- *Corresponding Author:
- N. Bolourchian
Department of Pharmaceutics, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran-19834, Iran
E-mail: bolourchian@sbmu.ac.ir
| Date of Submission | 26 May 2016 |
| Date of Revision | 09 November 2016 |
| Date of Acceptance | 20 January 2017 |
| Indian J Pharm Sci 2017; 79(1): 105-112 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Intraarticular administration of microspheres containing non-steroidal antiinflammatory drugs is beneficial in the treatment of rheumatoid arthritis. Microspheres could localize drug at the site of administration and control its release, resulting in improved therapeutic effects and decreased side effects. Therefore, the objective of the present study was to prepare controlled release meloxicam-loaded gelatin microspheres and evaluate the effect of various variables on their properties. Meloxicam-loaded microspheres were prepared by emulsion-congealing-chemical cross-linking method. Different amounts of polymer, emulsifier and cross-linking agent, as formulation variables, were evaluated. Microspheres were characterized in terms of yield value, encapsulation efficiency and the drug release pattern. The particle size, surface morphology and thermal behavior of microspheres were also investigated. According to the results, using glutaraldehyde as a cross-linking agent resulted in spherical microspheres with the yield value of 63-96% and encapsulation efficiency of 23-63%. The optimum formulation with the mean particle size of about 57 μm, showed slow drug release profile (64%) throughout 48 h. An appropriate polymer:cross-linker ratio must be used to obtain suitable particles with acceptable controlled released behavior. In summary the results of the present study supported the preparation of sustained release meloxicam-loaded microspheres using emulsion-congealing method along with the potential application of glutaraldehyde in improving the physical properties of prepared particles as well as the release profile of this low water soluble drug.
Keywords
Meloxicam, gelatin microspheres, controlled release, glutaraldehyde, intraarticular
Rheumatoid arthritis (RA) is one of the most common chronic autoimmune diseases that still does not have any curative treatment [1,2]. RA has three pathophysiological phases including immunological abnormalities, inflammation and proliferation of synovium. According to these phases, two types of drugs, which are commonly prescribed are antiinflammatory agents non-steroidal antiinflammatory drugs (NSAIDs) and glucocorticoids and disease modifying antirheumatic drugs (DMARDs). Using biological agents, immune suppressants and gene therapy are some newer strategies for the treatment of RA [3].
Using NSAIDs is the main strategy for pain relief in arthritic joints [4]. Recently, selective cyclooxygenase (COX-2) inhibitors have been more preferred due to their lower gastrointestinal side effects. It has also been reported that the efficacy of COX-2 inhibitors in the treatment of RA is similar to conventional NSAIDs [3]. Meloxicam (MX) is an oxicam derivative with analgesic, antipyretic and antiinflammatory effects. This drug is a preferential COX-2 inhibitor that is widely prescribed for treatment of osteoarthritis and RA [5].
Systemic administration of NSAIDs for a long period of time would cause significant gastrointestinal, cardiovascular and renal side effects; also low concentration of drug would reach to the inflammation site [6]. Local administration of these drugs is desirable due to producing higher concentration and lower systemic side effects. However, rapid clearance of drug from the joint cavity is a limiting factor [7]. Therefore, designing a controlled release drug delivery system for intra-articular administration could be beneficial [8,9]. In fact, incorporation of NSAIDs in biocompatible and biodegradable microspheres with appropriate size seems to be a logical strategy [7-16].
Gelatin is a biocompatible, biodegradable and nontoxic natural polymer that is widely used for preparation of controlled release systems [6,17,18]. Previous studies have indicated that intra-articular administration of gelatin microspheres does not induce any inflammatory reaction [19]. Recently, special attention has been focused on the preparation of gelatin microspheres containing NSAIDs for intraarticular administration [6,20,21]. The aim of the present study was to prepare and characterize MX-loaded gelatin microspheres. The effects of various variables on the microspheres properties were also investigated.
Materials and Methods
MX powder was supplied as a gift sample by Osveh Pharmaceutical Co., Iran. Fifty percent v/v glutaraldehyde (GA) solution was purchased from Sigma-Aldrich, St. Louis, MO, USA. Gelatin, liquid paraffin, Span 80, acetone and all other reagents were purchased from Merck, Germany. Materials and excipients used in preparing microspheres were of pharmacopoeial grades.
