the possible signalling pathway by which the fractions-induced apoptosis in THP-1 cells, the protein levels of various apoptosis-regulating proteins such as effector caspases (caspase-3 and -7) and poly(ADP-ribose) polymerase (PARP) were studied by western blotting. As depicted in Figure 4, the exposure of THP-1 cells to fractions for 24 h led to downregulation of procaspase-7 and 3, which signify the cleavage of caspases and therefore, the activation of apoptotic pathways. Moreover, these fractions-induced cleavage of PARP at 24 h that indicates the activation of caspase-7, as compared to control. Since the PARP is one of the biochemical markers of cells that undergo apoptosis, our results suggested that the process of apoptosis was triggered in THP-1 cells following the treatment with A. precatorius fractions.
Figure 4: Effect of fractions on expression of proapoptotic proteins in THP-1 cells
Cells were incubated with the indicated concentrations of fractions for 24 h and the whole cell lysates were analysed by western blotting using antibodies for procaspase-7, 3 and PARP. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. A representative blot was shown from three independent experiments
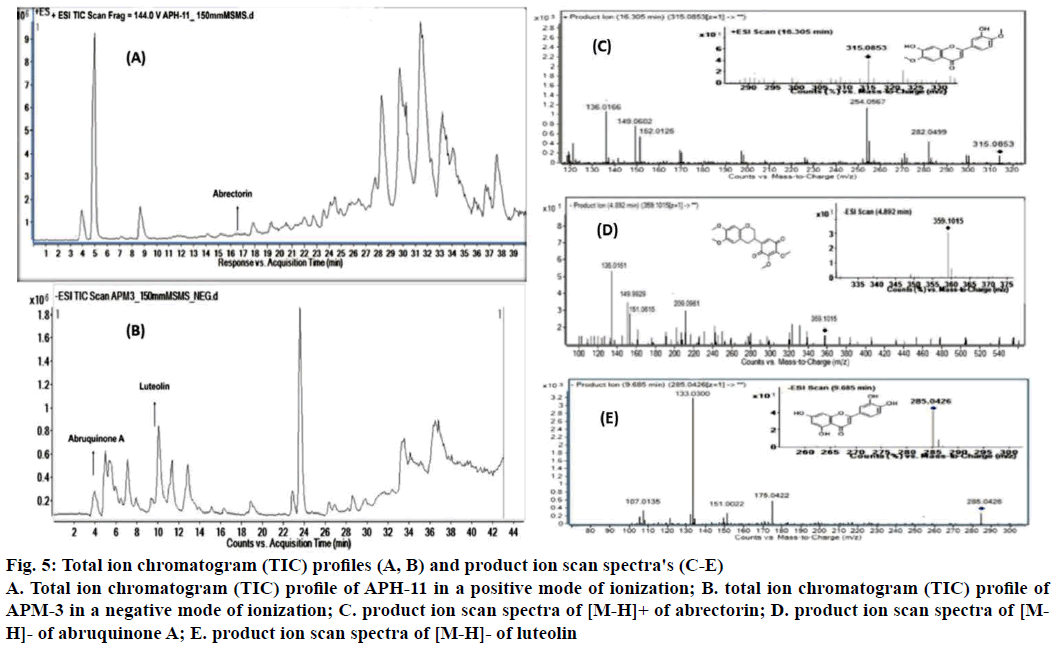
Since APH-11 and APM-3 showed significant antiproliferative activity, they were further investigated for identifying the possible bioactive metabolites present in active fractions using LC/MS-MS analysis. The information on molecular mass of the components can be revealed by LC-MS analysis of sample. MS2 dissociations give further structural information on the compounds (Table 2), complementing the highresolution LC/ESI-MSn, which were analysed with the support of Agilent Mass Hunter software and DNP and Mass Bank databases. In fraction APH-11, only one peak was identified in positive mode. According to LCESI- MS-MS spectrum of a compound 1 assigned it as abrectorin (Figure 5A), the positive ESI-MS (RT= 16.305 min, λ= 280 nm, MW= 314.2895) showed ion [M+H]+ with m/z 315.0853. MS/MS analysis found loss of water (H2O) molecule and a methyl group (CH3-) with 33 a.m.u., with m/z at ~282 and followed by loss of carbon monoxide (CO) with 28 a.m.u. with m/z at ~254. Ions peaks at ~151, ~148, ~135 correspond to cleavage of central (C) ring by 0,2B+, 1,3B+ and 1,2B+, respectively. Thus [M+H]+ along with its fragments suggest compound 1 as abrectorin [24]. In APM-3, two peaks were identified in negative ionisation mode. Compound 2 (RT= 4.892 min, λ= 280 nm, MW= 360.3579) had [MH]- at m/z 359.1015 and was identified as abruquinone A with the loss of one methyl group and loss of ring B benzoquinone with 15 amu, and 107 amu at m/z ~344 and ~151, respectively. Ions peaks at ~209, ~149 and ~135 correspond to cleavage of central (C) ring by 0,3B-, 0,3A-, and 0,4A-, respectively. Thus [M-H]- along with its fragments suggests compound 2 as abruquinone A [25] (Figure 5B). According to LC-ESI-MS-MS spectrum of compound 3 from APM-3 is assigned it as luteolin, the negative ESI-MS (Rt= 9.685 min, λ= 280 nm, MW= 286.2363) showed ion [M-H]- with m/z 285.0426. MS/ MS analysis found the loss of a B ring with 110 amu, m/z at ~175. Ions peaks at ~151, ~133, ~107 correspond to cleavage of central (C) ring by 1,3A-, 1,3B and 0,4A-, respectively. Thus [M-H]- along with its fragments suggests compound 3 as luteolin Figure 5C. Some of the LC-MS/MS peaks remained unidentified, because of lack of library data of corresponding compounds.
