- *Corresponding Author:
- I. Kim
Department of Bioengineering, College of Engineering, Hanyang University, Seoul 04763, Republic of Korea
E-mail: iwkim@hanyang.ac.kr
| Date of Received | 21 March 2022 |
| Date of Revision | 24 February 2023 |
| Date of Acceptance | 20 June 2023 |
| Indian J Pharm Sci 2023;85(3):806-814 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
As complex mixtures of carcinogenic metalloids, arsenic compounds have been reported to possess anticytotoxic and antitumor effects. In this study, we evaluated the in vitro protective effects of arsenic compounds tetraarsenic oxide and arsenic trioxide against 3-methylcholanthrene-induced toxicity in human keratinocytes. Human keratinocytes were treated with varying concentrations of arsenic compounds alone or in combination with 3-methylcholanthrene. Treatment with arsenic compounds did not significantly affect cell viability, whereas, 3-methylcholanthrene significantly reduced the viability of human keratinocytes. Furthermore, both tetraarsenic oxide and arsenic trioxide decreased the expression of cytochrome P4501A1 at messenger ribonucleic acid and protein levels in human keratinocytes cells treated with 3-methylcholanthrene. In addition, these arsenic compounds increased the expression of nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1, which was shown to be inhibited by 3-methylcholanthrene treatment. Together, these findings suggest that tetraarsenic oxide and tetraarsenic oxide significantly inhibit 3-methylcholanthrene-induced cytotoxicity in human keratinocytes by decreasing the expression of cytochrome P4501A1 and increasing the expression of nicotinamide adenine dinucleotide phosphate quinone oxidoreductase 1. Additionally, tetraarsenic oxide was found to be more effective than arsenic trioxide against 3-methylcholanthrene-induced cytotoxicity in vitro.
Keywords
3-methylcholanthrene, tetraarsenic oxide, arsenic trioxide, nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate dehydrogenase (Quinone), cytochrome P-450A1.
Complex mixtures of carcinogenic metalloids, such as arsenic, and Polycyclic Aromatic Hydrocarbons (PAHs) or halogenated aromatic hydrocarbons are common environmental contaminants[1]. Arsenic, in spite of its toxicity, has been used as a therapeutic agent against various pathologies, including inflammatory and protozoal diseases for centuries[2]. Arsenic Trioxide (As2O3), a trivalent inorganic form, has successfully undergone clinic trials and was recently approved by the United States Food and Drug Administration for the treatment of acute promyelocytic leukemia[3-7]. Additionally, As2O3 has been reported to possess marked antitumor properties toward hematological malignancies[8]. Furthermore, many experimental studies have demonstrated the toxic effects of As2O3 at low concentrations against malignant hematological cells and several solid tumor cell lines[9-12]. The mechanisms underlying the anti-cancer action of As2O3 are complex and involve partial cytodifferentiation, inhibition of cell proliferation, induction of apoptosis and inhibition of angiogenesis[13,14]. Tetraarsenic Oxide (As4O6), another trivalent arsenic compound has different physical and chemical properties from those of As2O3 [15,16]. The anticancer properties of As4O6 in vitro and in vivo have been previously reported[17]. Furthermore, As4O6 has been reported to inhibit the proliferation, invasion and migration of basic fibroblast growth factor-stimulated bovine capillary endothelial cells. Moreover, As4O6 potently induced apoptotic cell death of As2O3-resistant U937 leukemic cells via the generation of reactive oxygen species[15].
The cellular effects of arsenic, including apoptosis, are likely to be linked to oxidative stress[18,19]. Interestingly, the metalloid has also been known to regulate the expression of genes encoding xenobiotic metabolizing enzymes[20,21]; arsenate down-regulates Cytochrome P450 family of enzymes, including CYP1A1[22,23]. In this study, we investigated the protective effects of arsenic compounds As4O6 and As2O3 against 3-MC-induced cytotoxicity using HaCaT human keratinocytes.
Materials and Methods
Cell culture and chemical reagents:
Human keratinocyte HaCaT cells were purchased from ThermoFisher Scientific and incubated in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10 % fetal bovine serum and 1 % antibiotic-antimycotic at 37° in a humidified 5 % CO2 atmosphere. As2O3 was purchased from Sigma (MO, USA). As4O6 was obtained from Chonjisan, Co., Seoul, Korea. These chemicals were dissolved in Phosphate- Buffered Saline (PBS) to a final concentration of 10-3 M by continuous stirring and stored at 4° as a stock solution. 3-(4,5-Dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (MO, USA) and dissolved in PBS at a final concentration of 2 mg/ ml. 3-methylcholanthrene (3-MC) was purchased from Sigma and dissolved in Dimethylsulfoxide (DMSO; Sigma-Aldirch, MO, USA).
