- *Corresponding Author:
- V. B. Tatipamula
Center for Molecular Biology, College of Medicine and Pharmacy, Duy Tan University, Danang 550000, Vietnam
E-mail: vinaybharadwajtatipamula@duytan.edu.vn
| Date of Received | 01 July 2021 |
| Date of Revision | 25 September 2022 |
| Date of Acceptance | 16 February 2023 |
| Indian J Pharm Sci 2023;85(1):227-232 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Medicinal herb Bauhinia variegata L. is the main ingredient in “Gandmala Kandan Ras” herbomineral medicine used in Ayurveda for the treatment of swelling, inflammation and tumors. This study aimed to isolate the anti-inflammatory compounds from the methanolic extract of aerial parts of Bauhinia variegata. Through bioassay-guided isolation, three known flavonoids, namely kaempferol, ombuin and quercetin were identified from methanolic extract of aerial parts of Bauhinia variega. The primary screening by protein denaturation method revealed a significant percentage of inhibition of protein denaturation of compounds kaempferol and quercetin. The anti-inflammatory assays against COX-1/2 enzymes showed significant anti-inflammatory activity of these two compounds, compared to the standard drug, indomethacin. Of which, quercetin as a potent non-selective inhibitor of COX1 and COX2 with minimal inhibitory concentration values of 172.05±4.29 and 220.62±9.13 nM, respectively; while kaempferol showed selective COX2 inhibition with the IC50 of 154.86±5.60 nM. The results provided evidence that supports the Ayurvedic usage of Gandmala Kandan Ras formulation in the treatment of inflammation, which was attributed to the natural active kaempferol and quercetin. The results indicated that plant Bauhinia variegata could be considered as an excellent natural source of remedial medicine for inflammation.
Keywords
Bauhinia, protein denaturation method, cyclooxygenase enzymes, inhibitory assay
Bauhinia genus belongs to the family Fabaceae, well recorded in the flora of Brazil, India, Nepal and South Africa[1,2]. Traditionally, the bark, flowers and roots of the genus Bauhinia are most valued in Brazil, India and South Africa for treating various alignments such as diabetes, inflammation, tumors, gastrointestinal disorders and infectious diseases[3]. In Ayurveda, an herbo-mineral formulation named “Gandmala Kandan Ras” (GKR) is widely used for patients suffering from inflammation-related conditions such as swelling, cysts and tumors. The Sanskrit technical term “Gandmala” is “scrofula,” which is an inflammatory disorder indicating swelling of the neck and inflammation of lymph glands.
GKR formulation mainly contains ingredients from Bauhinia variegata (B. variegata) L. and Shuddha Guggulu[4].
B. variegate L. is a semi-evergreen tree, usually called “Camel’s foot creeper” in English, and “Kanchanara or Kachnar” in Sanskrit. In some parts of India, buds and flowers of B. variegata are used as vegetables and are cooked and eaten as a famous food named “Karalen Ki Sabji”[5]. Particularly, in the Indian tribes, the B. variegata has been using in the treatment of multiple conditions, including microbial infections, oxidative stress, chronic inflammation and cancer. Biologically, B. variegata has been reported for its antibacterial[6], antioxidant[6,7], anti-inflammation[3], anti-hyperlipidemic[7], anti- cancer[6], hepatoprotective properties[8], trypsin inhibitory[9] and immunomodulatory[10] activities. The broad spectrum of biological activities of B. variegata is mainly due to the presence of multiple bioactive compounds such as flavonoids, flavanones, bibenzyls, triterpenes, flavonol-glycosides, saponins and phenanthraquinones in its different parts[11,12].
In the current study, we aimed to investigate the anti-inflammatory agents present in aerial parts extract of B. variegata, one of the main ingredients of GKR formulation[4], using bio-guided isolation and screening its bioactive metabolites for anti- inflammatory activity against cyclooxygenase enzymes.
Materials and Methods
Collection:
The aerial parts of B. variegata L. were collected at Seshachalam hills, Tirupati, Andhra Pradesh, India, in October 2019 and a voucher specimen (DB- SVU-2019-3626) has deposited at the Department of Botany, Andhra University, Visakhapatnam, Andhra Pradesh, India.
