- *Corresponding Author:
- D. H. Ahn
Department of Food Science and Technology and Institute of Food Science,
Pukyong National University,
Busan 48513
E-mail: dhahn@pknu.ac.kr
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “54-63” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study investigated the effect of Chondria crassicaulis ethanol extract on degranulation of rat basophilic leukemia-2H3 cells and 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in Bagg Albino/c mice. General properties of Chondria crassicaulis (proximate analysis, total phenol and total flavonoid) were measured; moreover, beta-hexosaminidase release and cytotoxicity in rat basophilic leukemia-2H3 cells, severity of skin dermatitis, production of cytokines, and total immunoglobulin E content in an atopic dermatitis-like mouse model were estimated. Chondria crassicaulis ethanol extract decreased the secretion of beta-hexosaminidase without cytotoxicity in rat basophilic leukemia-2H3 cells and decreased the total immunoglobulin E content in serum. In addition, Chondria crassicaulis ethanol extract decreased the production of interleukin-4 and interleukin-5 in mouse splenocytes, whereas significantly increased the level of interferon gamma. Furthermore, Chondria crassicaulis ethanol extract alleviated skin lesions induced by atopic dermatitis without causing toxicity to mouse splenocytes. Therefore, the present study findings suggest that Chondria crassicaulis ethanol extract can relieve atopic dermatitis by regulating the activity of type 1 T helper and type 2 T helper cells that mediate cellular immune response and it can be used as an effective alternative therapy for atopic dermatitis.
Keywords
Chondria crassicaulis, atopic dermatitis, rat basophilic leukemia-2H3 cells, splenocytes, anti-atopic agent
Although the cause of Atopic Dermatitis (AD) has not been clearly elucidated, it is a multifactorial disease attributable to genetic, immunological, pharmacological, physiological and biochemical factors. It has been observed that patients with AD have increased expression of Langerhans cell surface receptors, which specifically recognize foreign antigens and deliver them to T lymphocytes, important immune cells that induce an immune response. T cells are activated and differentiated to Type 2 helper T (Th2) cells by Langerhans, and AD symptoms are caused and exacerbated by excessive secretion of immunological mediators involved in allergic immune responses such as Interleukin (IL)-4, IL-13 and IL-5.
In addition, naturally present antibacterial peptides on the skin of patients with AD are less functional than healthy individuals making it more susceptible to infections caused by bacteria, viruses and fungi. Increase in skin infection exacerbates AD and the symptomatic dry skin causes and worsens itching and consequently develops thick skin and wrinkles. This causes abnormality in the skin barrier and moisture in the body is released through the epidermis to the outside; the epidermal cells proliferate and the stratum corneum thickens due to changes in the ion concentration such as calcium in the epidermal cells and increase in the level of immune mediators such as cytokines.
The treatment strategy for AD caused by an immune response involves removal of antigens that cause irritation, which is complicated and difficult; therefore, it is usually treated with antibiotics, topical steroids and topical calcineurin inhibitors. However, there are several safety issues associated with the long-term use of these drugs[1]. Therefore, this study explored anti-atopic agent with high safety profile from Chondria crassicaulis (C. crassicaulis), a natural resource.
C. crassicaulisis red algal seaweed belonging to the family Rhodomelaceae; it inhabits in Busan, the southern coast of Korea and Jeju Island. C. crassicaulis is magenta or purplish-brown and has cartilaginous structure and slightly flat rod-shaped form with many branches in opposite or alternate forms at the edge[2]. C. crassicaulis possess antioxidant, antibacterial[3] and anticancer activities[4,5] showed that ethanol extract of C. crassicaulis modulated the expression of cyclooxygenase-2 and inducible nitric oxide synthase, and secretion of nitric oxide and pro-inflammatory cytokines via regulation of Nuclear Factor kappa B (NF-κB) activity. In addition, a recent study demonstrated anti-inflammatory effect of C. crassicaulis Ethanol Extract (CCEE) through inhibition of Mitogen-Activated Protein Kinase (MAPK) and NF-κB signaling in macrophages[6]. Kim et al.[7] performed structural analysis and reported the properties of antioxidants isolated from C. crassicaulis.
In this study, we confirmed the anti-atopic effect shown through the regulation of the activity of Type 1 helper T (Th1) cytokines and Th2 cytokines by using the CCEE and investigated the therapeutic use of C. crassicaulis in AD.
Materials and Methods
Preparation of CCEE:
C. crassicaulis was collected from Yeonhwa-ri, Busan in 2016. The seaweed was washed with fresh water to remove foreign substances, freeze-dried, powdered, vacuum-packed, storage at -20° until further use.
Dry powder of C. crassicaulis was extracted using 95 % ethanol (10 times) and stirring at room temperature for 24 h using a stirrer (H-0820, Dongwon Science Co., Busan, Korea). After centrifugation at 1977×g for 10 min using a centrifuge (UNION 32R, Hanil Co., Incheon, Korea), the supernatant was collected and the residue was extracted twice in the same manner. The extracted supernatant was concentrated at 37° using a vacuum concentrator (RE200, Yamoto Co., Tokyo, Japan); the concentrated and dried samples were stored at -20° until further use.
Animals and treatment:
4 w old male Bagg Albino (BALB)/c mice used in this study were purchased from Orient Bio (Orient Co., Seongnam, Korea) and pre-bred for 6 w in an animal room maintained at a temperature of 20°±2°, humidity of 50 %±10 % and 12/12 h light-dark cycle. All experimental protocols and animal care were performed according to the rules and regulations of the Animal Ethics Committee of Pukyong National University.
AD was induced by the method described by Ahn et al.[8] with slight modification. After shaving the back of 4 w old male BALB/c mice, they were left for 24 h to cure the wounds. After 24 h, 200 μl of 1 % (w/v) 2,4-Dinitrochlorobenzene (DNCB; Sigma Chemical Co., St. Louis, Missouri (MO), United States of America (USA)) dissolved in a mixture of acetone and olive oil (3:1) was applied to the back of the ears and back of the mice three times a week. After a week, 200 μl of 0.3 % (w/v) DNCB was evenly applied once a day to the same area. Further, 200 μl of the CCEE sample (30 mg/ml) was evenly applied to the back of the ears and back of the mice for 2 w, once a day at 12 h interval from 0.3 % (w/v) DNCB solution application.
