- *Corresponding Author:
- Shweta Jain
College of Veterinary Science and Animal Husbandry, Anjora, Durg, Dau Shri Vasudev Chandrakar Kamdhenu Vishwavidyalaya, Durg 481001, India
E-mail: drshwetavety@yahoomail.co.in
| Date of Received | 30 December 2025 |
| Date of Revision | 20 August 2025 |
| Date of Accepted | 12 March 2025 |
| Indian J Pharm Sci 2025;87(6):288-296 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
People and pharmaceuticals of world are attracting towards herbs and alternates for prophylactic and therapeutic uses based on their folklore utilization. Hygrophila spinosa plant was extensively used in traditional systems of medicine for treating various ailments like jaundice, rheumatism, anemia, inflammation, pain, urinary infections, oedema and gout. According to the Chinese pharmaceutical dictionary cow urine had been used as a medium for delivery of medicinal herbs to strengthen their effects. Cow urine distillate (ark) maintains well-being and increases the effectiveness of antimicrobial, antifungal and anticancer drugs. But studies on bio enhancing effect of cow urine distillate with herbs on hepatoprotection are not available. Therefore, present work is undertaken to study the bio enhancing potential of Kosli cow urine distillate in amelioration of carbon tetrachloride induced hepatotoxicity by Hygrophila spinosa Hydroalcoholic extract in Albino rats.
Keywords
Bio enhancing, traditional, hepatotoxicity, Hygrophila spinosa, cow urine distillate
Hygrophila spinosa (H. spinosa) plant is widely distributed and used as a folk medicine in tropical Africa, India, China, Nepal and Malaysia [1]. It is also known as Indian cuckoo and Asteracantha longifolia (L.) Nees [2]. Therapeutic value of plant has also been reported in Homeopathy, Unani and Siddha system of medicine, where whole plant as well as seeds, roots, flowers, leaves, stems and aerial parts have been utilized for various ailments and disorders [3]. Various parts of plant are extensively used in traditional systems of medicine for treating ailments like jaundice, rheumatism, anemia, inflammation, pain, urinary infections, oedema and gout.
Cow urine has been used extensively in Indian system of medicinal preparations since time immemorial as cited in ancient holy texts. In recent decades researches on Cow Urine Distillate (CUD) are gaining interest unveiling its therapeutic potential scientifically. On the basis of characterization through High-Performance Liquid Chromatography (HPLC), it has been proved that the urine of Indian cows is highly effective [4]. Kamadhenu Ark from the urine of Indian cows manufactured by Govigyan Anusandhan Kendra, Nagpur showed immunomodulatory properties in mice by increase in T and B lymphocyte blastogenesis and Immunoglobulin G (IgG), Immunoglobulin M (IgM) and Immunoglobulin A (IgA) antibody titres. The therapy also protected the Deoxyribonucleic Acid (DNA) from oxidative damage, responsible for ageing, cancer etc. [5] CUD is more effective as a bio enhancer than cow urine and increases the effectiveness of antimicrobial, antifungal and anticancer drugs [6]. According to the Chinese pharmaceutical dictionary “Shang Han Lun”, cow urine had been used as a medium for delivery of medicinal herbs to strengthen their effects [7]. Bio enhancing effect of cow urine has been investigated on antimicrobial, antibiotic and anticancer activity of various drugs but its ameliorative effect with herbs has not been researched on hepatoprotection.
Liver injury is a significant toxicological problem and protection of liver is a prime requisite to maintain health and safety of body. Carbon tetrachloride (CCl4) is a model drug to study xenobiotic-induced acute and chronic hepatotoxicity. It causes cellular damage in multiple organs, mostly in the liver, kidneys, and lungs. It induces oxidative damage, inflammation, fatty degeneration and fibrosis in the liver [8]. Several studies indicate that antioxidants can prevent the risk of liver diseases [9]. Considering the side effects of synthetic antioxidants, natural antioxidants are gaining much attention. Many plants and cow urine including its distillate exhibited antioxidants property, so may prove better options for hepatoprotection. With this view, present work was conducted to study bioenhancing effect of Kosli CUD in amelioration of hepatoprotection by H. spinosa in Albino rats.
Materials and Methods
Preparation of HSE and CUD: Leaves of H. spinosa from paddy fields of district Rajnandgaon, (C.G.) were collected, shade dried, powdered and Hydroalcoholic extract (30 % distilled water and 70 % methanol) was prepared by Soxhlet apparatus. Natural voiding, morning, mid-stream urine from Kosli cows of 2 y-3 y age was collected in a sterile container and distillate was prepared and stored at 4?.