Extraction and bioassay-guided isolation:
Approximately 250 g of the dried aerial parts of B. variegata was grounded and extracted by maceration method using methanol (3×500 ml×7 d) at room temperature. All the three fractions of extract were filtered using a muslin cloth and concentrated on a rotary evaporator (Shimadzu Rotation evaporator QR 2005-S, Japan) under reduced pressure at 40° thereby provided a crude methanol extract of the aerial parts of B. variegata (ME) 4.5 g, 18 % w/w as black solid.
Approximately 3.0 g of ME was subjected to column chromatography (sintered disc column, 600 mm×45 mm; Product code: 6101067, Borosil, India) over silica gel (230-400 mesh, CAS No.: 112926-00-8, Merck) by employing a mobile phase hexane-ethyl acetate gradient (0-100 %) yielded eight fractions (F1-8). These fractions were concentrated in a vacuum and screened for their protein denaturation capacity against bovine serum albumin (Sigma-Aldrich, India). The fractions that exhibited prominent inhibitory action against protein denaturation were further purified by column chromatography (sintered disc column, 300 mm×18 mm; Product code: 6101062, Borosil, India) over silica gel (230-400 mesh) using mobile phase hexane-chloroform gradient (0-100 %) and thin layer chromatography (pre-coated glass silica plates, TLC-Silica gel 60 GF254, CAS: 7631-86-9, Merck, Germany) to obtain the bioactive compounds. Further, 1H- Nuclear magnetic resonance (NMR) and 13C-NMR (Bruker Avance 400 Spectrometer, Germany), Mass (LC/MS Triple Quad Portfolio, Agilent, China) spectral analyses, melting point (m.p.) determination (M-560/565 m.p. Apparatus, Buchi, Switzerland) and CNHS (2400 CHNS Organic Elemental Analyzer, PerkinElmer, USA) analyses were applied for the structure elucidation of isolated compounds.
In vitro anti-inflammatory assays:
Preliminary screening by protein denaturation method: In the present study, in vitro anti-inflammatory activity of ME and its fractions (F1-8) was evaluated against bovine serum albumin protein using the protein denaturation method[13]. Briefly, the bovine serum albumin protein (1 %) was prepared using 50 mM sodium phosphate buffer (pH 6.4). To 0.2 ml of the above solution, added 0.1 ml of the tested samples at three different concentrations 100, 200 or 400 μg/ml. The final volume was adjusted to 5 ml with buffer and incubated at 37° for 20 min. The tubes were heated in a steam bath at 95° for 20 min, then cooled to room temperature. Finally, the turbidity in the cooling tubes was measured at 660 nm by Ultraviolet-Visible Spectrophotometer (Model SL 210, Elico India Ltd.). The experiment was performed in triplicate (n=3) and the quantitative value was expressed as the mean±standard deviation (SD).
Cyclooxygenase (COX-1/2) inhibitory assay: The abilities of compounds (kaempferol, ombuin and quercetin) to inhibit isoenzymes COX-1/2 were performed using (ovine/human) COX inhibitor assay kit (Cayman, No.: 560131)[3]. To 10 μl of either COX1 or COX2 added 960 μl of 0.1 M Tris-HCl buffer and one of the three concentrations of the test samples and incubated for 10 min at 37°. After 2 min, add 10 μl of 100 μM arachidonic acid, 50 μl of 1 M HCl and Ellman’s reagent. The absorbance was noted spectrophotometrically at 410 nm against the blank. The percentage of inhibition was calculated with the optical density values and the IC50 values were determined by linear regression.