Measurement of proximate composition contents of C. crassicaulis:
Proximate analysis was performed according to the Association of Official Analytical Chemists (AOAC) method [9]. The sample was dried at 105° to determine the moisture content; ash content was measured at 550°. In addition, protein content was estimated using the Kjeldahl method (BUCHI Distillation Unit K-350/355, Hwashin Instrument Co., Seoul, Korea), and the lipid content was assessed by Soxhlet extraction (EAM4202, Dongwon Science Co., Busan, Korea). All data are expressed as percentage. Carbohydrates were calculated by subtracting the contents of moisture, crude protein, crude lipid and ash from 100. The total sugar content was determined using the method by Dubois et al.[10] with slight modification. Briefly, 1 ml of 25 % Hydrochloric Acid (HCl) and 9 ml of distilled water were added to 0.5 g of the sample, followed by acid decomposition in an aqueous phase at 95°-100° for 2 h and filtration through a filter paper. Using this as a sample, phenol-Sulfuric Acid (H2SO4) method was performed, which involved addition of 5 % phenol and concentrated H2SO4 to the sample and measurement of absorbance (Optical Density (OD)) after 30 min at 490 nm[11]; the total sugar content was calculated using a standard calibration curve of standard D-glucose solution.
Measurement of total polyphenol and flavonoid contents of CCEE:
Total polyphenols and flavonoids present in C. crassicaulis were extracted by adding 50 ml of 75 % ethanol to 1 g of the freeze-dried sample, stirring for 18 h, filtering through Whatman paper and adjusting the volume to 50 ml with ethanol. The total polyphenol content of the CCEE (5 mg/ml) was determined using the Folin-Denis method[12]. The sample (0.5 ml) was added to 6.5 ml of distilled water, then 0.5 ml of Folin-Ciocalteu’s solution was mixed and reacted for 3 min. After completion of the reaction, 1 ml of anhydrous saturated Sodium Carbonate (Na2CO3) solution and distilled water were added to make the total volume to 10 ml; the solution was left at room temperature for 1 h and the OD was measured at 765 nm using Ultraviolet (UV)/visible spectrophotometer (GENESIS 10 UV, Rochester, New York (NY), USA). Total Phenolic Content (TPC) was quantified from the standard curve prepared by the same method using gallic acid as the standard. Total Flavonoid Content (TFC) was determined using the aluminum nitrate method[13] published in the current health functional foods code. In brief, 0.5 ml of the sample (5 mg/ml), 1.5 ml of ethanol, 0.1 ml of 10 % (w/v) aluminum nitrate solution, 0.1 ml of 1 M potassium acetate solution and 2.8 ml of distilled water were mixed and stirred thoroughly, and the mixture was left at room temperature for 40 min. The OD was measured at 415 nm using UV/visible spectrophotometer (GENESIS 10 UV). Distilled water was used as a control solution and the flavonoid content was calculated from the calibration curve prepared using the difference in the OD of the sample and the OD obtained by adding 0.1 ml of distilled water instead of 10 % (w/v) aluminum nitrate solution.
Measurement of pH and color values:
The pH of the CCEE (5 mg/ml) was measured using a pH meter (HM-30V, TOA, Kobe, Japan). The chromaticity of the extract (0.5 mg/ml) was measured using a colorimeter (JC 801 Color technosystem Co., Japan) and repeated three times. The chromaticity of the extract was expressed as the L*, a* and b* values.
Rat Basophilic Leukemia-2H3 (RBL-2H3) cell culture:
RBL-2H3 cells were purchased from the Korea Cell Line Bank (KCLB 40071, Seoul, Korea) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Grand Island, Nebraska, USA) with 10 % inactivated Fetal Bovine Serum (FBS, Hyclone, Logan, Utah, USA) and 1 % penicillin-streptomycin (Hyclone, Logan, UT, USA) at 37° and 5 % Carbon Dioxide (CO2).
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2HTetrazolium Bromide (MTT) assay:
The MTT assay was performed to evaluate the cytotoxicity of the sample. RBL-2H3 cells were seeded in a 96-well plate at a concentration of 1×106 cells/ml and incubated for 20 h; different concentrations (0.1, 1, 10, 50 and 100 μg/ml) of the CCEE sample were added and incubated for 24 h. Then, 5 mg/ml MTT (Sigma- Aldrich Chemical Co., Louis, MO, USA) solution was added and incubated for 2 h. The medium was then discarded and Dimethylsulfoxide (DMSO; Sigma- Aldrich Co., St. Louis, MO, USA) was added to each well and absorbance was measured at 540 nm with a microplate reader (Model 550, Bio-Rad, Richmond, California (CA), USA). The cell proliferation index was calculated using the following formula:
Proliferation index (%)=(Sample OD/Control OD)×100
Beta (β)-hexosaminidase assay:
We used the method described by Hwang et al.[14] to measure the amount of β-hexosaminidase secreted by RBL-2H3 cells. RBL-2H3 cells were stimulated by incubating with 0.5 μg/ml anti-Dinitrophenyl (DNP) Immunoglobulin E (IgE) (Sigma-Aldrich Co., St. Louis, MO, USA) for 20 h in a 24-well microplate (2×105 cells/well) and washed with Piperazine-N,N'-bis(2-Ethanesulfonic acid) (PIPES) buffer (25 mM PIPES, 119 mM Sodium Chloride (NaCl), 5 mM Potassium Chloride (KCl), 5.6 mM glucose, 0.4 mM Magnesium Chloride (MgCl2), 1 mM Calcium Chloride (CaCl2), 40 mM Sodium Hydroxide (NaOH), and 0.1 % Bovine Serum Albumin (BSA); pH 7.2); then CCEE (10-100 μg/ml) or Dexamethasone (DEXA) (1 μM) was added, incubated for 1 h, dispensed 50 μg/ml DNP-BSA (Invitrogen, Life Technologies Co., Oregon, USA), and incubated for 24 h for stimulation. The supernatant (25 μl) of the culture medium and the same amount of 5 μM substrate solution (p-nitrophenyl-N-acetyl-β-D-glucosaminide and 0.2 M sodium citrate buffer; pH 4.5) were mixed and reacted at 37° for 2 h; next, 200 μl of stop solution (0.1 M Na2CO3/Sodium Bicarbonate (NaHCO3); pH 10.0) was dispensed to terminate the reaction. The OD was measured at 405 nm using a microplate reader.