Preparation of microspheres
MX-loaded gelatin microspheres (MX-GMs) were prepared by emulsion-congealing-chemical crosslinking method. Briefly, 100 mg of MX powder was dissolved in 0.2 M sodium hydroxide solution; then different concentrations of gelatin aqueous solutions (5 ml) were prepared using above MX solution under stirring at 70°. Gelatin containing drug solutions were drop wise added to 75 ml of preheated liquid paraffin and Span 80 mixture, by a syringe fitted with 22 G needle. The biphasic system was stirred under a mechanical stirrer (IKA, Germany) at a speed of 600 rpm at 70° for 10 min to form a w/o emulsion. The emulsion was cooled in an ice bath to 8° and continuously stirred for 15 min. In the next step, 75 ml of acetone was gradually added to the emulsion by stirring for an additional 5 min. The resulting microspheres were collected by filtration and washed several times with acetone until the whole paraffin was removed. Finally, the microspheres were dried at room temperature for 12 h. It is worth mentioning that the stability of MX at alkaline pH has been previously indicated [22].
Two different methods were used for cross-linking of microspheres: (1) for the first group, 100 mg of prepared microspheres were transferred to a beaker containing 50 ml of GA solution and stirred magnetically for 4 h at room temperature. Then microspheres were collected and washed with distilled water. The resulting microspheres were dried at room temperature (Fa group). (2) For the second group, 1 ml of the GA solution was added to the w/o emulsion and stirred for 5 min before congealing it to 8°. The other steps were performed as was mentioned above (Fb group). The composition of the prepared formulations was shown in Table 1.
| Formulationa | Gelatin | Span 80 | GA solution |
|---|---|---|---|
| (% w/v) | (% v/v) | (% v/v) | |
| Fa1 | 25 | 0.2 | 1 |
| Fa2 | 25 | 0.2 | 5 |
| Fb1 | 25 | 0.2 | 1 |
| Fb2 | 35 | 0.2 | 1 |
| Fb3 | 45 | 0.2 | 1 |
| Fb4 | 25 | 0.2 | 5 |
| Fb5 | 35 | 0.2 | 5 |
| Fb6 | 45 | 0.2 | 5 |
| Fb7 | 45 | 0.2 | 10 |
| Fb8 | 25 | 0.3 | 5 |
Table 1: Composition of Mx-Gm Formulations
Characterization of microspheres
The prepared microspheres were characterized in terms of yield value, encapsulation efficiency (EE), size distribution, in vitro drug release, morphology and thermal analysis. The yield value of each formulation was calculated using the following Eqn., [23] yield value (%) = (weight of dried microspheres/total solid material amount in the dispersed phase)×100.
Drug content
Ten milligrams of MX-GMs was accurately weighted and transferred to a beaker containing 20 ml of 1 M sodium hydroxide solution and stirred for 12 h to dissolve microspheres completely. The prepared solution was analysed for drug content using a UV/ Vis spectrophotometer at 362 nm (Shimadzu, Japan). The probable interference of gelatin with the UV absorbance of MX was studied and no interaction was observed.
The drug loading and EE were calculated using the following Eqns [21]: drug loading (%) = (weight of drug in microspheres/weight of microspheres)×100; drug EE (%) = (drug loading/theoretical drug loading)×100.
In vitro drug release
Drug release studies were carried out in phosphate buffer solution (PBS, pH=7.4) at 37°. A sample of 10 mg of MX-GMs were suspended in 25 ml of dissolution medium (sink condition) and stirred on a magnet stirrer at 30 rpm. At certain time intervals, 1 ml of solution was sampled and centrifuged at 5000 rpm for 5 min. The supernatant was collected and analysed spectrophotometrically at 362 nm and the sediment remaining in centrifuge tubes was returned to the release medium. After each sampling, fresh buffer solution was replaced in order to maintain sink conditions. All experiments were performed in triplicate for each formulation.