Results and Discussion
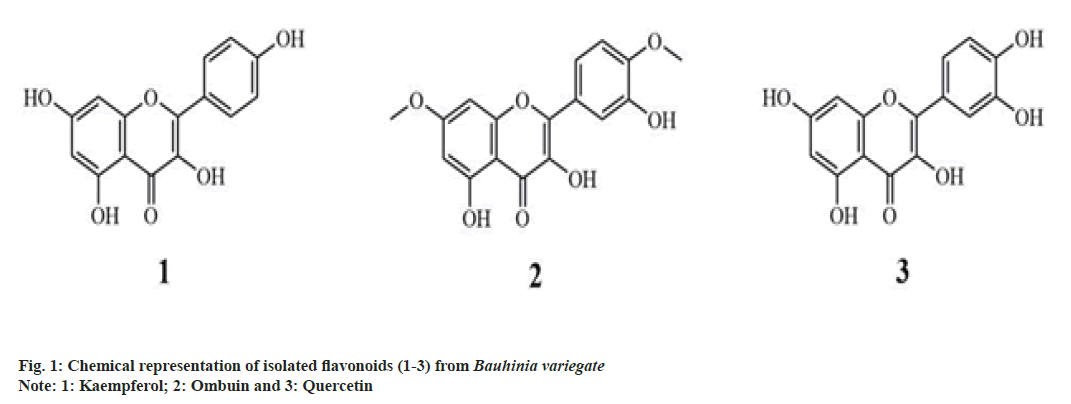
The preliminary anti-inflammatory assay revealed the good potential of protein denaturation inhibition of ME, Fraction (F)3, and F5 with the percentage of protein denaturation inhibition found to be 92.76±1.36 %, 92.00±5.12 % and 70.65±1.52 %, respectively (Table 1). By using chromatography and analyses of their spectral NMR data and elemental composition, three known flavonoids, namely kaempferol (1), ombuin (2) and quercetin (3) were successfully identified from F3 and F5 two most active fractions (fig. 1). Particularly, F3 (620 mg) yielded compound 1 (100 mg) as a yellow solid and F5 (250 mg) yielded compounds, 2 (60 mg) as a pale yellow powder and 3 (50 mg) as a shiny yellow powder. At 400 mg/ml concentration, compounds 1 (92.95±1.22 %) and 3 (93.16±2.67 %) exhibited profound anti-inflammatory potency, which was substantially higher than that of compound 2 (44.65±2.70 %) (Table 1). Notably, the percentage of inhibition of compounds 1 and 3 was also very high at 200 mg/ml and substantially lower at 100 mg/ml concentration (Table 1).
| Sample | Yield (mg) | % Inhibition of protein denaturation* | ||
|---|---|---|---|---|
| 100 μg/ml | 200 μg/ml | 400 μg/ml | ||
| ME | 4500 | 41.24±1.97 | 69.38±3.62 | 92.76±1.36 |
| F1 | 800 | 3.45±0.21 | 14.94±3.75 | 25.97±1.42 |
| F2 | 450 | 9.45±1.73 | 19.87±2.28 | 27.93±1.00 |
| F3 | 620 | 67.78±2.34 | 72.97±6.57 | 92.00±5.12 |
| F4 | 250 | 11.02±3.10 | 25.93±3.52 | 27.90±1.76 |
| F5 | 310 | 35.55±6.43 | 54.06±5.26 | 70.65±1.52 |
| F6 | 200 | 6.57±1.40 | 19.01±1.09 | 27.31±0.36 |
| F7 | 550 | 13.45±0.72 | 25.69±2.74 | 41.14±1.43 |
| F8 | 140 | 3.45±1.66 | 6.86±1.72 | 7.67±1.72 |
| 1 | 100 | 66.10±1.87 | 90.60±1.29 | 92.95±1.22 |
| 2 | 60 | 20.92±2.59 | 32.95±1.63 | 44.65±2.70 |
| 3 | 50 | 70.11±2.41 | 88.88±1.30 | 93.16±2.67 |
Note: *mean±SD (n=3)
Table 1: Yield and Inhibitory Effects of Methanol Extract (ME), Fractions (F1-8), and Compounds (1-3) from Bauhinia variegata against Bovine Albumin Protein Denaturation
Compound 1 (Kaempferol) (fig. 1)[14] is a yellow solid, m.p.: 275-276°; (Retention factor) Rf: 0.6 (hexane:ethyl acetate 1:1); 1H NMR (400 MHz, Dimethyl Sulfoxide (DMSO)-d6): 2.27 (s, 1H, OH), 2.98 (s, 1H, OH), 3.93 (s, 1H, OH), 5.03 (s, 1H, OH), 6.35 (s, 1H, Ar-H), 6.45 (s, 1H, Ar-H), 7.12-7.13 (d, 2H, J=4 Hz, Ar-H), 7.61-7.63 (d, 2H, J=8 Hz, Ar-H). 13C NMR (400 MHz, DMSO-d6): 94.96 (C-8), 100.15 (C-6), 104.82 (C-4), 116.49 (C-12/14), 122.48 (C-10), 131.48 (C-11/15), 136.89 (C-2), 146.42 (C-1), 158.13 (C-9), 160.36 (C- 13), 160.49 (C-5), 164.68 (C-7), 175.78 (C-3). CHNS analysis for C15H10O6: calcd. C-62.94 %, H-3.52 %, found C-62.96 %, H-3.54 %. Electrospray Ionisation- Mass Spectrometry (ESI-MS): calcd. m/z for C15H10O6: 286.05 [M], found 285.14 [(M+H+), positive mode], 287.24 [(M-H+), negative mode].