Cell viability assay:
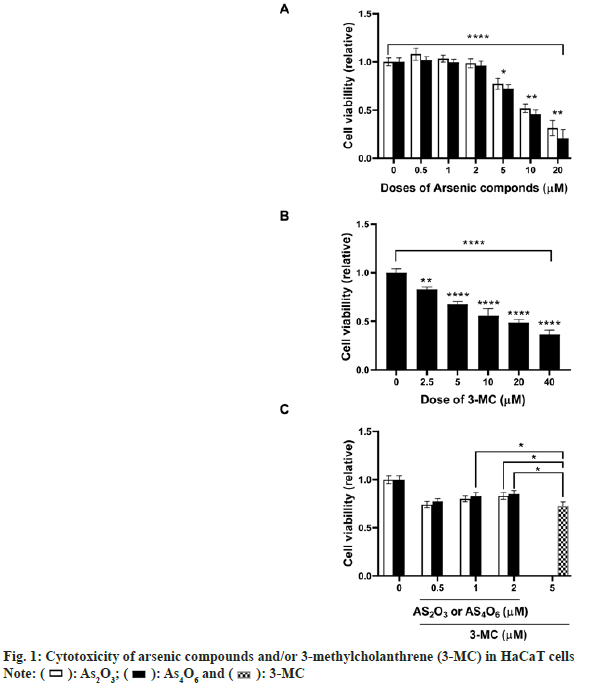
HaCaT cells (5×103 cells/well) were seeded into 96-well plates containing 100 μl of DMEM. After 24 h of incubation, cells were treated with varying concentrations of arsenic compounds (As4O6 or As2O3, 0.5-20 μM), 3-MC (2.5-40 μM), or arsenic (As2O3, 0.5-2 μM) and 3-MC (5 μM) together. Following 24 h incubation, 20 μl of MTT solution was added to each well and the cells were further incubated for 4 h at 37°. The cell media were replaced with 100 μl of DMSO per well. The plate was shaken for 10 s, and then optical density was measured at 570 nm using an enzyme linked immunosorbent assay reader (Spectromax 250, Molecular Devices, CA, USA). The growth inhibition rate (%) was calculated as follows:
Cell viability (%)=[A570 nm absorbance of treated cells/A570 nm absorbance of control cells]×100
Reverse transcription-Polymerase Chain Reaction (RT-PCR) analysis:
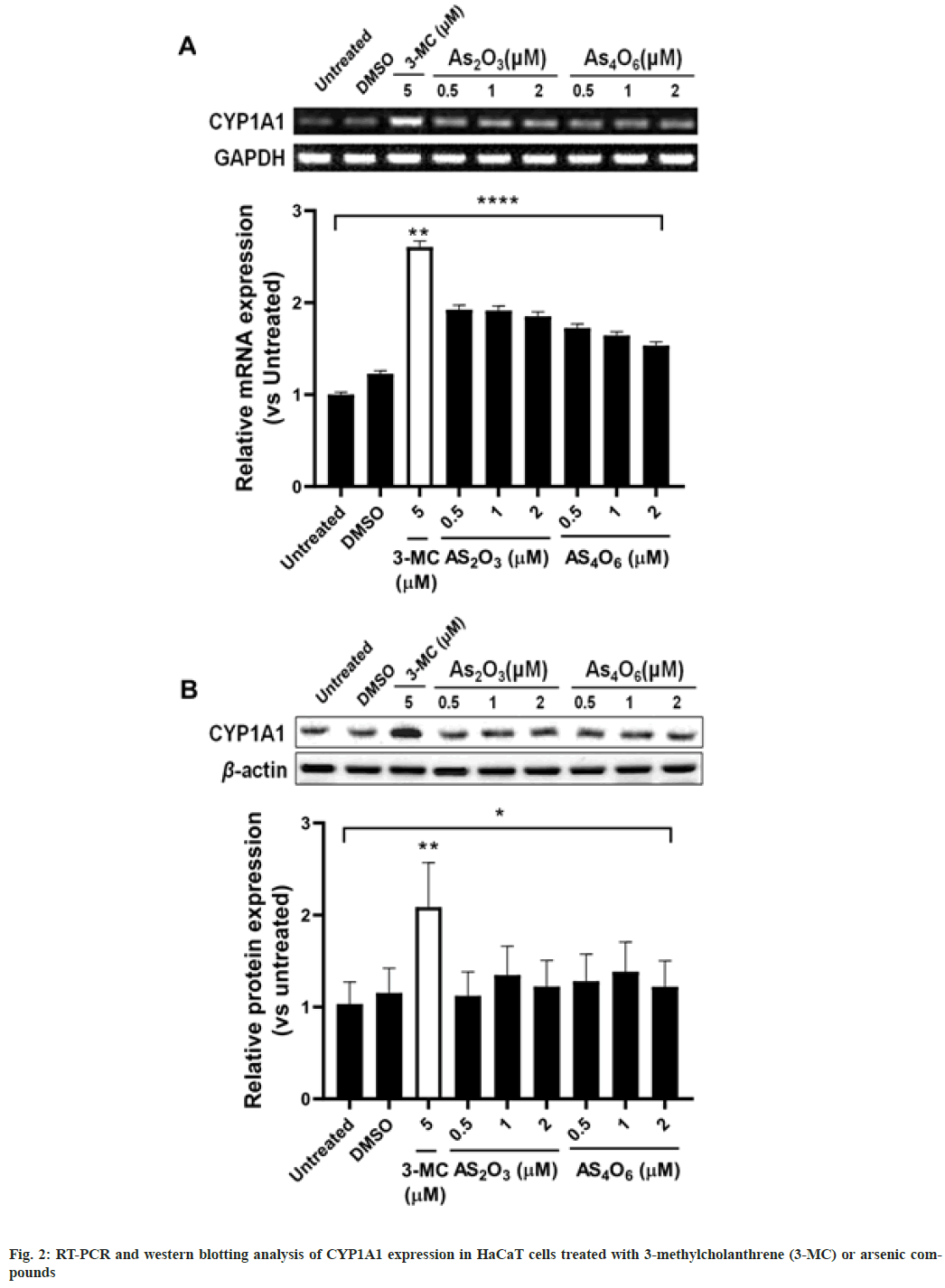
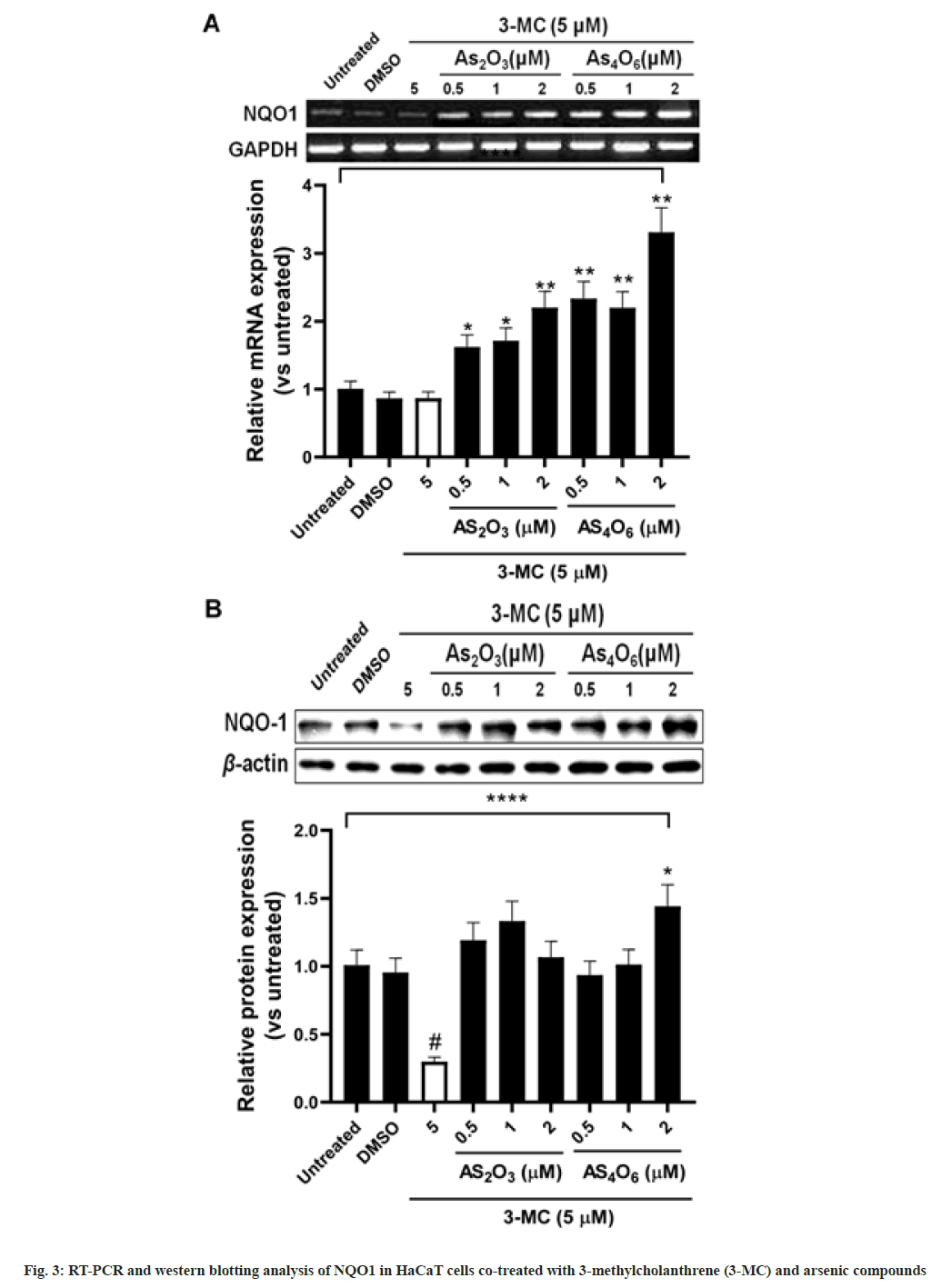
The expression of CYP1A1 and Nicotinamide adenine dinucleotide phosphate Quinone Oxidoreductase 1 (NQO1) at messenger Ribonucleic acid (mRNA) levels was detected by RT-PCR. Total RNA was extracted from HaCaT cells using TRIzol (Invitrogen, Carlsbad, USA) according to the manufacturers’ protocol. Briefly, 1 ml of TRIzol reagent was added to HaCaT cells and incubated for 5 min at room temperature to permit the complete dissociation of nucleoprotein complex. Then 0.2 ml of chloroform (Sigma, MO, USA) was added, samples were vigorously shaken for 15 s, incubated for 2-3 min and centrifuged at 12 000 rpm for 15 min at 4°. Total RNA in the upper aqueous phase was precipitated by mixing the solution with twice the volume of isopropanol (Sigma). The mixture was then incubated for 1 h at -70° and centrifuged at 12 000 rpm for 10 min at 4°. The pellet was washed with 75 % ethanol, dried, and dissolved in RNase-free water. The concentration of the total RNA was estimated by measuring the absorbance at 260 nm using NanoDrop VD-1000 UV/Vis spectrophotometer (NanoDrop, MA, USA).