Particle size analysis and surface morphology of microspheres
A small amount of microspheres was suspended in absolute ethanol and the average particle size was determined using a particle size analyser (Mastersizer 2000 Malvern, UK). Shape and surface morphology of microparticles were evaluated by scanning electron microscopy (SEM, Philips XL30, Netherlands). Microspheres were attached to a specimen holder and coated by gold sputter coater (BAL-TEC SCD 005, Switzerland) before observation.
Thermal analysis of microspheres
Differential scanning calorimetry (DSC) (Shimadzu DSC 60, Japan) was performed to evaluate cross linking of gelatin. Drug free microspheres (8-10 mg) were placed in sealed aluminium pans and heated at the rate of 10°/min for the range of 20-250°. An empty aluminium pan was used as reference.
Statistical analysis
Statistical analysis was performed using SPSS software. For comparing each variable, Student’s t-test or analysis of variance (ANOVA), followed by Tukey’s post hoc test were used. The differences of P<0.05 were interpreted as statistically significant.
Results and Discussion
The characteristics of the prepared microspheres were presented in Table 2. Based on the results, except for Fb7, all compositions led to microspheres formation. The yield value for Fb group was acceptable [11] and higher than Fa group. EE and average size of microspheres in Fb group were in the range of 23.6- 63.3% and 34.6-148.2 μm, respectively. For Fa group, because of the very low yield and EE, other properties such as particle size were not evaluated.
| Formulation | Yield value (%) |
Drug loading (%) |
EEa (%) |
Average size (µm) |
|---|---|---|---|---|
| Fa1 Fa2 Fb1 Fb2 Fb3 Fb4 Fb5 Fb6 Fb7 Fb8 |
21.3 29.4 86.2 80.7 72.5 88.1 71.0 63.7 -d 96.2 |
0.4±0.03c 0.7±0.08 2.3±0.09 2.2±0.15 2.4±0.05 2.6±0.03 3.0±0.45 2.7±0.06 - 1.7±0.11 |
5.1±3.4c 9.8±4.1 31.0±2.1 40.7±1.3 54.7±1.8 35.0±1.1 55.5±0.9 63.3±2.3 - 23.6±2.7 |
NDb ND 102.0±1.8c 130.5±2.1 148.2±2.0 57.5±0.9 92.8±0.8 104.4±1.0 - 34.6±1.3 |
Table 2: Physicochemical Properties of Mx-Gms, (N=3)
As was mentioned earlier, two different methods were used for crosslinking of gelatin microspheres. For group Fa, in which dried untreated microspheres were transferred to GA solution, yield value was decreased about 70% in comparison to the initial untreated particles. EE of Fa1 and Fa2 formulations after crosslinking step, was also decreased significantly (%EE of Fa1 and Fa2 formulations before crosslinking process were 25.2 and 29.5%, respectively). Also the shape of these microspheres was not spherical. According to the results, the above mentioned method could not be considered as an appropriate method. It seems that during the crosslinking step, a large portion of microspheres were eroded and the encapsulated drug was released. Increasing the concentration of GA solution from 1 to 5% v/v had no significant effect on yield and EE values.
On the other hand, using GA solution before congealing step (group Fb) resulted in spherical microspheres with higher yield values and encapsulation efficiencies compared to the formulations Fa. Adding GA to the emulsion before congealing step resulted in faster cross-linking of gelatin and consequently reducing drug leaching from droplets; therefore %EE of formulations Fb1 and Fb4 were 6 and 3.5 folds higher than that of Fa1 and Fa2, respectively. Also 2.6-4 folds increase in yield values was observed for formulations Fb1 and Fb4 in comparison to Fa1 and Fa2. Therefore this method was used for the preparation of MX-GMs.
Polymer amount and crosslinking agent concentration are two important parameters that could affect microspheres properties including yield value, drug loading and particle size. As shown in Table 2, by increasing gelatin concentration from 25 to 45% w/v in Fb1-Fb3 and Fb4-Fb6 formulations, yield value was decreased. In overall, the same trend was observed by increasing GA concentration from 1 to 5% v/v. It seems that by using higher polymer concentration, viscosity of dispersed phase and in turn adhesion of polymer to the surfaces increases, resulting in lower yield value. Also application of GA solution with higher concentration 10% v/v in Fb7, resulted in a rigid mass of polymer created around the impeller with no spherical particles. This indicates that an optimum GA concentration must be applied in order to obtain appropriate microspheres.