Splenocytes culture:
Splenocytes were isolated using the method described by Mishell et al.[15] with slight modification. On the last day of the experiment, five male BALB/c mice from each experimental group (Normal, DNCB; DNCB only, DEXA; DNCB and DEXA, and CCEE; DNCB and CCEE) were anesthetized with diethyl ether and sacrificed. The spleen was extracted aseptically and washed with Roswell Park Memorial Institute (RPMI) 1640 medium, (Grand Island Biological Company (GIBCO), NY, USA), and then homogenized using a tissue grinder to release cells. After centrifuging the cell suspension at 1800 rpm and 4° for 5 min, the cells were allowed to stand in Red Blood Cell (RBC) lysis buffer (Tris-buffered Ammonium Chloride (NH4Cl); 0.87 % (w/v) NH4Cl; pH 7.2) for 10 min to remove RBCs. Thereafter, the spleen cell suspension was diluted to a concentration of 2×106 cells/ml by adding 10 % (w/v) FBS-RPMI medium and cultured at 37° in a CO2 incubator (MCO-15 AC, Sanyo, Osaka, Japan) for 72 h.
Proliferation of splenocytes:
The splenocytes suspension was seeded in a 96-well plate at a concentration of 2×106 cells/ml and then cultured at 37° in a CO2 incubator for 72 h. After incubation, 5 mg/ml MTT solution was added and the cells were re-cultured for 2 h to induce formazan crystal formation. After centrifugation at 2000 rpm and 4° for 10 min, the supernatant was removed, DMSO was added and a microplate reader was used to measure OD at 540 nm. The proliferation of splenocytes was calculated using the following formula:
Proliferation index (%) = (Sample OD/Control OD)×100.
Measurement of IL-4, IL-5 and Interferon gamma (IFN-γ) secretion in splenocytes:
The cytokines (IL-4, IL-5 and IFN-γ) secreted from splenocytes were measured using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (Mouse ELISA set; BD Biosciences, San Diego, CA, USA). The microplate was captured with anti-mouse IL-4, IL-5 and IFN-γ and coated overnight at 4°. It was washed with Phosphate Buffered Saline-Tween 20 (PBST; 0.01 M PBS and 0.05 % (w/v) Tween 20; pH 7.2), and then blocked at room temperature for 1 h with 10 % (w/v) FBS solution. The plate was then washed with PBST and the culture supernatant was added and reacted for 2 h at room temperature, and the plate was washed again with PBST. Next, diluted biotinylated anti-mouse IL-4, IL-5 and IFN-γ detection antibody and streptavidin-horseradish peroxidase conjugate were dispensed and reacted at room temperature for 1 h. After the reaction, it was washed again with PBST and then O-Phenylenediamine (OPD; Sigma Chemical Co., St. Louis, MO, USA) and citrate buffer (pH 5.0) containing Hydrogen Peroxide (H2O2) was added, followed by a dark reaction at room temperature for 30 min. After terminating the reaction with 2 N H2SO4, the OD was measured at 490 nm using a microplate reader.
Measurement of total IgE in mice serum:
At the end of the experiment, mice were anesthetized with diethyl ether and approximately 1.0 ml of blood was collected from the aorta using a disposable syringe (Sungshim Medical Co., Ltd., Bucheon, Korea). Blood was centrifuged at 10 000 rpm and 4° for 5 min to separate the serum. The separated serum was stored at -20° until further use and the total IgE content in mouse serum was measured using an ELISA kit. Anti-mouse IgE was dispensed on an ELISA microplate and coated overnight at 4°. After washing with PBST, it was blocked with 2 % (w/v) BSA solution (Sigma Chemical Co.) at room temperature for 1 h. After an hour, it was washed with PBST, the culture supernatant was dispensed and reacted for 2 h at room temperature, and the plate was washed again with PBST. Further, diluted biotinylated anti-mouse IgE detection antibody and streptavidin-horseradish peroxidase conjugate were dispensed and reacted at room temperature for 1 h. After the reaction, it was washed again with PBST and then OPD and citrate buffer containing H2O2 were added, followed by a dark reaction at room temperature for 30 min. After terminating the reaction with 2 N H2SO4, the OD was measured at 490 nm using a microplate reader.
Evaluation of severity of skin dermatitis:
After application of the test substances, severity of skin dermatitis was determined visually on d 0, 7 and 14 using a clinical evaluation method commonly used in AD that indicates the degree of severity of AD as the sum of the scores evaluated for each of the following four conditions: Erythema/hemorrhage, scarring/dryness, edema and excoriation/erosion. Each condition was graded as none (0), mild (1), moderate (2), or severe (3), and then summed to give a score between 0 and 15 points[8].
Statistical analysis:
Data are expressed as mean±Standard Error of the Mean (SEM) (n=3). Statistical evaluation was carried out using Analysis of Variance (ANOVA) with Statistical Analytical System (SAS) program (SAS V8.2, SAS Institute Inc., Cary, North Carolina (NC), USA), one way ANOVA method was used to analyze variance and the significance test between survey substances was performed at p<0.05 level by Duncan’s multiple range test.