The Complementary Deoxyribonucleic Acid (cDNA) was synthesized from 1 μg of the total RNA and 1 μM of oligonucleotide primers (oligo-dT15) using the M-MLV reverse transcriptase enzyme (Promega, CO, USA). Using the recombinant Taq DNA polymerase kit (Takara, Shiga, Japan), cDNA was amplified in a final volume of 20 μl containing 2 μg of the firststrand cDNA and 10 pmol/μl of the forward and reverse primers. The following primers were used for the amplification of the genes; CYP1A1, 5'-TGGATGAGAACGCCAATGTC-3' (forward) and 5'-TGGGTTGACCCATAGCTTCT-3' (reverse); NQO1, 5'-AGGCTGGTTTGAGCGAGT-3' (forward) and 5'-TTGAATTCGGGCGTCTGCT-3' (reverse); Glyceraldehyde-3 Phosphate Dehydrogenase (GAPDH), 5'-ACCACCATGGAGAAGGCTGG-3' (sense) and 5'-CTCAGTGTAGCCCAGGATGC-3' (anti sense). GAPDH was used as the internal control for estimating the relative mRNA levels of CYP1A1 and NQO1 genes. Amplification was performed in a thermal cycler (PTC-100, MJ research, USA) with the following settings: 94° for 5 min followed by 94° for 30 s, 60° (CYP1A1 and NQO1) or 56° (GAPDH) for 30 s, and 72° for 1 min. The expected product sizes of the genes were 392 bp (CYP1A1), 286 bp (NQO1), and 527 bp (GAPDH). DNA size markers (Fermentas, USA) were run in parallel to validate the predicted sizes of the amplified bands. The densities of the amplified bands were analyzed using an Image Documentation System (GelDoc 2000; Bio-Rad, USA) with image analysis software (Quantity One; Bio-Rad, USA).
Western blotting assay for detecting protein expression:
HaCaT cells were treated with arsenic compounds (As4O6 or As2O3, 0.5-2 μM), 3-MC (5 μM) or together with arsenic compounds (0.5-2 μM) and 3-MC (5 μM) for 24 h. The cell lysates (approximately 30 μg of protein) were separated in 12 % polyacrylamide sodium dodecyl sulfate gel electrophoresis and transferred onto the nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). The membranes were then treated with blocking buffer (5 % skim milk and 0.1 % Tween 20 in PBS, pH 7.4) for 1 h at room temperature followed by incubation with primary antibodies (SantaCruz Biotechnology, Inc., CA, USA), such as rabbit polyclonal anti-CYP1A1 (1:1000), mouse monoclonal anti-NQO1 (1:1000) and anti-β-actin (1:5000) in a blocking buffer solution overnight at 4°. Following the incubation, the membranes were probed with horseradish peroxidase-conjugated anti-rabbit Immunoglobulin G antibody (1:5000) in PBS-T buffer (0.05 % Tween 20 and 5 % skim milk powder) for 1 h at room temperature. The proteins were detected by an enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK) and the bands were visualized by autoradiography using X-ray film (Amersham). The densities of protein bands were analyzed using an Image Documentation System (GelDoc 2000; Bio-Rad, USA) with image analysis software (Quantity One; Bio-Rad, USA).
Statistical analyses:
Independent t-test was used for the comparison between two groups, whereas one-way analysis of variance was used for the comparison among three and more groups. Statistical significance was defined as p<0.05 or p<0.01. The statistical tests were performed using IBM SPSS statistics version 25 (SPSS, Chicago, IL, USA). All the results are expressed as mean±standard deviation.
To investigate whether As4O6 could attenuate 3-MC-induced cytotoxicity in human keratinocytes, the HaCaT cells were treated with increasing concentrations of As4O6, As2O3, 3-MC alone or with arsenic compounds and 3-MC in combination for 24 h. As shown in fig. 1, both As4O6 and As2O3 had no effect on the growth of HaCaT cells at a concentration of <2 μM. However, the growth of HaCaT cells was found to be attenuated by 3-MC in a dose-dependent manner. When the cells were co-treated with arsenic compounds and 3-MC, the arsenic compounds significantly decreased 3-MCinduced cytotoxicity in HaCaT cells. The cell viability was shown to be significantly higher in cultures treated with >1 μM of As4O6 (p<0.05) or >2 μM of As2O3 (p<0.05) than those treated with 3-MC.