Compound 2 (Ombuin) (fig. 1)[15] is a pale yellow powder; m.p.: 202-203°; Rf: 0.5 (hexane:ethyl acetate 1:1); 1H NMR (400 MHz, DMSO-d6): 2.97 (s, 1H, OH), 3.55 (s, 1H, OH), 3.85 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 5.03 (s, 1H, OH), 6.23 (d, 1H, J=1 Hz, Ar-H), 6.24-6.25 (d, 1H, J=4 Hz, Ar-H), 6.85-6.87 (d, 1H, J= 8 Hz, Ar-H), 7.02-7.06 (m, 2H, Ar-H). 13C NMR (400 MHz, DMSO-d6): 57.07 (C-10), 57.81 (C-17), 93.54 (C-8), 98.70 (C-6), 106.03 (C-4), 113.24 (C-15), 116.39 (C-12), 121.00 (C-16), 124.86 (C-11), 138.13 (C-2), 146.92 (C-13), 147.87 (C-1), 150.82 (C-14), 159.09 (C- 9), 161.63 (C-5), 166.42 (C-7), 176.27 (C-3). CHNS analysis for C17H14O7: calcd. C-61.82 %, H-4.27 %, found C-61.76 %, H-4.24 %. ESI-MS: calcd. m/z for C17H14O7: 330.07 [M], found 331.63 [(M+H+), positive mode], 329.20 [(M-H+), negative mode].
Compound 3 (Quercetin) (fig. 1)[14] is a shiny yellow powder; m.p.: 316-317°; Rf: 0.4 (hexane:ethyl acetate 1:1); 1H NMR (400 MHz, DMSO-d6): 2.77 (s, 1H, OH), 3.01 (s, 1H, OH), 3.50 (s, 1H, OH), 3.87 (s, 1H, OH), 5.01 (s, 1H, OH), 6.19 (d, 1H, J= 1 Hz, Ar-H), 6.19- 6.20 (d, 1H, J= 4 Hz, Ar-H), 6.77-6.78 (d, 1H, J= 4 Hz, Ar-H), 6.98-7.01 (m, 2H, Ar-H). 13C NMR (400 MHz, DMSO-d6): 94.81 (C-8), 99.99 (C-6), 104.66 (C-4), 116.36 (C-11), 116.67 (C-14), 122.01 (C-15), 122.19 (C-10), 137.48 (C-2), 145.68 (C-12), 147.21 (C-1), 148.73 (C-13), 157.97 (C-9), 160.34 (C-5), 164.52 (C- 7), 175.62 (C-3). CHNS analysis for C15H10O7: calcd. C-59.61 %, H-3.34 %, found C-59.62 %, H-3.34 %. ESI-MS: calcd. m/z for C15H10O7: 302.04 [M], found 303.66 [(M+H+), positive mode], 301.25 [(M-H+), negative mode].