Results and Discussion
To determine the composition of C. crassicaulis, moisture, ash, crude protein, crude lipid, carbohydrate and total sugar contents were measured (Table 1). The moisture content of the freeze-dried C. crassicaulis powder was found to be 7.59 % on a dry basis, whereas ash, crude protein, crude lipid and total sugar contents were 46.16 %, 19.41 %, 0.82 % and 26.29 mg Glucose Equivalents (GE)/g, respectively. Although we determined the composition of general components in C. crassicaulis used in this experiment, additional research should be carried out because the components of seaweed generally differ depending on the species, production area, and collection period.
| Seaweed | Composition contents (%) | Total sugar (mg GE/g) | TPC (mg GAE/g) | TFC (mg QE/g) | |||
|---|---|---|---|---|---|---|---|
| Crude lipid | Crude protein | Moisture | Ash | ||||
| C. crassicaulis | 0.82±0.09 | 19.41±0.03 | 7.59±0.51 | 46.16±16.09 | 26.29±0.94 | 1.20±0.02 | 0.59±0.00 |
Note: GE: Glucose Equivalents; TPC: Total Phenolic Content; TFC: Total Flavonoid Content; GAE: Gallic Acid Equivalents; QE: Quercetin Equivalent
Table 1: Proximate Composition, TPC and TFC of C. Crassicaulis
Polyphenolic compounds are present in several natural products and are known to exist mainly in vacuoles and cell membranes as natural pigments[16]. Representative polyphenol compounds present in plants include sesamol in sesame oil, gossypol in cottonseed oil and catechin in green tea. They exhibit various physiological effects such as antioxidant, anti-inflammatory and anticancer effects owing to the presence of a phenolic ring that stabilizes free radicals[17-19]. In addition, flavonoids are mainly composed of anthocyanidins, flavanols, flavones, catechins and flavanones, and they have been reported to possess various physiological activities such as antioxidant and antibacterial properties based on their structures [20]. The contents of total polyphenols and total flavonoids in C. crassicaulis are shown in Table 1. The total polyphenol content was 1.20 mg Gallic Acid Equivalents (GAE)/g, which is similar to thatof Laminaria japonica. The TFC was 0.59 mg Quercetin Equivalents (QE)/g, which is similar to the content of Porphyra terera[21]. These results suggest that the total polyphenol and TFC of C. crassicaulis are abundant, similar to those of other seaweeds.
The pH and color values of natural products is an important factor for their use as natural food, since pH affects the contamination and quality of food by being involved in the growth of microorganisms and activity of enzymes [22], and color affects the sensory aspect of the food; therefore, pH and color values of the extract should be considered.
The pH of CCEE (Table 2) was measured as 4.49, which shows weak acidic behavior. Regarding color, the brightness was high and the yellowness was higher than the redness, which might be due to elution of hydrophobic pigment components such as chlorophyll a, c and carotenoids by ethanol extraction of C. crassicaulis[23,24]. Thus, high brightness and weak acidity of CCEE is favorable for its use as a food material and functional food.
| Seaweed | Color | pH | ||
|---|---|---|---|---|
| L* | a* | b* | ||
| C. crassicaulis | 80.01±0.01 | -6.02±0.02 | 38.77±0.09 | 4.49±0.01 |
Table 2: Color and pH Values of CCEE
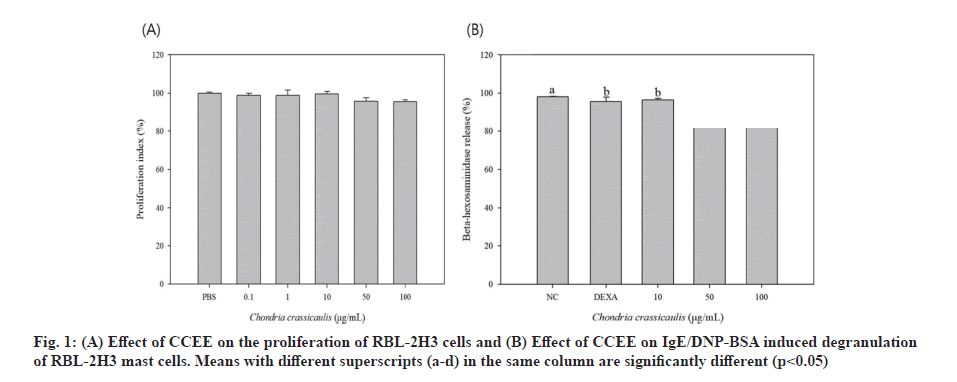
Type 1 allergic reactions are categorized into early and late responses. The early response occurs rapidly within a few minutes and mast cells secrete already produced mediators such as histamine, β-hexosaminidase, serotonin, and mediators of newly synthesized fat components. The late response occurs within a few hours and results in synthesis and secretion of Th2 cytokine to sustain the inflammatory responses[25]. RBL-2H3 cells are mast cells derived from neutrophils of rats and are widely used in research and development of anti-inflammatory and anti-allergic drugs because of the presence of IgE receptors on their surface, similar to human mast cells; moreover, they possess similar characteristics such as cytokine expression by IgE stimulation, histamine release and secretion of inflammatory substances. Fig. 1A shows the results of evaluating the toxicity of CCEE in RBL-2H3 cells. CCEE was nontoxic for RBL-2H3 cells at concentration range of 0.1-100 μg/ml. Therefore, subsequent experiments were performed at CCEE concentration of 100 μg/ml or less, which did not affect cell survival and proliferation.
Histamine secreted by mast cells is a major indication of degranulation in the early allergic response. RBL-2H3 cells exhibit the characteristics of mucosal mast cells and express numerous IgE receptors on their surface[26]. To check the effect of CCEE on IgE/DNP-BSA-induced degranulation of RBL-2H3 cells, it is important to measure the amount of histamine released from mast cells sensitized to IgE. However, the concentration of histamine secreted from mast cells was low and difficult to measure, so an experiment that released β-hexosaminidase by antigen-antibody reaction was performed. β-hexosaminidase, a lysosomal enzyme, is used as an indicator of degranulation because it is present in histamine-containing granules of basophils or mast cells and secreted from the cell sensitized by an allergic reaction. DEXA, used as a control, significantly reduced the secretion of β-hexosaminidase activated by DNP-IgE and DNP-BSA, and the experimental group treated with CCEE at different concentrations also showed a significant decrease (fig. 1B). Atopic diseases are result of hypersensitivity reactions caused by an abnormal immunological mechanism. In the early stages of the disease, chemical mediators such as histamine and proteoglycan are released from the stored granules in basophils and mast cells mainly by antigen stimulation and chronic inflammatory atopic and allergic reactions are induced by promoting the synthesis of arachidonic acid metabolites or cytokines (IL-4, Tumour Necrosis Factor alpha (TNF-α) and granulocyte-macrophage colony-stimulating factor). This result was similar to that of Hwang et al.[14], who found that green tea extract reversed the increase in β-hexosaminidase in a house dust mite antigen-induced atopic mouse model. Therefore, these results suggest that CCEE inhibits the initial atopic inflammatory response in mast cells.