Gelatin and GA solution concentration also had significant effect on EE of microspheres. Based on the results, increasing the polymer concentration from 25 to 45% w/v in Fb1-Fb3 and Fb4-Fb6 resulted in an improvement about 76-80% in EE value. As indicated by Esposito et al., the lipophilicity of drug molecule has significant effect on EE. During the emulsification step, hydrophobic drugs (such as MX) might diffuse from the aqueous internal phase to the external continuous oil phase [24]. It seems that the viscosity enhancement due to the higher gelatin concentration reduce drug diffusion from gelatin droplets to the external oil phase leading to improvement of the EE values. Also increasing GA concentration had a positive effect on the EE values. Probably, due to the high concentration of GA solution, a rigid network was formed that restricts leaching of the drug molecules during preparation procedure [25].
With regard to the particle size, as shown in Table 2, by increasing gelatin amount at both low and high levels of GA solution, a 1.5-1.8 fold increase in the average size of microspheres was observed (P<0.05). This result can be rationalized that the presence of more gelatin and thus higher viscosity of internal phase, causes formation of larger droplets in continuous phase and increase microspheres size. Also, as was reported by previous studies, at constant amount of gelatin, employing higher GA concentration led to reduction of size of microspheres, which could be attributed to the formation of more rigid network structure in the presence of higher cross-linking agent and in turn more shrinkage of prepared microspheres [25,26]. On the other hand, according to Saravanan et al., drug loading enhancement could increase average size of microparticles [20].
In the emulsioncongealing-chemical crosslinking method, which was used in the present study, presence of emulsifier has critical role in the formation of emulsion. Also type and concentration of emulsifier have significant effect on the properties of internal phase droplets. As shown in Table 2, by increasing Span 80 concentration from 0.2% Fb4 to 0.3% in Fb8 formulation, yield value was increased to 96.2%. The presence of higher emulsifier concentration in the preparation medium could decrease surface tension of gelatin droplets and adhesion of them to the surfaces, leading to improvement of the yield value. However, 32.6% and 39.8% reduction was observed in EE and average particle size values, respectively, by increasing emulsifier concentration (P<0.05). As was previously reported, by increasing emulsifier concentration, solubility of a lipophilic drug in the external phase increases and as a result, EE of drug would decrease [27,28]. Besides, diffusion of hydrophobic compound to the external phase would be facilitated due to the formation of smaller droplets of internal phase in the presence of higher Span 80 concentration [24]. On the other hand, by decreasing emulsifier concentration, stability of internal phase droplets may decrease and droplets tend to coalescence, resulting in larger microparticles [27].
Release profile of drug from microspheres has significant effect on drug bioavailability and its therapeutic efficacy. Cumulative release percent of MX from different formulations in PBS at 15 min, 1 h, 4 h, 8 h and 24 h were shown in Table 3. According to the results, MX was released with high initial burst from all microparticles except for Fb4. By comparing Q15 for Fb1-Fb3 and Fb4-Fb6, initial burst effect was increased significantly (P<0.05). Microspheres with high EE (%) showed higher initial burst effect and vice versa. This relationship between microparticles drug loading and drug release rate has been reported previously [20]. It is probable that in microparticles with higher EE values, more drug particles were present on the surfaces, leading to marked burst release. In addition, dissolution of drug from microspheres with more EE values could facilitate drug diffusion through interconnected channels [29,30]. Based on the previous studies, concentration of both gelatin and GA solution has significant effect on release profile of microspheres [25,26]. In fact the ratio of crosslinking agent to polymer can change drug release profile. The results suggest that for Fb4, this ratio was appropriate, so that release of MX from microparticles was slower than other formulations. Also the colour of Fb4 microspheres was darker than other formulations, indicating higher crosslinking density in these microparticles and as a result, slower drug diffusion rate [31]. It should be mentioned that although Fb4 had smaller particle size than Fb1, but due to the use of higher cross linking agent and more rigid structure, its drug release rate was lower significantly (P<0.001). Fb8 formulation had higher drug release rate in comparison to Fb4, which could be attributed to the smaller particle size of Fb8 microspheres.