| Fraction | Compounds | Rt (min) | Molecular Weight |
Molecular Formula |
Precursor ion (m/z) |
Product ions (m/z) | Ref. |
|---|---|---|---|---|---|---|---|
| APH-11 | Abrectorin** | 16.305 | 314.2895 | C19H20O7 | [M+H]+ 315.0853 | 136,149,152,254,282 | [24] |
| APM-3 | Abruquinone A* | 4.892 | 360.3579 | C17H14O6 | [M-H]- 359.1015 | 135,149,151,209 | [25] |
| APM-3 | Luteolin* | 9.685 | 286.2363 | C15H9O6 | [M-H]- 285.0426 | 107,133,151,175 | Mass bank |
Table 2: Retention Time, Precursor Ions and Product Ions (for Qualitative Confirmation of Compound(S) by LC-MS/MS Analysis
Figure 5: Total ion chromatogram (TIC) profiles (A, B) and product ion scan spectra's (C-E)
A. Total ion chromatogram (TIC) profile of APH-11 in a positive mode of ionization; B. total ion chromatogram (TIC) profile of APM-3 in a negative mode of ionization; C. product ion scan spectra of [M-H]+ of abrectorin; D. product ion scan spectra of [MH]-of abruquinone A; E. product ion scan spectra of [M-H]- of luteolin
Cancer progression is dependent on the delicate balance between cell proliferation and cell death. The induction of apoptosis might be a useful method for the discovery of chemopreventive and chemotherapeutic agents in the treatment of cancer cells [26]. Compounds from plants, which act as apoptotic inducers have been studied by several groups in the recent past [27,28]. In this investigation, we demonstrated for the first time that semi-purified fraction from A. precatorius induces apoptosis in human monocytic leukaemia cells in vitro. The extracts (APE and APA) and their subsequent fractions (APH-11 and APM-3) were able to inhibit the cell viability in a concentration-dependent manner in the studied cell line, the fractions being manifold potent than their parent extracts. According to US National Cancer Institute (NCI), the criteria of cytotoxicity for crude/semi-purified fraction is the value of an IC50< 30 μg/ml in the preliminary assays [29]. The results showed that the fractions increased the rate of cell death at lesser concentrations than that of parent crude extracts and satisfied the criteria of a promising anticancer drug as per the NCI guidelines. Since it is important for an anticancer agent to exhibit cytotoxicity that is selective for cancer cells [30], keeping this in view, the effect was studied on normal cells (HEK-293 and mouse peritoneal macrophages). The results showed that normal cells were not markedly affected when exposed to the same fractions. The relatively high selectivity index indicates that these extracts may be useful for the design and development of anticancer drugs in future and therefore, we proceeded with the study of cytotoxicity mechanism.
Apoptosis has long been recognised as a novel therapeutic strategy for identification of anticancer agents owing to its role in cellular destruction during the maintenance of physiological and cellular homeostasis in organisms [31]. This mode of cell death is a strictly controlled process, characterized by chromatin condensation, DNA fragmentation, membrane blebbing, cell shrinkage and compartmentalization of dead cells into apoptotic bodies, along with various biochemical changes [32].