We examined the effect of arsenic compounds (As4O6 and As2O3) and 3-MC on the expression of CYP1A1 at mRNA and protein levels, fig. 2. The expression of CYP1A1 was shown to be significantly induced at both mRNA (p<0.01, fig. 2A), and protein levels (p<0.01) in HaCaT cells treated with 3-MC (5 μM) compared to that of untreated cells. However, treatment with the arsenic compounds had no significant change in the expression of CYP1A1. Further, we investigated whether the co-treatment of HaCaT cells with arsenic compounds and 3-MC had any effect on CYP1A1 expression. The mRNA expression of CYP1A1 was significantly higher in cells co-treated with 3-MC and As2O3 (<1 μM, p<0.01) or As4O6 (<0.5 μM, p<0.01) than in the untreated cells (fig. 3). However, no significant increase in CYP1A1 expression was observed when the cells were co-treated with 3-MC and higher concentrations of As2O3 (>2 μM) or As4O6 (>1 μM), indicating that 3-MCinduced CYP1A1 mRNA expression is inhibited by higher concentrations of arsenic compounds. Similarly, 3-MC-induced CYP1A1 protein expression was inhibited by arsenic compounds in a dose-dependent manner. Treatment with >1 μM of As4O6 significantly down-regulated 3-MC-induced CYP1A1 protein expression (p<0.05).
We investigated the expression of NQO1 at mRNA and protein levels in HaCaT cells cotreated with arsenic compounds and 3-MC (fig. 4). As4O6 and As2O3, respectively, at concentrations of >0.5 and 2 μM significantly increased the mRNA expression of NQO1 (p<0.01). However, the mRNA expression of NQO1 was significantly down-regulated by 3-MC (p<0.05). When the HaCaT cells were co-treated with 3-MC and arsenic compounds, a significant increase in NQO1 was observed by As2O3 or As4O6 in a dose-dependent manner. Further, the expression of NQO1 protein inhibited by 3-MC was recovered by co-treatment with 3-MC and arsenic compounds. However, the increase in NQO1 protein expression by arsenic compounds was not dose-dependent, which was in contrast to that of mRNA expression. The NQO1 protein expression inhibited by 3-MC was significantly up-regulated (p<0.05) by 2 μM of As4O6 treatment.
Both arsenic and 3-MC are well-documented hazardous substances. Arsenic compounds have been widely accepted as chemotherapeutic drugs; they trigger apoptosis in tumor cells. However, not all cell types show the same response to arsenic compounds due to the heterogeneity of their cellular susceptibility to these cytotoxic agents[24-26].
The interactions between arsenic compounds and 3-MC are not well defined. Further, chemicals usually reciprocally affect each other by altering their activation and/or detoxification[27]. Both simultaneous and sequential exposure to arsenic and 3-MC potentially occurs in human populations through contaminated drinking water and coal. The aim of the present study was to examine the potential toxicological interactions between arsenic and 3-MC.
To the best of our knowledge, this is the first study to demonstrate the ability of a new arsenic compound, As4O6 in attenuating carcinogen toxicity. As2O3 and As4O6 have been shown to reduce the growth of various solid and hematological tumors[5,28,29]. However, relatively little is known about various bioactivities of As4O6, including its role as an antitumor agent and in detoxification. Our results showed that As4O6 along with As2O3 participate in a sequential detoxification process in HaCaT cells.
CYP1A1 is a major phase I metabolic enzyme involved in the bioactivation of PAHs into mutagenic and carcinogenic metabolites[30,31]. Owing to its low level expression in humans, the significance of CYP1A1 in carcinogenesis remains debated. Among the xenobiotic metabolizing enzymes, CYP1A1 has a distinct substrate specificity, and it bioactivates a number of procarcinogens and toxicants[32]. Exposure to PAHs leads to the induction of CYP1A1 in humans[33,34]. Epidemiology studies showed that individuals with a high level of CYP1A1 expression are at a greater risk of cancer development[35,36]. Further, diverse metalloids have been reported to induce NQO1 expression at the transcriptional level through the activation of antioxidant response element proteins[37,38]. NQO1 decreases the toxicity of various metabolites by modifying their redox status that is then excreted out of the cells via transport proteins[39]. The effects of arsenic compounds on CYP1A1 expression in our study are in agreement with those reported in arsenite treated primary rat and human hepatocytes, as well as HepG2 cells co-treated with various PAHs[22,40,41]. However, arsenite was not found to prevent CYP1A1 induction in the mouse Hepa-1 and the human lung adenocarcinoma CL2 cell lines[42,43]. Furthermore, an increase in 2,3,7,8-tetrachlorodibenzo-p-dioxin-dependent CYP1A1 mRNA levels and a decrease in CYP1A1 enzyme activity was observed in Hepa1c1c7 cells pretreated with arsenite[44]. Another study showed a significant reduction (45 %) in CYP1A1 mRNA levels in HepG2 hepatoma cells co-treated with aromatic hydrocarbon receptor ligands and arsenic[45]. Thus, these data suggest that the cellular effect of arsenic involves complex mechanisms and could be restricted to specific species and/or organs.