| Formulation | Q15(%) | Q1h (%) | Q4h (%) | Q8h(%) | Q24h(%) |
|---|---|---|---|---|---|
| Fb1 Fb2 Fb3 Fb4 Fb5 Fb6 Fb8 |
51.2±0.11 80.0±0.07 88.0±0.06 29.3±0.09 77.5±0.04 80.4±0.10 79.1±0.11 |
63.4±0.17 83.2±0.06 89.2±0.20 33.3±0.09 79.0±0.15 83.1±0.21 82.0±0.07 |
70.1±0.12 84.1±0.14 93.0±0.07 35.4±0.07 80.1±0.10 86.1±.14 85.3±0.10 |
74.5±0.03 87.1±0.25 95.1±0.06 37.1±0.06 82.4±0.08 87.1±0.10 88.4±0.05 |
80.3±0.10 92.5±0.23 98.7±0.21 41.1±0.13 86.3±0.09 94.0±0.13 94.2±0.09 |
Table 3: Cumulative Release Percent of Meloxicam from Mx-Gms (mean±Sd; N=3)
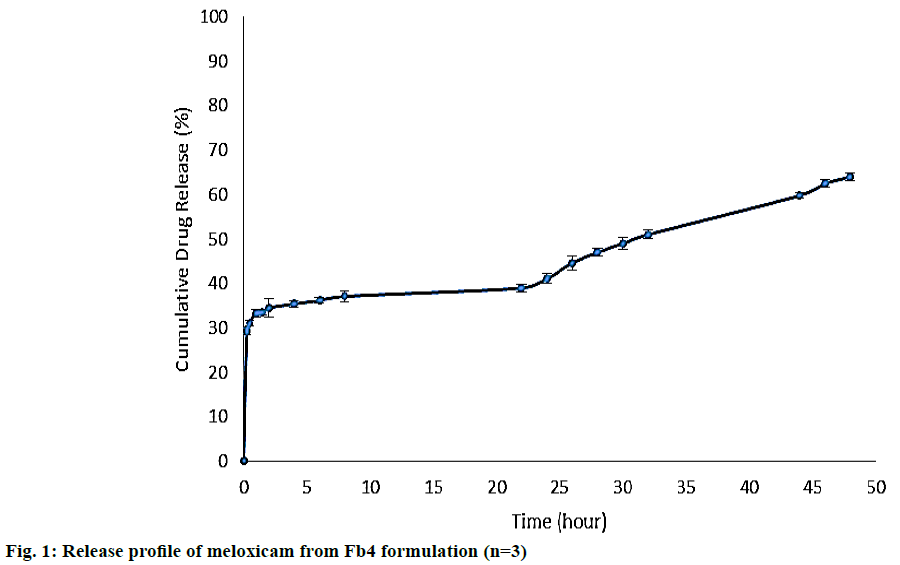
Because of lower drug release rate from Fb4 microspheres, the release study was exceeded up to 48 h. As shown in Figure 1. The burst drug release was approximately 30% and reached to 64% after 48 h. The initial burst effect seems to be due to drug molecules that are not entrapped but adsorbed on the surface of microspheres [11] and the later slower release is related to the degradation of micro particles. As was reported by previous studies, micro particles absorb high amounts of water in the release medium and swell noticeably. After reaching to swelling equilibrium, disintegration of microspheres starts, followed by degradation. By increasing GA amount, the degradation and drug release rate will be slower [26]. This release behaviour is preferred for MX microspheres, because initial burst can produce an immediate therapeutic effect, followed by the prolonged release of drug that could keep this effect [20].