Our results demonstrated that THP-1 cells displayed early as well as late phases of apoptosis-like activation of caspase-7/-3 and PARP, nuclear fragmentation in response to treatment with fractions. One of the remarkable and easily distinguishable features of cells undergoing apoptosis is the fragmentation of DNA [33]. The results demonstrated that DNA fragmentations were clearly detectable in THP-1 cells, at the level of treated concentrations of fractions for 48 h via TUNEL assay, which is a direct indicator of cellular apoptosis. Many anticancer molecules show growth inhibition and/or apoptotic cell death of cancer cells by modulating the cell cycle regulatory molecules [34], hence, targeting cell cycle is an important approach in cancer therapy [35,36]. Results obtained from the flow cytometry analysis revealed that both the fractions (APH-11 and APM-3) were able to alter the cell cycle. However, it is worth to note that there was not any considerable change in the cell cycle pattern or cell cycle arrest at lower concentrations below than 50 μg/ml. Such phenomenon of differential effect of various concentrations of natural products on cell cycle is not uncommon [37,38]. A time-dependent increase in the percent sub-G0/G1 cell population was quite evident following the treatment of these fractions. The ability of APH-11 and APM-3 to alter the cell cycle and to induce apoptosis in THP-1 is believed to contribute to the antiproliferative activity of these fractions. The activation of aspartate-specific cysteine protease is an essential step of apoptosis induction elicited by drugs and cleavage of PARP by caspase-3 is considered to be one of the hallmarks of apoptosis [39]. PARP, a critical signalling nuclear protein is involved in catalysing the synthesis of poly(ADP-ribose) binding to DNA strand breaks and altering the nuclear proteins in the process of DNA repair. However, the cleavage of PARP inhibits DNA repair mechanism as it is a downstream substrate of caspase-3 and is an indicator of apoptosis. In this study, APH-11 and APM- 3 fractions were able to cause caspase-7/3 cleavage, and PARP activation (Figure 4). The possible mechanism of the induction of apoptosis by the active fractions in THP-1 cells might be a caspase-dependent pathway in which caspase-7/3 are activated sequentially which leads to PARP cleavage. These findings were observed in earlier reports in which plant extracts/compounds were shown to induce apoptosis through the activation of caspases in different human cancer cell lines [26,40]. As the activation of caspases-7/3 and PARP being shown as an early event in the DNA fragmentation and TUNEL assays during APH-11/APM-3-induced apoptosis in THP-1 cells, further study on initiator caspase 8 and 9 would be vital to describe the extrinsic and intrinsic apoptotic pathways [41]. Nevertheless, the findings revealed that APH-11 and APM-3 affect the THP-1 cells by activating the caspases in apoptotic pathway. The fragmentation pattern observed in MS-MS spectra found to match with the literature of/from DNP and public domain. The MassBank spectral database supports the possibility of presence of abruquinone A, luteolin and abrectorin metabolites in active fractions. These identified compounds are classified under polyphenols (flavonoids), which are known to exhibit numerous biological activities. Many recent epidemiological studies have revealed that polyphenols from plants are promising nutraceuticals, which reduce the risk of some chronic diseases like cardiovascular diseases, neurodegenerative disorders and neoplastic diseases [42,43]. Furthermore, the plant-derived polyphenolic compounds have shown anticancer properties against different types of cancers [44,45], which explains the high interest and initiation of many studies to evaluate the biological activities as well as bioavailabilities of polyphenolic compounds. Abruquinone A has been isolated previously from A. precatorius roots and was shown to possess antiinflammatory, antiallergic and cardiovascular effects in several studies [46]. Luteolin exhibits widespread pharmacological benefits including antioxidant, hypertension, inflammatory disorders, cancer and other chemopreventive effects [47]. Abrectorin has been confirmed in A. precatorius and other plant species with medicinal properties [48].
In conclusion, the present study demonstrated for the first time the antiproliferative effect of fractions of A. precatorius leaves that induced programmed cell death via induction of DNA fragmentation, alteration of cell cycle and activating a caspase cascade. In addition, we could hypothesize that these fractions might be preferably involved in the intrinsic apoptotic pathway. However, such specificity needs to be proved by additional analyses, and the mechanism of targeting apoptotic pathway remains unknown. However, further studies are needed to elucidate the active principle and validate these findings in various in vivo settings so as to develop it as a potential therapeutic agent for treating acute monocytic leukaemia.