A decrease in the activity of carcinogens due to the modulation of the CYP1A1 enzyme has been proposed as a possible chemopreventive mechanism. Our results showed that As2O3 and As4O6 had no effect on the expression of CYP1A1 in HaCaT cells. Further, As4O6 was more effective in suppressing 3-MC-induced CYP1A1 expression in vitro than As2O3. Additionally, CYP1A1 protein expression was found to be reduced in cells co-treated with 3-MC and >1 μM of As4O6. Currently, there are no studies supporting these results. However, we aim to perform further studies exploring different protective mechanisms associated with arsenic compounds As2O3 and As4O6 in HaCaT cells.
In particular, the mRNA expression of NQO1, associated with the detoxification of carcinogens, was found to be significantly higher in As4O6- treated than in As2O3-treated cells. Further, the down-regulation of NQO1 by 3-MC at mRNA and protein levels was rescued by co-treatment with As2O3 or As4O6 and 3-MC in HaCaT cells. These data indicate that As4O6 could be a more potent detoxicant in human keratinocytes owing to its role in decreasing CYP1A1 expression and increasing NQO1 expression. NQO1 plays a role in cancer prevention and acts as a therapeutic target for a few anticancer drugs[46,47]. Several human NQO genes have been identified; however, NQO1 is considered to be the most important for the prevention of carcinogen-induced tumors[46,48]. It is ubiquitous in nature; however, its expression levels vary in different human tissues[49]. Further, as shown previously, NQO1 is regulated by other signaling pathways as well, specifically those involving oxidative stress[50]. The dioxin induction of the human NQO1 gene has been reported to be mediated by antioxidant responsive element proteins[51]. Furthermore, an increase in the expression of NQO1 and activity of phase I enzymes ensures either the reduction of oxidized intermediates or the addition of hydrophilic groups to these intermediates and thus their elimination[39]. It has been reported that arsenic disrupts the Nrf2/Keap1 complex and recruits Nrf2/Maf to ARE, which in turn induces NQO1 expression[52]. Further, the long isoforms of NRF1 are associated with the transcriptional regulation of a few antioxidant response element containing genes, contributing to arsenic induced antioxidant response as shown in human keratinocytes treated with inorganic arsenic[53]. In the future, we aim to study the mechanisms associated with the protective effects of arsenic compounds against genomic alterations and mutations induced by 3-MC in HaCaT cells.
In conclusion, our study suggests that the arsenic compounds As2O3 and As4O6 prevent 3-MC-induced cytotoxicity in HaCaT human keratocytes by decreasing CYP1A1 expression and increasing NQO1 expression. The results of the current study may provide a novel insight into their protective effects against various carcinogens.
Acknowledgements:
We thank Dr. Woong-Shick Ahn and Mr. Il- Ju Bae for supporting technical assistance and experimental processes.
Conflict of interests:
The authors declared no conflict of interests.
References
- Yu H. Environmental carcinogenic polycyclic aromatic hydrocarbons: Photochemistry and phototoxicity. J Environ Sci Health Part C 2002;20(2):149-83.
[Crossref] [Google Scholar] [PubMed]
- McConville MJ, Ralph SA. Chronic arsenic exposure and microbial drug resistance. Proc Natl Acad Sci 2013;110(49):19666-7.
[Crossref] [Google Scholar] [PubMed]
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood J Ame Soc Hematol 1997;89(9):3354-60.
[Crossref] [Google Scholar] [PubMed]
- Soignet SL. Clinical experience of arsenic trioxide in relapsed acute promyelocytic leukemia. Oncologist 2001;6(S2):11-6.
[Crossref] [Google Scholar] [PubMed]
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol 2001;19(18):3852-60.
[Crossref] [Google Scholar] [PubMed]
- Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. New Engl J Med 1998;339(19):1341-8.
[Crossref] [Google Scholar] [PubMed]
- Zhao WL, Chen SJ, Shen Y, Xu L, Cai X, Chen GQ, et al. Treatment of acute promyelocytic leukemia with arsenic trioxide: Clinical and basic studies. Leukemia Lymphoma 2001;42(6):1265-73.
[Crossref] [Google Scholar] [PubMed]
- Gregory J, Feusner J. Acute promyelocytic leukemia in childhood. Curr Oncol Rep 2009;11(6):439-45.
- Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S, et al. Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: Role of Akt and nuclear factor-κB. Cancer Lett 2011;301(1):75-84.
[Crossref] [Google Scholar] [PubMed]
- Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: Overview of the national cancer institute cooperative research and development studies. Oncologist 2001;6(S2):22-8.
[Crossref] [Google Scholar] [PubMed]
- Wu DD, Xiao YF, Geng Y, Hou J. Antitumor effect and mechanisms of arsenic trioxide on subcutaneously implanted human gastric cancer in nude mice. Cancer Genet Cytogenet 2010;198(2):90-6.
[Crossref] [Google Scholar] [PubMed]
- Alex AA, Ganesan S, Palani HK, Balasundaram N, David S, Lakshmi KM, et al. Arsenic trioxide enhances the NK cell cytotoxicity against acute promyelocytic leukemia while simultaneously inhibiting its bio-genesis. Front Immunol 2018;9:1357.
[Crossref] [Google Scholar] [PubMed]
- Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist 2001;6(S2):3-10.