The release kinetics of Fb4 formulation was investigated for two release phases (1-22 h and 22-48 h) separately, ignoring the burst release, using three different models including zero order, first order and Higuchi equation [32]. Based on the squared correlation coefficient (R2) depicted in Table 4, the first phase of release profile was in accordance to the Higuchi model. However, there is no evidence to specify the dominant kinetics model for the second phase of release profile.
| Release phase | Zero order | First order | Higuchi equation | |||
|---|---|---|---|---|---|---|
| R2 | k(mg.h-1) | R2 | k (h-1) | R2 | k(mg.h-0.5) | |
| Phase 1 | 0.837 | 0.259 | 0.847 | 0.004 | 0.943 | 1.5924 |
| Phase 2 | 0.982 | 0.888 | 0.983 | 0.018 | 0.985 | 10.47 |
Table 4: Correlation Coefficient (R2) and Release Rate Constant (K) of Meloxicam from Optimum Formulation Based on Various Models
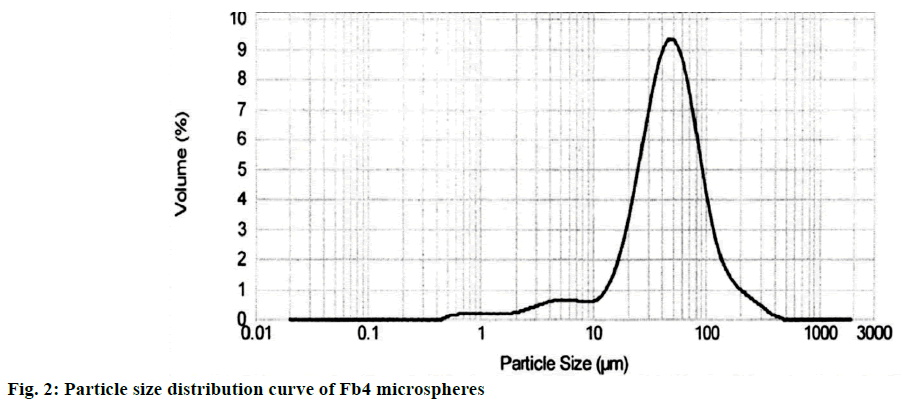
According to the results of release studies and particle size analysis, Fb4 was selected as an optimum formulation. As was reported previously, the best range of particle size that can be injected by using a conventional needle with maximum retention at the injected joint is in the range of 1-70 μm [20]. The average size of Fb4 microspheres was obtained 57.5 μm with narrow particle size distribution curve (Figure 2).
Surface morphology of selected microspheres (Fb4) was determined by SEM analysis. To investigate the effect of cross-linking on surface morphology of micro particles, another formulation (Fu) was prepared, in which all preparation parameters were similar to Fb4 except that cross-linking agent was not used. As shown in Figure 3, both microspheres had spherical geometry. However, untreated microspheres showed wrinkled surfaces, but GA treated micro particles had smooth surfaces indicating presence of more rigid network structures [33].
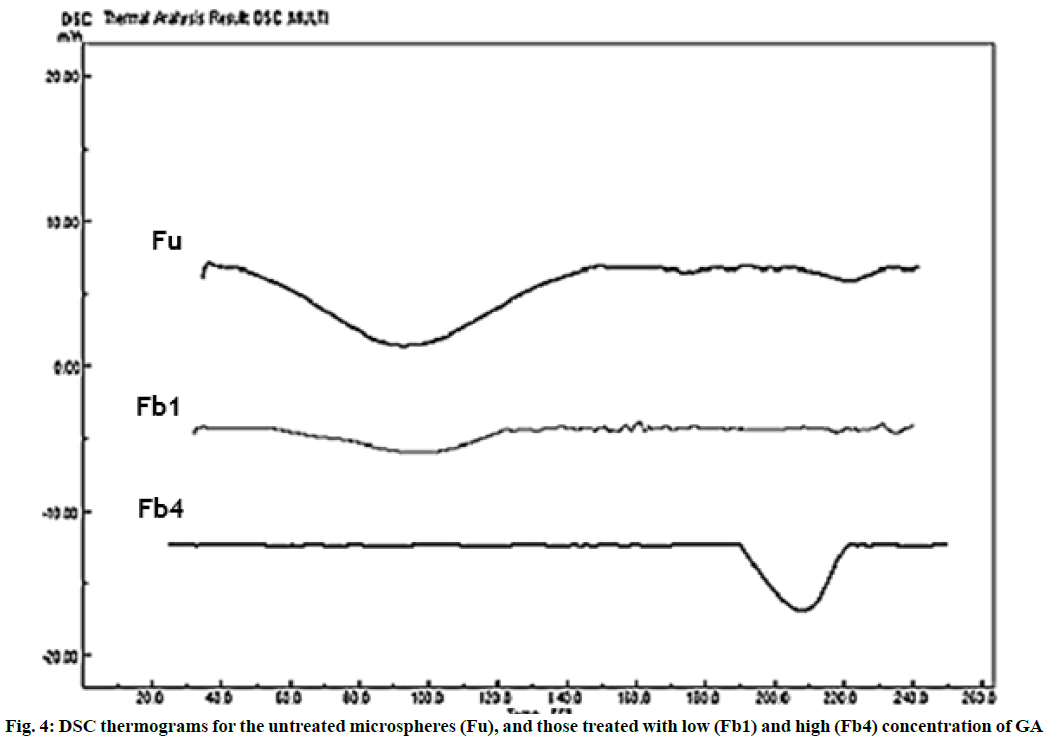
Crosslinking of gelatin would change its thermal behaviour and shift polymer glass transition temperature (Tg) to a higher value. In this study, thermal behaviours of drug-free microparticles were evaluated. As shown in Figure 4. Tg of Fu, Fb1 and Fb4 microspheres could be observed at 92.08, 96.75 and 208.1°, respectively. In these three formulations, gelatin concentration and all other variables were similar and only the amount of crosslinking agent used during the preparation process was different. As the crosslinking density increases, thermal stability of microspheres would improve, therefore the Tg of gelatin, shifts to higher values [33,34]. Tg value of gelatin for Fb1 was close to the untreated microspeheres, which may be described by a very low degree of crosslinking in this formulation. The higher Tg value of gelatin for Fb4, could explain the slower release rate of drug from these microparticles.