Acknowledgements
The authors would like to thank Central Research Institute of Unani Medicine, Hyderabad, Telangana for providing the plant material. The authors acknowledge the financial support in the form of Senior Research Fellowship (SRF) from CSIR and UGC, India and Department of Science and Technology, New Delhi in the form of DST-WOSA award. Authors are thankful to DBT-CREBB and DIST-FIST for funding UoHmetabolomics facility and flow cytometry facility at Department of Animal Biology, UoH, respectively. Authors would also like to thank DBT-CREBB, DSTFIST level I & II, DST-PURSE Phase I & II and UGCSAP- CAS for supporting infrastructural facilities of Department of Plant Sciences and School of Life Sciences, University of Hyderabad. The funding given by UGC-sponsored University of Hyderabad-UPE phase-II to IAG lab is gratefully acknowledged.
Conflicts of interest
There are no conflicts of interest.
Financial support and sponsorship
Nil.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86.
- Zhu K, Fukasawa I, Furuno M, Inaba F, Yamazaki T, Kamemori T, et al. Inhibitory effects of herbal drugs on the growth of human ovarian cancer cell lines through the induction of apoptosis. Gynecol Oncol 2005;97:405-09.
- Kviecinski MR, Felipe KB, Schoenfelder T, de Lemos Wiese LP, Rossi MH, Gonçalez E, et al. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J Ethnopharmacol 2008;117:69-75.
- Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F. Antiproliferative activity against human tumour cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol 2008;46:3325-32.
- Schwartsmann G, Ratain MJ, Cragg GM. Anticancer drug discovery and development throughout the world. J Clin Oncol 2002;20:47S-59.
- Johnson IT. Phytochemicals and cancer. Proc Nutr Soc 2007;66:207-15.
- Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int 2014;2014:150845.
- Raff MC. Social controls on cell survival and cell death. Nature 1992;356(6368):397-400.
- Gosslau A, Chen KY. Nutraceuticals, apoptosis and disease prevention. Nutrition 2004;20:95-02.
- Schafer ZT, Kornbluth S. The apoptosome: physiological, developmental, and pathological modes of regulation. Dev Cell 2006;10(5):549-61.
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 2005;5(3):231-37.
- Denmeade SR, Isaacs JT. Programmed Cell Death (Apoptosis) and Cancer Chemotherapy. Cancer Control 1996 3(4):303-09.
- Hemaiswarya S, Doble M. Potential synergism of natural products in the treatment of cancer. Phytother Res 2006;20(4):239-49.
- Dias DA, Urban S, Roessner U. Historical overview of natural products in drug discovery. Metabolites 2012;2:303-36.
- Morton JF. Plants poisonous to people in Florida and other warm areas. Miami, Florida: Hallmark Press; 1982.
- Gul MZ, Ahmad F, Kondapi AK, Qureshi IA, Ghazi IA. Antioxidant and antiproliferative activities of Abrus precatorius leaf extracts - an in vitro study. BMC Complement Altern Med 2013;13:53.
- Ayesh BM, Abed AA, Faris DM1. In vitro inhibition of human leukemia THP-1 cells by Origanum syriacum L. and Thymus vulgaris L. extracts. BMC Res Notes 2014;7:612.
- Roy MK, Kobori M, Takenaka M, Nakahara K, Shinmoto H, Isobe S, et al. Antiproliferative effect on human cancer cell lines after treatment with nimbolide extracted from an edible part of the neem tree (Azadirachta indica). Phytother Res 2007;21(3):245-50.
- Bhattacharjee S, Gupta G, Bhattacharya P, Mukherjee A, Mujumdar SB, Pal A, et al. Quassin alters the immunological patterns of murine macrophages through the generation of nitric oxide to exert antileishmanial activity. J Antimicrob Chemother 2009;63:317-24.
- Gul MZ, Chandrasekaran S, Manjulatha K, Bhat MY, Maurya R, Qureshi IA, Ghazi IA. Bioassay-guided fractionation and in vitro antiproliferative effects of fractions of Artemisia nilagirica on THP-1 cell line. Nutr Cancer 2016;68(7):1210-24.
- Ait-Mohamed O, Battisti V, Joliot V, Fritsch L, Pontis J, Medjkane S, et al. Acetonic extract of Buxus sempervirens induces cell cycle arrest, apoptosis and autophagy in breast cancer cells. PLoS One 2011;6:e24537.