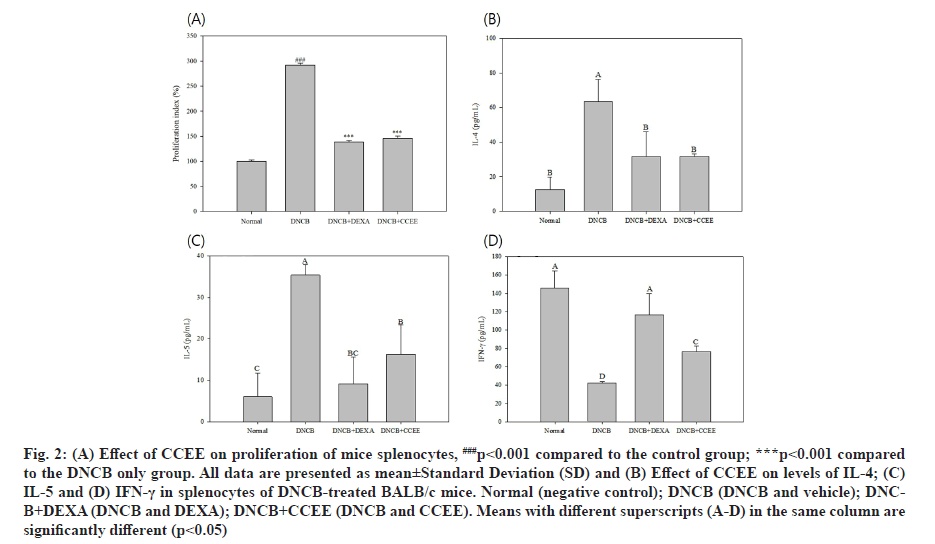
The MTT assay uses the principle of reducing yellow water-soluble substrate MTT tetrazolium to blue-violet water-insoluble MTT formazan by mitochondrial dehydrogenase in living cells[27,28]. The absorbance of MTT formazan is maximum at a wavelength of 540 nm and the OD measured at this wavelength reflects the concentration of viable and metabolically active cells. Accordingly, the present study investigated the effect of CCEE application on splenocyte proliferation ability using the MTT assay, which was performed by applying CCEE to DNCB-induced AD-like mouse model for 2 w, followed by separation and culture of splenocytes (fig. 2A). The DEXA and CCEE groups did not show a significant effect on the proliferation of splenocytes compared to the DNCB group in which AD was induced and immune cells were activated.
Cytokines are water-soluble proteins produced by immune and non-immune cells, and play role in biological defenses such as immunity, inflammation and hematopoietic response by regulating the growth, differentiation, proliferation, and activity of immune cells. Among the cells of the immune system, the cytokines produced by T cells are divided into two subtypes, Th1 and Th2 [29]. AD is result of allergic reactions involving T cells, eosinophils and mast cells in a complex manner. In particular, Th2 cytokines such as IL-4 and IL-5 expressed by Th2 cells and excessive production of IgE are known to be major factors in the pathogenesis of AD[30]. However, recent studies have reported that there is a difference in the immune mechanism involved in the acute and chronic phases depending on the onset of atopy[31,32]. In acute-phase lesions, antigen-recognized T cells secrete IL-4, IL-5 and IL-13 cytokines to regulate differentiation of naive Cluster of Differentiation (CD) 4+ T cells into Th2 cells, and induce transformation of B cells into plasma cells, which not only increase the production of IgE and Immunoglobulin G1 (IgG1), but also increase the levels of vascular adhesion molecules that increase eosinophil infiltration and reduce the expression of Th1 cytokines such as IFN-γ and IL-12. In patients with AD, IL-4 is mainly secreted by allergen-specific T cells; however, cytokines secreted by Th2 cells are also observed in bronchial biopsies of patients with asthma, skin biopsies of patients with atopy and nasal mucosa of patients with rhinitis[33,34]. Conversely, in chronic-phase lesions, IFN-γ and IL-12 cytokines are significantly increased compared to that in acute-phase lesions, and IFN-γ promotes the differentiation of naive CD4+ Th cells into Th1 cells, inhibits the proliferation of Th2 cells, and inhibits the production of IgE by antagonizing IL-4[35,36]. In addition, it induces an immune response by activating macrophages and enhancing the cytotoxicity of natural killer cells and allergen-specific cytotoxic T cells, and it mainly promotes the production of IgG2a[37]. This study investigated the changes in immunological factors to alleviate AD by CCEE in an atopic-induced mouse model. The splenocytes of the mice were isolated and cultured after application of DNCB. Then, the levels of Th1 cytokine IFN-γ and Th2 cytokines IL-4 and IL-5 in the culture medium were measured. As shown in fig. 2, the secretion of Th1 cytokine was significantly decreased in the DNCB-induced mouse model, whereas the secretion was increased significantly in the sample and DEXA groups compared to the untreated group. Conversely, the DNCB group confirmed increase in Th2 cytokine secretion, whereas it was significantly reduced in the CCEE and DEXA groups. When CCEE was applied, the secretion of IL-4 (fig. 2B) and IL-5 (fig. 2C) decreased, and the secretion of IFN-γ (fig. 2D) increased. These results are consistent with a study on AD alleviation effect of Sargassum fulvellum ethanol extract and grasshopper ketone derived from them[38]. It has been suggested that CCEE relieved AD symptoms by regulating the secretion of T cell cytokines. In vitro experiments demonstrated direct activation of cells by CCEE; however, in vivo experiments did not reveal direct action on splenocytes, rather it penetrated into the skin and caused complex factors, which can be understood by additional experiments.