In the present study, MX loaded gelatin microspheres were prepared by emulsion-congealing-chemical crosslinking method. GA was used as chemical crosslinking agent and its concentration had significant effect on microspheres properties. Gelatin concentration and polymer to cross-linker ratio also altered the physicochemical properties of micro particles such as EE, size and drug release profile. The optimized microspheres (Fb4) showed slow and biphasic MX release that is suitable for using as an intra-articular drug delivery system for RA treatment.
Financial support and sponsorship
Nil.
References
- Butoescu N, Jordan O, Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: a review of the factors influencing their performance. Eur J Pharm Biopharm 2009;73:205-18.
- Fernández-Carballido A, Herrero-Vanrell R, Molina-Martinez IT, Pastoriza P. Biodegradable ibuprofen-loaded PLGA microspheres for intraarticular administration: effect of labrafil addition on releasein vitro. Int J Pharm 2004;279:33-41.
- Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, et al. Intra‐articular depot formulation principles: Role in the management of postoperative pain and arthritic disorders. J Pharm Sci 2008;97:4622-54.
- Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.Cyclooxygenase-2 selective non-steroidal antiinflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economicevaluation. Health Tachnol Assess 2008;12:1-278.
- AHFS Drug Information. American Society of Health-System Pharmacists 2011;2164-66.
- Kawadkar J, Jain R, Kishore R, Pathak A, Chauhan MK. Formulation and evaluation of flurbiprofen-loaded genipin cross-linked gelatin microspheres for intra-articular delivery. J Drug Target 2013;21:200-10.
- Edwards SH. Intra-articular drug delivery: the challenge to extend drug residence time within the joint. Vet J 2011;190:15-21.
- Sam M, Gayathri D, Prasanth V, Vinod B. NSAIDs as microspheres. Internet J Pharmacol 2007;6:13.
- Atkinson TJ, Fundin J, Jahn HL, Kubotera N, Rennick AL, Rhorer M. What's new in NSAID pharmacotherapy: oral agents to injectables. Pain Med 2013;14:11-7.
- Thakkar H, Sharma RK, Mishra AK, Chuttani K, Murthy RS. Celecoxib incorporated chitosan microspheres: in vitroand in vivoevaluation. J Drug Target 2004;12:549-57.
- Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA.Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations andin vitro/in vivo evaluation. Int J Pharm 2000;195:179-88.
- Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA. In vitro andin vivo evaluation of diclofenac sodium loaded album in microspheres. J Microencapsul 2000;17:145-55.
- BozdagS,Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA. In vitro evaluation and intra-articular administration of biodegradable microspheres containing naproxen sodium. J Microencapsul 2001;18:443-56.
- Zhang Z, Bi X, Li H, Huang G. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Del 2011;18:536-44.
- KawadkarJ,Chauhan MK.Intra-articular delivery of genipin cross-linked chitosan microspheres of flurbiprofen: Preparation, characterization, in vitroand in vivostudies. Eur J Pharm Biopharm 2012;81:563-72.
- Zhang Z, Huang G. Intra-articular lornoxicam loaded PLGA microspheres: enhanced therapeutic efficiency and decreased systemic toxicity in the treatment of osteoarthritis. Drug Del 2012;19:255-63.
- Dinarvand R, Rahmani E, Farbod E.Gelatin microspheres for the controlled release of all-trans-retinoic acid topical formulation and drug delivery evaluation. Iran J Pharm Res 2010;2:47-50.
- Atyabi F, Vahabzadeh R, Dinarvand R.Preparation of ethylcellulose coated gelatin microspheres as a multiparticulate colonic delivery system for 5-aminosalicilic acid. Iran J Pharm Res 2010;3:81-6.
- Zhang Z, Huang G. Micro-and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J Nanotech 2012;1-11.
- Saravanan M,Bhaskar K, Maharajan G, Pillai KS. Development of gelatin microspheres loaded with diclofenac sodium for intra-articular administration. J Drug Target 2011;19:96-103.
- Lu Y, Zhang G, Sun D, Zhong Y. Preparation and evaluation of biodegradable flubiprofen gelatin micro-spheres for intra-articular administration. J Microencapsul 2007;24:515-24.
- Starek M, Krzek J. TLC determination of meloxicam in tablets and after acidic and alkaline hydrolysis. ActaPolon Pharm. Drug Res 2012;69:225-35.
- Kılıçarslan M, Baykara T.The effect of the drug/polymer ratio on the properties of the verapamil HCl loaded microspheres. Int J Pharm 2003;252:99-109.
- Esposito E, Cortesi R, Nastruzzi C. Gelatin microspheres: influence of preparation parameters and thermal treatment on chemico-physical and biopharmaceutical properties. Biomaterials 1996;17:2009-20.
- Kumar P, Singh I.Formulation and characterization of tramadol-loaded IPN microgels of alginate and gelatin: Optimization using response surface methodology. Acta Pharm 2010;60:295-310.
- Muvaffak A, Gurhan I, Hasirci N. Prolonged cytotoxic effect of colchicine released from biodegradable microspheres. J Biomed Mater Res B Appl Biomater 2004;71:295-304.
- Dinarvand R, Mirfattahi S, Atyabi F. Preparation, characterization and in vitrodrug release of isosorbide dinitrate microspheres. J Microencapsul 2002;19:73-81.
- Fu X, Ping Q, Gao Y.Effects of formulation factors on encapsulation efficiency and release behaviour in vitroof huperzine A-PLGA microspheres. J Microencapsul 2005;22:705-14.
- Forni F, Vandelli MA, Cameroni R. Influence of drug loading level on drug release and dynamic swelling of crosslinked gelatin microspheres. J Microencapsul 1992;9:29-39.
- Egbaria K,Friedman M. Sustained release albumin microspheres containing antibacterial drugs: effects of preparation conditions on kinetics of drug release. J Control release 1990;14:79-94.
- Cortesi R, Esposito E, Osti M, Squarzoni G, Menegatti E, Davis SS, et al. Dextran cross-linked gelatin microspheres as a drug delivery system. Eur J Pharm Biopharm 1999;47:153-60.
- Shaikh HK, Kshirsagar RV, Patil SG. Mathematical models for drug release characterization: a review. WJPPS 2015;4:324-38.
- Cortesi R, Nastruzzi C, Davis SS. Sugar cross-linked gelatin for controlled release: microspheres and disks. Biomaterials 1998;19:1641-49.
- Bigi A, Cojazzi G, Panzavolta S, Rubini K, Roveri N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001;22:763-8.