- Ravi A, Mallika A, Sama V, Begum AS, Khan RS, Reddy BM. Antiproliferative activity and standardization of Tecomella undulata bark extract on K562 cells. J Ethnopharmacol 2011;137:1353-59.
- Alessandro L, Franco D, Giovanni Battista M, Deise Lia Barros C, Ivan Leoncio D. Abruquinones: new natural isoflavanquinones. Gazz Chem Ital 1979;109:9-12.
- Bhardwaj DK, Bisht MS, Mehta CK. Flavonoids from Abrus precatorius. Phytochem 1980;19:2040-41.
- Wong, FC, Woo CC, Hsu A, Tan, BKH. The Anti-cancer activities of Vernonia amygdalina extract in human breast cancer cell lines are mediated through caspase-dependent and p53-independent pathways. PLoS One 2013;8:e78021.
- Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod 2003;66:1022-37.
- Yacob NS, Hamzah N, Nik-Mohamed KNN, Zainal Abidin SA, Lai CS, Navaratnam V, Norazmi MN. Anticancer activity of a sub-fraction of dichloromethane extract of Strobilanthes crispus on human breast and prostate cancer cells in vitro. BMC Complement Altern Med 2010;10:42.
- Suffness M, Pezzuto JM. Assays related to cancer drug discovery. In: Hostettmann K, editor. Methods in Plant Biochemistry: Assays for Bioactivity. London: Academic Press; 1990. p. 71-133.
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, et al. Selective killing of oncogenically transformed cells through an ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006;10:241-52.
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 2005;5:876-85.
- Mohan S, Abdul AB, Abdelwahab SI, Al-Zubairi AS, Sukari MA, Abdullah R, et al. Typhonium flagelliforme induces apoptosis in CEMss cells via activation of caspase-9, PARP cleavage, and cytochrome c release: its activation coupled with G0/G1 phase cell cycle arrest. J Ethnopharmacol 2010;131:592-600.
- Cohen GM, Sun XM, Snowden RT, Dinsdale D, Skilleter DN. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J 1992;286:331-34.
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer 2002;2:331-41.
- Dickson MA, Schwartz GK. Development of cell cycle inhibitors for cancer therapy. Curr Oncol 2009;16:36-43.
- Wang ZY, Wang DM, Loo TY, Cheng Y, Chen LL, Shen JG, et al. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J Ethnopharmacol 2011;133:751-58.
- Hsieh TC, Wu P, Park S, Wu JM. Induction of cell cycle changes and modulation of apoptogenic/anti-apoptotic and extracellular signaling regulatory protein expression by water extracts of I'm-Yunity (PSP). BMC Complement Altern Med 2006;6:30.
- Hsu CP, Lin CC, Huang CC, Lin YH, Chou JC, Tsia YT. Induction of apoptosis and cell cycle arrest in human colorectal carcinoma by Litchi seed extract. J Biomed Biotechnol 2012;2012:341479.
- Singh RP, Agrawal P, Yim D, Agarwal C, Agarwal R. Acacetin inhibits cell growth and cell cycle progression and induces apoptosis in human prostate cancer cells: structure activity relationship with linarin and linarin acetate. Carcinogenesis 2005;26:845-54.
- Hsieh YJ, Chang CJ, Wan CF, Chen CP, Chiu YH, Leu YL, et al. Euphorbia formosana root extract induces apoptosis by caspase-dependent cell death via Fas and mitochondrial pathway in THP-1 human leukemic cells. Molecules 2013;18:1949-62.
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 1999;6:1028-42.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2009;2:270-78.
- Vauzour D, Rodriguez-Mateos A, Corona G, Oruna-Concha MJ, Spencer JPE. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010;2:1106-31.
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011;10:12.
- Rodríguez ML, Estrela JM, Ortega AL. Natural polyphenols and apoptosis induction in cancer therapy. J Carcinogene Mutagene 2013;S6:004.
- Kuo SC, Chen SC, Chen LH, Wu JB, Wang JP, Teng CM. Potent antiplatelet, anti-inflammatory and anti-allergic isoflavanquinones from the roots of Abrus precatorius. Planta Medica 1995;61:307-12.
- Lin Y, Shi R, Wang X, Shen HM. Luteolin, a flavonoid with potentials for cancer prevention and therapy. Curr Cancer Drug Targets 2008;8:634-46.
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochem 2000;55:481-504.