Fig. 2: (A) Effect of CCEE on proliferation of mice splenocytes, ###p<0.001 compared to the control group; ***p<0.001 compared to the DNCB only group. All data are presented as mean±Standard Deviation (SD) and (B) Effect of CCEE on levels of IL-4; (C) IL-5 and (D) IFN-γ in splenocytes of DNCB-treated BALB/c mice. Normal (negative control); DNCB (DNCB and vehicle); DNCB+ DEXA (DNCB and DEXA); DNCB+CCEE (DNCB and CCEE). Means with different superscripts (A-D) in the same column are significantly different (p<0.05)
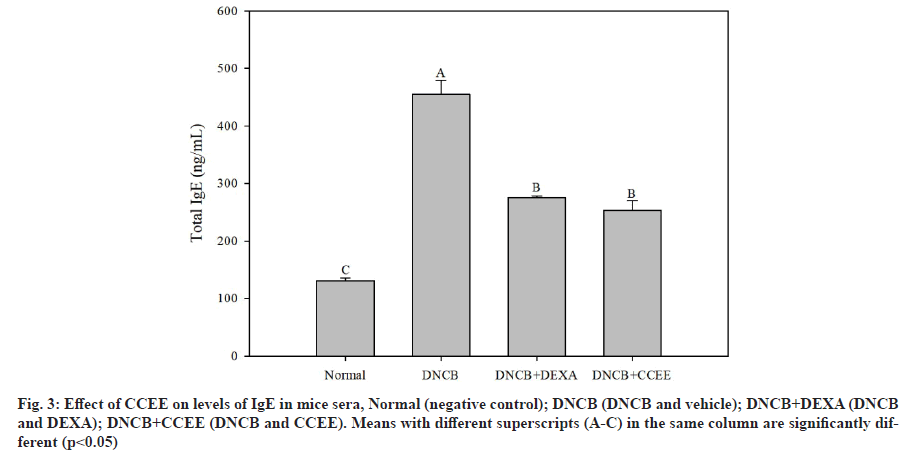
Total IgE content in serum is elevated in 80 %-85 % of patients with AD[7] and excessive production of IgE causes mast cells to secrete enzymes such as histamine, chymase, tryptase and serine esterase, and induces destruction of matrix proteins and tissues[39,40]. IgE is also known to mediate immediate-type hypersensitivity reactions associated with conditions of hay fever, asthma, hives and anaphylactic shock[35,41]. Therefore, it is important to identify and control the production, regulation mechanism of IgE for the treatment of AD and allergic diseases. In this study, the total IgE content of serum was investigated to determine the effect of CCEE treatment on IgE secretion. As shown in fig. 3, the total IgE secretion in the DNCB-induced AD-like mouse model was significantly increased, whereas the DEXA group showed a significant decrease. The CCEE group showed decreased IgE secretion to a degree similar to that in the DEXA group. The findings of this study suggest that CCEE suppressed the secretion of IgE by decreasing IL-4 cytokine level. This is similar to a previous study[42] in which the secretion of serum IgE was reduced through regulation of IL-4 cytokine level by application of an ethanol extract of red algae Callophyllis japonica to atopic-induced mice. It has been shown that CCEE alleviates the symptoms of AD by reducing the secretion of various inflammatory mediators from basophils and mast cells.
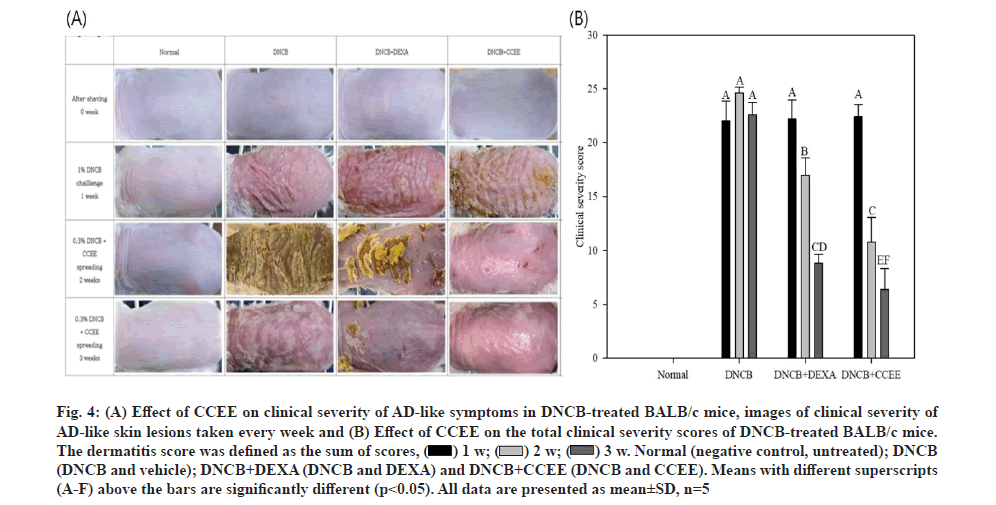
In the acute phase of AD, symptoms such as erythema, papule and exudation appear due to rapid increase in Th2 cytokines such as IL-4, IL-5 and IgE in the serum. In the chronic phase, Th1 cytokines, such as IFN-γ and IL-12 are increased, resulting in the death of keratinocytes and symptoms such as lichenification, scaling and dryness. In addition, pruritus occurs during the overall AD process, leading to scratching and secondary skin symptoms such as wounds and secondary infections[6]. Therefore, in this study, DNCB, a hapten-forming substance, was applied to the back of BALB/c mice, a species that is specialized for Th2 and widely used in the field of immunology, to artificially induce dermatitis in an AD-like mouse model and to investigate the effect of CCEE on AD symptoms. As shown in fig. 4A, immediately after shaving, there was no significant difference between the experimental groups; however, when AD was induced with DNCB, external changes in the skin (erythema, itchiness, dry skin, edema, erosion, and lichenification) were observed in all groups from the 1st w. Moreover, when CCEE was applied to the AD-like mouse model, the symptoms were gradually alleviated. Based on these results, it is expected that CCEE could reduce AD symptoms and thus have the potential to be used in external preparation for the treatment of AD; nevertheless, further research is needed to validate its use as a food material.