Based on their protein denaturation inhibition capability, active compounds 1 and 3 at a concentration range of 25 to 100 μg/ml were further evaluated for in vitro anti-inflammatory activity against COX-1/2 isoenzymes using (ovine/human) inhibitor assay kit (Table 2). The IC50 values were calculated against COX-1/2 through dose-response assay and the results were presented in Table 3. IC50 values were taken as the lowest concentration of compounds that exhibited inhibition of 50 %, relative to the inhibition control without compounds.
| Sample | Percentage inhibition at different concentration* | |||
|---|---|---|---|---|
| 25 µg/ml | 50 µg/ml | 75 µg/ml | 100 µg/ml | |
| Cyclooxygenases 1 (COX1) inhibitory assay | ||||
| 1 | 16.33±3.00 | 28.31±3.10 | 42.45±1.68 | 48.83±0.64 |
| 3 | 39.29±0.70 | 48.86±1.03 | 61.71±2.64 | 69.18±2.30 |
| Indomethacin | 41.64±0.45 | 62.29±1.22 | 68.34±2.99 | 83.92±2.27 |
| Cyclooxygenases 2 (COX2) inhibitory assay | ||||
| 1 | 40.78±0.59 | 52.72±0.83 | 60.23±3.47 | 66.33±3.06 |
| 3 | 34.31±0.97 | 43.23±1.79 | 53.26±0.71 | 66.08±2.77 |
| Indomethacin | 48.93±0.95 | 62.77±1.40 | 79.98±1.20 | 88.33±2.39 |
Note:*mean±SD (n=3)
Table 2: Percentage Inhibition of Compounds 1 and 3 against Cyclooxygenase Enzymes
| Sample | IC50 values (nM)* | |
|---|---|---|
| COX1 | COX2 | |
| 1 | - | 154.86±5.60 |
| 2 | - | - |
| 3 | 172.05±4.29 | 220.62±9.13 |
| Indomethacin | 110.35±2.91 | 84.26±4.40 |
Note: *Mean±SD values (n=3), “-” indicates not found up to 100 μg/ml concentration
Table 3: IC50 Values Of Compounds 1-3 on COX1/2 Enzymes
The IC50 values of compound 3 against COX1 and COX2 enzymes were noted to be 172.05±4.29 and 220.62±9.13 nM respectively, higher than that of the reference drug indomethacin (IC50 values: 110.35±2.91 and 84.26±4.40 nM respectively). Compound 1, on the other hand was selectively active against the COX2 enzyme with the IC50 values of 154.86±5.60 nM (Table 3), whereas its inhibition against COX1 at the concentration of 100 µg/ml reached 48.83±0.64 % only (Table 2). Taken together, our data revealed the potent inhibition efficiency of the compounds 1 and 3 on COX enzymes.
GKR is a herbo-mineral formulation that is well-known for its anti-inflammatory effects such as Gandamala (lymphadenopathy), Galaganda (goitre) and Arbuda (tumor)[4,5]. The general composition of “GKR” formulation is B. variegata (144 g), shuddha guggulu (144 g), mandur bhasma (36 g), sonth (Zingiber officinale rhizome, 24 g), kali mirch (Piper nigrum, 24 g), pippali (Piper longum, 24 g), shuddha parad (12 g), shuddha gandhak (6 g), tamra bhasma (18 g), sendha namak (6 g), and cow’s ghee (quantity sufficient). This oral formulation (tablets) is recommended for adults (250-1000 mg per day) and children (8 mg per kg body weight per day) suffering from different types of inflammation[4].
The previous studies on the chemical and biological properties have identified multiple secondary metabolites from B. variegata (e.g. flavonoids, flavanones, triterpenes, saponins, etc.)[11,12] and shuddha guggulu (guggulusterone, naringenin, myrrhanol, etc.) [16-20], which were noted for their anti-inflammatory activities. Besides, anti-inflammatory studies were also well-established for other GSK-included plants and multiple key chemical constituents were reported comprising ginger and gingerol derivatives (from sonth, (Zingiber officinale rhizome))[21] and piperine derivatives (from kali mirch (Piper nigrum) and pippali (Piper longum))[22-24]. On the other hand, mandur bhasma is well-acknowledged as an iron supplement[25] and shuddha parad and shuddha gandhak act as detoxifying agents[26]. Tamra bhasma is used as an adjuvant and sendha namak (rock salt) offers trace minerals in Ayurveda formulations[27,28].
With this in mind, we investigated the main chemical constituents present in B. variegata that are responsible for the anti-inflammatory actions. The in vitro preliminary screening of ME and its fractions (F1-8) revealed its aptitude to treat inflammation of ME and fractions F3 and F5. The bioassay-guided isolation of these two fractions has yielded three known secondary metabolites (1-3) (fig. 1). This finding provides new insights into the phytochemical profile of B. variegata.