[Crossref] [Google Scholar] [PubMed]
- Sun Z, Li M, Bai L, Fu J, Lu J, Wu M, et al. Arsenic trioxide inhibits angiogenesis in vitro and in vivo by upregulating FoxO3a. Toxicol Lett 2019;315:1-8.
[Crossref] [Google Scholar] [PubMed]
- Park IC, Park MJ, Woo SH, Lee HC, An S, Gwak HS, et al. Tetraarsenic oxide induces apoptosis in U937 leukemic cells through a reactive oxygen species-dependent pathway. Int J Oncol 2003;23(4):943-8.
[Crossref] [Google Scholar] [PubMed]
- Šlejkovec Z, Falnoga I, van Elteren JT. Arsenic trioxide vs. tetraarsenic oxide in biomedical research: Misunderstandings and misinterpretations. Biometals 2012;25(1):231-5.
[Crossref] [Google Scholar] [PubMed]
- Park MJ, Park IC, Bae IJ, Seo KM, Lee SH, Hong SI, et al. Tetraarsenic oxide, a novel orally administrable angiogenesis inhibitor. Int J Oncol 2003;22(6):1271-6.
[Crossref] [Google Scholar] [PubMed]
- Hu Y, Li J, Lou B, Wu R, Wang G, Lu C, et al. The role of reactive oxygen species in arsenic toxicity. Biomolecules 2020;10(2):240.
[Crossref] [Google Scholar] [PubMed]
- Shen S, Li XF, Cullen WR, Weinfeld M, Le XC. Arsenic binding to proteins. Chem Rev 2013;113(10):7769-92.
- Rajpoot DS, Prakash A, Mandil R, Rahal A, Garg SK. Differential modulation of xenobiotic-metabolizing enzymes in rats following single and concurrent exposure to chlorpyrifos, arsenic, and ascorbic acid. J Toxicol Environ Health Part A 2013;76(24):1354-65.
[Crossref] [Google Scholar] [PubMed]
- Mondal P, Shaw P, Bandyopadhyay A, Bhowmik AD, Chakraborty A, Sudarshan M, et al. Mixture effect of arsenic and fluoride at environmentally relevant concentrations in zebrafish (Danio rerio) liver: Expression pattern of Nrf2 and related xenobiotic metabolizing enzymes. Aquatic Toxicol 2019;213:105219.
[Crossref] [Google Scholar] [PubMed]
- Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang QY, et al. Effect of metals on polycyclic aromatic hydrocarbon induction of CYP1A1 and CYP1A2 in human hepatocyte cultures. Toxicol Appl Pharmacol 2001;170(2):93-103.
[Crossref] [Google Scholar] [PubMed]
- Chao HR, Tsou TC, Li LA, Tsai FY, Wang YF, Tsai CH, et al. Arsenic inhibits induction of cytochrome P450 1A1 by 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin in human hepatoma cells. J Hazardous Mater 2006;137(2):716-22.
[Crossref] [Google Scholar] [PubMed]
- Abudoureyimu A, Muhemaitibake A. Arsenic trioxide regulates gastric cancer cell apoptosis by mediating cAMP. Eur Rev Med Pharmacol Sci 2017;21(3):612-7.
[Google Scholar] [PubMed]
- Hu J, Dong Y, Ding L, Dong Y, Wu Z, Wang W, et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduction Targeted Ther 2019;4(1):28.
- Moon Y, Park G, Kim Y, Kim M, Lim J, Pai SH, et al. Arsenic trioxide (As2O3) sensitivity of carcinoma cell lines and cancer cells from patients with carcinomatosis peritonei. Ann Clin Lab Sci 2004;34(3):271-6.
[Google Scholar] [PubMed]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol 2017;68:3-3.
[Crossref] [Google Scholar] [PubMed]
- Griffin RJ, Lee SH, Rood KL, Stewart MJ, Lyons JC, Lew YS, et al. Use of arsenic trioxide as an antivascular and thermosensitizing agent in solid tumors. Neoplasia 2000;2(6):555-60.
[Crossref] [Google Scholar] [PubMed]
- Park SG, Jung JJ, Won HJ, Kang MS, Seo SK, Choi IW, et al. Tetra-arsenic oxide (Tetras) enhances radiation sensitivity of solid tumors by anti-vascular effect. Cancer Lett 2009;277(2):212-7.
[Crossref] [Google Scholar] [PubMed]
- Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: A bio-historical perspective with updates. Carcinogenesis 2001;22(12):1903-30.
[Crossref] [Google Scholar] [PubMed]
- Wohak LE, Krais AM, Kucab JE, Stertmann J, Øvrebø S, Seidel A, et al. Carcinogenic polycyclic aromatic hydrocarbons induce CYP1A1 in human cells via a p53-dependent mechanism. Arch Toxicol 2016;90(2):291-304.
[Crossref] [Google Scholar] [PubMed]
- Munro AW. Cytochrome P450 1A1 opens up to new substrates. J Biol Chem 2018;293(50):19211-2.