The results of the severity test performed every week on the AD-like mouse model are shown in fig. 4B. According to the clinical evaluation method, each score was visually evaluated for each of four conditions: Erythema/hemorrhage, scarring/dryness, edema and excoriation/erosion, and the total scores were expressed. The group administered DEXA to the AD-like mouse model significantly reduced the symptoms of AD over 3 w and it was confirmed that the score significantly decreased even after application of CCEE.
Fig. 4: A) Effect of CCEE on clinical severity of AD-like symptoms in DNCB-treated BALB/c mice, images of clinical severity of AD-like skin lesions taken every week and (B) Effect of CCEE on the total clinical severity scores of DNCB-treated BALB/c mice. The dermatitis score was defined as the sum of scores, Normal (negative control, untreated); DNCB (DNCB and vehicle); DNCB+DEXA (DNCB and DEXA) and DNCB+CCEE (DNCB and CCEE). Means with different superscripts (A-F) above the bars are significantly different (p<0.05). All data are presented as mean±SD, n=5
Normal (negative control, untreated); DNCB (DNCB and vehicle); DNCB+DEXA (DNCB and DEXA) and DNCB+CCEE (DNCB and CCEE). Means with different superscripts (A-F) above the bars are significantly different (p<0.05). All data are presented as mean±SD, n=5
DEXA is an Adrenocorticotropic Hormone (ACTH) with anti-inflammatory action and is one of the most widely used topical steroids for the treatment of AD[27,43]. The anti-inflammatory action of DEXA involves suppression of immune response by reducing phosphorylation and activation of MAPKs such as Extracellular Signal-Regulated Kinase 1/2 (ERK 1/2), Jun N-terminal Kinase (JNK), and p38 and NF-κB pathways among the mechanisms contributing to the expression of inflammation, inhibiting the migration of polymorphonuclear leukocytes, reducing the increased permeability of capillaries, blocking the production of arachidonic acid, a main precursor of prostaglandin, reducing the ability of immune-related cells such as white blood cells to relieve inflammation and decrease the activity of the lymphatic system[44-46]. When various irritants penetrate the skin through the damaged skin barrier as a result of AD, it causes more severe pruritus and an inflammatory response. These results suggest that the symptoms of AD can be effectively alleviated by applying CCEE to the skin.
Acknowledgements:
This research was a part of the project entitled “Bioactive material for algae-based bio-health care substantiation”, funded by the Ministry of Oceans and Fisheries, Korea.
Conflict of interests:
The authors declare that there are no conflicts of interest.
References
- Stander S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: Cutaneous elicitation of itch. Arch Dermatol 2003;139(11):1463-70.
[Crossref] [Google Scholar] [PubMed]
- Vairappan CS, Suzuki M, Abe T, Masuda M. Halogenated metabolites with antibacterial activity from the Okinawan Laurenciaspecies. Phytochemistry 2001;58(3):517-23.
[Crossref] [Google Scholar] [PubMed]
- Bae SJ. Studies on the antioxidative and antimicrobial effects of Chondria crassicaulis. J Life Sci 2004;14(3):411-6.
- Jeon KH, Shin MO, Bae SJ. A study on the effects of anticarcinogenic activity of Chondria crassicaulis. J Nutr Health 2005;38(7):503-11.
- Kim MJ, Kim KB, Park SH, Choi JS, Ahn DH. Anti-inflammatory effect of Chondriacrassicaulis ethanol extract on MAPKs and NF-kB signaling pathway in LPS-induced RAW 264.7 macrophages. Korean Soc Biotechnol Bioeng J 2017;32(4):352-60.
- Kim YK, Jeong EJ, Lee MS, Yoon NY, Yoon HD, Kim JI, et al. Ethanolic extract of Chondria crassicaulis inhibits the expression of inducible nitric oxide synthase and cyclooxygenase-2 in LPS-stimulated RAW 264.7 macrophages. Fish Aquat Sci 2011;14(4):275-82.
- Lee GS. Characterization of antioxidative compounds from Laurencia sp. Master Thesis. University of Gwandong; 2010.
- Ahn JY, Im LR, Kim JH, Park JH, Kim DK, Lee YM. Effects of Rumecis radix water extract on development of atopic dermatitis in BALB/c mice. Korean J Pharmacogn 2009;40(3):218-23.
- AOAC international. The Association of Official Agricultural Chemists. 17th ed. Official Method of Analysis: Washington DC; 2000. p. 17-24.
- Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem 1956;28(3):350-6.
- Wolfrom ML. Methods in carbohydrate chemistry: Reactions of Carbohydrates. New York: Academic Press; 1963.
- Swain T, Hillis WE. The phenolic constituents of Prunus domestica. I.-The quantitative analysis of phenolic constituents. J Sci Food Agric 1959;10(1):63-8.
- Korea Food and Drug Administration. Food Code. KS7053 Korea, Republic of FAIRS Country Report Annual 2007; 2007.
- Hwang Y, Chang B, Kim T, Kim S. Ameliorative effects of green tea extract from tannase digests on house dust mite antigen-induced atopic dermatitis-like lesions in NC/Nga mice. Arch Dermatol Res 2019;311(2):109-20.
[Crossref] [Google Scholar] [PubMed]
- Mishell BB, Shiigi SM. Selected methods in cellular immunology. UK: WH Freeman and Co. Oxford; 1980. p. 486.
- Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. J Am Oil Chem Soc 1975;52(2):59-63.
[Crossref] [Google Scholar] [PubMed]
- Isemura M, Saeki K, Kimura T, Hayakawa S, Minami T, Sazuka M. Tea catechins and related polyphenols as anti?cancer agents. Biofactors 2000;13(1?4):81-5.