COXs are key enzymes that catalyse the production of prostaglandins and thromboxanes, which are commonly involved in the regulation of inflammation, from arachidonic acid[29]. Thus, routes of non-selective COX1 and COX2 inhibition are widely chosen for the treatment of inflammation[30,31]. Our in vitro tests have justified the potent inhibition against COX1/2 enzymes of compounds 1 and 3 (Table 1), of which compound 3 acts as a non-selective COX inhibitor and compound 1 is selective COX2 inhibitor (Table 2 and Table 3). This observation is in accordance with the previous study, which uncovered the significant inhibition against the COXs and 15-LOX enzymes of the leaf extract of B. variegata[3]. Taken together, the overall anti-inflammatory activity exhibited by compounds 1 and 3 obtained from the ME extract of B. variegata was significant, although lower than that of the standard drug, indomethacin. These findings further supported the folklore usage of the herbo-mineral formulation GKR in the treatment of inflammation.
To conclude, the present work provides the first evidence for the presence of anti-inflammatory compounds from B. variegata, the main ingredient in herbo-mineral formulation, GKR. First, we reported the bioassay-guided isolation of compounds 1-3 from the aerial parts of B. variegata that possessed significant in vitro inhibition against COX-1/2. The key metabolites responsible for anti-inflammatory activities were noted to be compounds 1 and 3 by acting against COX1/2 proteins. The results provided evidence that supports the Ayurvedic use of the GKR formulation. Also, these findings suggested that plant B. variegata can be a good natural source of remedial medicine for inflammation.
Conflict of interest:
The authors have no conflict of interest to declare.
References
- Burlakoti C, Kunwar RM. Folk herbal medicines of Mahakali watershed Area, Nepal. Med Plants Nepal An Anthol Contemp Res 2008:187-93.
- dos Santos FJ, Moura DJ, Péres VF, de Moura Sperotto AR, Caramão EB, Cavalcante AA, et al. Genotoxic and mutagenic properties of Bauhinia platypetala extract, a traditional Brazilian medicinal plant. J Ethnopharmacol 2012;144(3):474-82.
- Ahmed AS, Elgorashi EE, Moodley N, McGaw LJ, Naidoo V, Eloff JN. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four South African Bauhinia species used traditionally to treat diarrhoea. J Ethnopharmacol 2012;143(3):826-39.
[Crossref] [Google Scholar] [PubMed]
- Babu MSS. Yoga Ratnakara: The 'A' to 'Z' classic on ayurvedic formulations practices and procedures. Chowkhamba Sanskrit Series Office 2008: pp. 859-70.
- Nariyal V, Sharma PK. Kanchnar (Bauhinia variegata) as a medicinal herb: A systematic review. Int J Adv Res 2017;5(9):587-91.
- Mishra A, Sharma AK, Kumar S, Saxena AK, Pandey AK. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Research International. 2013;2013:1-10.
[Crossref] [Google Scholar] [PubMed]
- Rajani G, Ashok P. In vitro antioxidant and antihyperlipidemic activities of Bauhinia variegata Linn. Indian J Pharmacol 2009;41:227-32.
- Bodakhe SH, Ram A. Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku zasshi. 2007;127(9):1503-7.
[Crossref] [Google Scholar] [PubMed]
- Fang EF, Wong JH, Bah CS, Lin P, Tsao SW, Ng TB. Bauhinia variegata var. variegata trypsin inhibitor: From isolation to potential medicinal applications. Biochem Biophys Res Commun 2010;396(4):806-11.
[Crossref] [Google Scholar] [PubMed]
- Patil JK, Jalalpure SS, Hamid S, Ahirrao RA. In vitro immunomodulatory activity of extracts of Bauhinia vareigata Linn bark on human neutrophils. Iran J Pharmacol Ther 2010;9:41-6.
- Lim TK. Edible medicinal and non-medicinal plants. Dordrecht, The Netherlands: Springer; 2012 pp. 754-65.
- Al-Snafi AE. The pharmacological importance of Bauhinia variegata: A Review. Int J Pharm Sci Res 2013;4(12):160-4.