[Crossref] [Google Scholar] [PubMed]
- Wincent E, Le Bihanic F, Dreij K. Induction and inhibition of human cytochrome P4501 by oxygenated polycyclic aromatic hydrocarbons. Toxicol Res 2016;5(3):788-99.
- Moorthy B, Chu C, Carlin DJ. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol Sci 2015;145(1):5-15.
[Crossref] [Google Scholar] [PubMed]
- Alexandrov K, Cascorbi I, Rojas M, Bouvier G, Kriek E, Bartsch H. CYP1A1 and GSTM1 genotypes affect benzo [a] pyrene DNA adducts in smokers' lung: Comparison with aromatic/hydrophobic adduct formation. Carcinogenesis 2002;23(12):1969-77.
[Crossref] [Google Scholar] [PubMed]
- Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009;9:187.
- Tripathi DN, Jena GB. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: Role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res 2010;696(1):69-80.
[Crossref] [Google Scholar] [PubMed]
- Jaiswal AK. Regulation of genes encoding NAD (P) H: Quinone oxidoreductases. Free Radic Biol Med 2000;29(3-4):254-62.
[Crossref] [Google Scholar] [PubMed]
- Marchand A, Barouki R, Garlatti M. Regulation of NAD (P) H: Quinone oxidoreductase 1 gene expression by CYP1A1 activity. Mol Pharmacol 2004;65(4):1029-37.
[Crossref] [Google Scholar] [PubMed]
- Jacobs JM, Nichols CE, Andrew AS, Marek DE, Wood SG, Sinclair PR, et al. Effect of arsenite on induction of CYP1A, CYP2B, and CYP3A in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol 1999;157(1):51-9.
[Crossref] [Google Scholar] [PubMed]
- Vakharia DD, Liu N, Pause R, Fasco M, Bessette E, Zhang QY, et al. Polycyclic aromatic hydrocarbon/metal mixtures: Effect on PAH induction of CYP1A1 in human HEPG2 cells. Drug Metabol Dispos 2001;29(7):999-1006.
[Google Scholar] [PubMed]
- Ho IC, Lee TC. Arsenite pretreatment attenuates benzo [a] pyrene cytotoxicity in a human lung adenocarcinoma cell line by decreasing cyclooxygenase-2 levels. J Toxicol Environ Health Part A. 2002;65(3-4):245-63.
[Crossref] [Google Scholar] [PubMed]
- Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo [a] pyrene genotoxicity. Mutat Res 2002;517(1-2):101-11.
[Crossref] [Google Scholar] [PubMed]
- Elbekai RH, El-Kadi AO. Modulation of aryl hydrocarbon receptor-regulated gene expression by arsenite, cadmium, and chromium. Toxicology 2004;202(3):249-69.
[Crossref] [Google Scholar] [PubMed]
- Bessette EE, Fasco MJ, Pentecost BT, Kaminsky LS. Mechanisms of arsenite-mediated decreases in benzo [k] fluoranthene-induced human cytochrome P4501A1 levels in HepG2 cells. Drug Metabol Dispos 2005;33(3):312-20.
[Crossref] [Google Scholar] [PubMed]
- Oh ET, Park HJ. Implications of NQO1 in cancer therapy. BMB Rep 2015;48(11):609.
[Crossref] [Google Scholar] [PubMed]
- Zhang K, Chen D, Ma K, Wu X, Hao H, Jiang S. NAD (P) H: Quinone oxidoreductase 1 (NQO1) as a therapeutic and diagnostic target in cancer. J Med Chem 2018;61(16):6983-7003.
[Crossref] [Google Scholar] [PubMed]
- Cui X, Li L, Yan G, Meng K, Lin Z, Nan Y, Jin G, Li C. High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer 2015;15(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Belinsky M, Jaiswal AK. NAD (P) H: Quinone oxidoreductase 1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metast Rev 1993;12(2):103-17.
[Crossref] [Google Scholar] [PubMed]
- Dhakshinamoorthy S, Long II DJ, Jaiswal AK. Antioxidant regulation of genes encoding enzymes that detoxify xenobiotics and carcinogens. Curr Top Cell Regul 2001;36:201-16.
[Crossref] [Google Scholar] [PubMed]
- Radjendirane V, Jaiswal AK. Antioxidant response element-mediated 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) induction of human NAD (P) H: Quinone oxidoreductase 1 gene expression. Biochem Pharmacol 1999;58(10):1649-55.
[Crossref] [Google Scholar] [PubMed]
- He X, Chen MG, Lin GX, Ma Q. Arsenic induces NAD (P) H-quinone oxidoreductase I by disrupting the Nrf2· Keap1· Cul3 complex and recruiting Nrf2· Maf to the antioxidant response element enhancer. J Biol Chem 2006;281(33):23620-31.
[Crossref] [Google Scholar] [PubMed]
- Zhao R, Hou Y, Xue P, Woods CG, Fu J, Feng B, et al. Long isoforms of NRF1 contribute to arsenic-induced antioxidant response in human keratinocytes. Environ Health Perspect 2011;119(1):56-62.
[Crossref] [Google Scholar] [PubMed]
 ): As2O3; (
): As2O3; ( ): As4O6 and (
): As4O6 and ( ): 3-MC
): 3-MC