[Crossref] [Google Scholar] [PubMed]
- Naczk M, Shahidi F. Phenolic compounds in plant foods: Chemistry and health benefits. Prev Nutr Food Sci 2003;8(2):200-18.
- Yoon JM, Jun JJ, Lim SC, Lee KH, Kim HT, Jeong HS, et al. Changes in selected components and antioxidant and antiproliferative activity of peppers depending on cultivation. J Korean Soc Food Sci Nutr 2010;39(5):731-6.
- Middleton E, Kandaswami C. Potential health-promoting properties of citrus flavonoids. Food Technol 1994;48(11):115-9.
- Kim JH, Kang HM, Lee SH, Lee JY, Park LY. Antioxidant and α-glucosidase inhibition activity of seaweed extracts. Korean J Food Preserv 2015;22(2):290-6.
- Lim SI. Purification and characterization of protease produced by Aspergillus wentti isolated from Korean traditional Meju. Korean J Food Sci Technol 2000;32(1):161-7.
- Hong SK, Chun BS, Park SY. Extraction of Pigment from Sea Mustard (Undaiia pinnatinda) using supercritical carbon dioxide and entrainer. Korean J Fish Aquat Sci 2001;34(3):213-7.
- Sangeetha RK, Bhaskar N, Baskaran V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol Cell Biochem 2009;331(1):59-67.
[Crossref] [Google Scholar] [PubMed]
- Chang TW, Shiung YY. Anti-IgE as a mast cell-stabilizing therapeutic agent. J Allergy Clin Immunol 2006;117(6):1203-12.
[Crossref] [Google Scholar] [PubMed]
- Park SB, Kang KH, Yoon HJ, Ko WS. Inhibitory effect of Ulmus davidiana on β-hexosaminidase release and cytokine production in RBL-2H3 cells. J Korean Med Ophthalmol Otolaryngol Dermatol 2011;24(1):86-95.
- Gerlier D, Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 1986;94(1-2):57-63.
[Crossref] [Google Scholar] [PubMed]
- Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 1987;56(3):279-85.
- Vilcek J, Le J. Immunology of cytokines: An introduction. In: The cytokine handbook. 2nd ed. London: Academic Press Limited; 1994.
- Leung DY. Atopic dermatitis: New insights and opportunities for therapeutic intervention. J Allergy Clin Immunol 2000;105(5):860-76.
[Crossref] [Google Scholar] [PubMed]
- Leung DY, Bieber T. Atopic dermatitis. Lancet 2003;361(9352):151-60.
[Google Scholar] [PubMed]
- Sehra S, Tuana FM, Holbreich M, Mousdicas N, Tepper RS, Chang CH, et al. Scratching the surface: Towards understanding the pathogenesis of atopic dermatitis. Crit Rev Immunol 2008;28(1):15-43.
[Crossref] [Google Scholar] [PubMed]
- O'Byrne PM, Inman MD, Adelroth E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol Sci 2004;25(5):244-8.
- Robinson DS, Larché M, Durham SR. Tregs and allergic disease. J Clin Invest 2004;114(10):1389-97.
[Crossref] [Google Scholar] [PubMed]
- Mayr SI, Zuberi RI, Liu FT. Role of immunoglobulin E and mast cells in murine models of asthma. Braz J Med Biol Res 2003;36(7):821-7.
- Robinson DS. New therapies for asthma: Where next? Pediatr Pulmonol 2003;36(5):369-75.
[Crossref] [Google Scholar] [PubMed]
- Asano Y, Kaneda K, Hiragushi J, Tsuchida T, Higashino K. The tumor-bearing state induces augmented responses of organ-associated lymphocytes to high-dose interleukin-2 therapy in mice. Cancer Immunol Immunother 1997;45(2):63-70.
[Crossref] [Google Scholar] [PubMed]
- Kang BK, Kim MJ, Ahn DH. In vivo and in vitro inhibitory activity of an ethanolic extract of Sargassum fulvellum and its component grasshopper ketone on atopic dermatitis. Int Immunopharmacol 2016;40:176-83.
[Crossref] [Google Scholar] [PubMed]
- Janeway CA, Travers P, Walport M, Shlomchik MJ. An introduction to immunobiology and innate immunity. New York: Garland Science Publishing; 2005. p. 1-35.
- Robinson DS. Th-2 cytokines in allergic disease. Br Med Bull 2000;56(4):956-68.
[Crossref] [Google Scholar] [PubMed]
- Robinson DS. T-cell cytokines: What we have learned from human studies. Paediatr Respir Rev 2004;5(1):53-8.
[Crossref] [Google Scholar] [PubMed]
- Park WS, Lee KS, Chun JH, Urm SH, Lee DS, Lee DY, et al. Investigation of the antiasthmatic properties of ethanol extract of Callophyllis japonica in mice. Trop J Pharm Res 2013;12(6):981-7.
- Matsumoto K, Mizukoshi K, Oyobikawa M, Ohshima H, Sakai Y, Tagami H. Objective evaluation of the efficacy of daily topical applications of cosmetics bases using the hairless mouse model of atopic dermatitis. Skin Res Technol 2005;11(3):209-17.
[Crossref] [Google Scholar] [PubMed]
- Watts AM, Cripps AW, West NP, Cox AJ. Modulation of allergic inflammation in the nasal mucosa of allergic rhinitis sufferers with topical pharmaceutical agents. Front Pharmacol 2019;10:294.
[Crossref] [Google Scholar] [PubMed]
- Tsurufuji S, Sugio K, Takemasa F. The role of glucocorticoid receptor and gene expression in the anti-inflammatory action of dexamethasone. Nature 1979;280(5721):408-10.
[Crossref] [Google Scholar] [PubMed]
- Zakar T, Olson DM. Dexamethasone stimulates arachidonic acid conversion to prostaglandin E2 in human amnion cells. J Dev Physiol 1989;12(5):269-72.
[Crossref] [Google Scholar] [PubMed]