- Tatipamula VB, Vedula GS. Fibrinolytic, anti-inflammatory and cytotoxic potentialities of extracts and chemical constituents of manglicolous lichen, Graphis ajarekarii Patw. & CR Kulk. Nat Prod J 2020;10(1):87-93.
- de Souza LA, Tavares WM, Lopes AP, Soeiro MM, de Almeida WB. Structural analysis of flavonoids in solution through DFT 1H NMR chemical shift calculations: Epigallocatechin, Kaempferol and Quercetin. Chem Phys Lett 2017;676:46-52.
- Salem MM, Hussein SR, El-Sharawy R, El-Khateeb A, Ragab EA, Dawood KM, et al. Antioxidant and antiviral activities of the aqueous alcoholic leaf extract of Boscia angustifolia A: Rich (Capparaceae) and its major component'ombuin'. Egypt Pharm J 2016;15(1):1-5.
- Shishodia S, Aggarwal BB. Guggulsterone inhibits NF-κB and IκBα kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J Biol Chem 2004;279:47148-58.
[Crossref] [Google Scholar] [PubMed]
- Manjula N, Gayathri B, Vinaykumar KS, Shankernarayanan NP, Vishwakarma RA, Balakrishnan A. Inhibition of MAP kinases by crude extract and pure compound isolated from Commiphora mukul leads to down regulation of TNF-α, IL-1β and IL-2. Int Immunopharmacol 2006;6(2):122-32.
[Crossref] [Google Scholar] [PubMed]
- Ebeling W. Healing with plants in the American and Mexican West. West Hist Q 1997;28:583-4.
- Kimura I, Yoshikawa M, Kobayashi S, Sugihara Y, Suzuki M, Oominami H, et al. New triterpenes, myrrhanol A and myrrhanone A, from guggul-gum resins, and their potent anti-inflammatory effect on adjuvant-induced air-pouch granuloma of mice. Bioorg Med Chem Lett 2001;11(8):985-9.
[Crossref] [Google Scholar] [PubMed]
- Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS, Parmar NS. Anti-inflammatory activity of Commiphora molmol. Agents and Actions. 1986;17:381-2.
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol 2008;46(2):409-20.
[Crossref] [Google Scholar] [PubMed]
- Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L. (black pepper): A review. Asian Pac J Trop Biomed 2012;2:S1945-53.
- Vaghasiya Y, Nair R, Chanda S. Investigation of some Piper species for anti-bacterial and anti-inflammatory property. Int J Pharmacol 2007;3(5):400-5.
- Guo Z, Xu J, Xia J, Wu Z, Lei J, Yu J. Anti-inflammatory and antitumour activity of various extracts and compounds from the fruits of Piper longum L. J Pharm Pharmacol 2019;71(7):1162-71.
- Pal D, Sahu CK, Haldar A. Bhasma: The ancient Indian nanomedicine. J Adv Pharm Technol Res 2014;5(1):4-12.
[Crossref] [Google Scholar] [PubMed]
- Mythili Krishna J, Gaude R. Significance of parad in Rasashastra: A review. J Ayu Herb Med 2017;3(3):169-74.
- Chaudhari SY, Jagtap CY, Galib R, Bedarkar PB, Patgiri B, Prajapati PK. Review of research works done on Tamra bhasma [incinerated copper] at Institute for post-graduate teaching and research in Ayurveda, Jamnagar. Ayu 2013;34(1):21-5.
[Crossref] [Google Scholar] [PubMed]
- Mooss NS. Salt in ayurveda I. Anc Sci Life 1987;6:217-37.
- Sud’ina GF, Pushkareva MA, Shephard P, Klein T. Cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) selectivity of COX inhibitors. Prostaglandins Leukot Essent Fatty Acids 2008;78(2):99-108.
[Crossref] [Google Scholar] [PubMed]
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis 2003;62(6):501-9.
[Crossref] [Google Scholar] [PubMed]
- Chandrasekaran CV, Deepak HB, Thiyagarajan P, Kathiresan S, Sangli GK, Deepak M, et al. Dual inhibitory effect of Glycyrrhiza glabra (GutGardTM) on COX and LOX products. Phytomedicine 2011;18(4):278-84.
[Crossref] [Google Scholar] [PubMed]