Experimental animals: Thirty-six adult healthy Albino rats of either sex weighing 150 g to 200 g from Laboratory Animal House, College of Veterinary Science and A. H., Anjora, Durg were randomly allocated in six groups for 28 d study. The animals were housed in polypropylene cages and maintained under standard laboratory conditions (27?±2? temperature and 12/12 h light/dark cycle) throughout the experiment. The study was carried out with prior approval by the Institutional Animal Ethical Committee (IAEC), College of Veterinary Science and Animal Husbandry, Durg (C.G.), India (No. 445/ GO/ReBi/S/01/CPCSEA) and followed the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Following treatments were given to different groups of animals; Group I control: Normal saline orally; Group II CCl4 1.0 ml/kg i. p. Once in every 72 h (negative control); Group III CCl4 1.0 ml/kg i. p once in every 72 h+Sylimarin (50 mg/kg, p.o.) once a day (positive control); Group IV CCl4 1.0 ml/kg i. p once in every 72 h+HSE@ 400 mg/kg b.wt orally daily; Group V CCl4 1.0 ml/kg i.p once in every 72 h+CUD 5 ml/kg b.wt orally once daily and Group VI CCl4 1.0 ml/kg i.p once in every 72 h+HSE @ 400 mg/kg b.wt orally once daily+CUD 5 ml/kg b.wt orally daily. CCl4 (30 % in olive oil) was used to induce hepatotoxicity. The rats were monitored daily for general health, mortality, behavioural abnormality, signs and symptoms of toxicity. Body weight changes of rats were recorded at weekly intervals for 4 w (on d 0, 7, 14, 21 and 28 of experiment).
Collection of samples: On d 29 of experiment, blood samples were collected from all the animals from retro-orbital plexus. Serum was harvested for biochemical analysis. For estimation of antioxidant parameters blood was collected with Acid citrate dextrose @ 1.5 ml/10 ml blood. Tissue samples of rats were collected after humane sacrifice by cervical dislocation under light anaesthesia. Liver was collected rapidly, some part was immediately deep frozen for analysing lipid per oxidation and some part is preserved in 10 % formalin solution for histopathological examination.
Haematological parameters: Haemoglobin, Packed Cell Volume (PCV), Total Erythrocyte Count, Total Leucocyte Count, Differential Leucocyte Count, Erythrocyte indices (Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH) and Mean Corpuscular Haemoglobin Concentration (MCHC)) were recorded [10].
Biochemical parameters: Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALP), Blood Urea Nitrogen (BUN), Creatinine, Cholesterol, Total protein, Bilirubin, Albumin, Albumin: Globulin ratio and Calcium were analysed using standard diagnostic kits (BIOLAB DIAGNOSTICS, India) by semi-auto analyser (Elitech Microlab 300).
Gross and histopathology: Small pieces of liver fixed in 10 % neutral formalin were dehydrated in ascending grades of alcohol and embedded in paraffin wax. Paraffin sections (5 μm thick) were stained for routine histological study using Haematoxylin and Eosin (H&E).
Anti-oxidant status: Lipid peroxidation [11], Reduced Glutathione (GSH) [12] and Glutathione Peroxidase (GSH-Px) [13] were analysed in Blood sample. Lipid peroxidation was also analysed in Liver tissue.
Statistical analysis: Data obtained was analysed statistically by using IBM Statistical Package for Social Sciences (SPSS) version 25 software and presented as a mean±standard error of the mean (M±SEM) at (p≤0.05) level of significance.
Results and Discussion
Effect of H. spinosa hydro-alcoholic extract, CUD and their combination on body weight of rats with CCl4 induced hepatotoxicity is presented in Figure 1 and Table 1. Decrease in body wt. of Group II rats on 21st and 28th d indicated toxic response to CCl4, whereas significant (p≤0.05) increase in body wt. of group I and III; progressive increase in body wt. in Group IV, V and VI animals indicated the protective effect of these treatments against CCl4 intoxication. High dose of Hydroalcoholic extract of H. auriculata (200 mg/kg) also provided hepatoprotection revealed by significant increase in body weight against significant decrease in body weight of rats was found with induced hepatotoxicity [14].
| Group | I | II | III | IV | V | VI | Level of significance | |
|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | CCl4 (1.0 ml/kg) | CCl4 (1.0 ml/kg)+Silymarine 50 mg/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg | CCl4 (1.0 ml/kg) +CUD 5 ml/kg | CCl4 (1.0 ml/kg)+CUD: 400 mg/kg+HSE 5 ml/kg | ||
| Average body weight (g) | 0 d | 164.000±8.629 | 164.833±3.885 | 167.000±5.865 | 165.667±4.279 | 166.667±2.716 | 163.167±5.540 | NS |
| 7 d | 172.833±7.998 | 164.667±3.756 | 177.167±7.791 | 175.833±4.989 | 174.500±3.085 | 174.333± 6.286 | NS | |
| 14 d | 177.833±7.282 | 166.333±5.493 | 185.333±8.015 | 180.667±5.731 | 182.500±2.717 | 181.833±7.888 | NS | |
| 21 d | 185.500±7.535ab | 170.667±5.377b | 191.500±7.680a | 186.833±6.101ab | 187.167±2.358ab | 186.000±6.658ab | * | |
| 28 d | 196.833±7.791a | 173.500±4.303b | 197.167±7.364a | 190.500±5.408ab | 191.167±4.045ab | 191.500±6.206ab | * | |
Note: The values are Mean±Standard error of mean of n=6 animals. Different superscripts show significant difference (*p<0.05) in a row. NS: Non Significant
Table 1: Effect of H. Spinosa Hydro-alcoholic extract, CUD and their combination on average body weight (g) of rats with CCl4
The results of administration of H. spinosa hydroalcoholic extract, CUD and their combination on haemato-biochemical parameters of rats with CCl4 induced hepatotoxicity are presented in Table 2 and Table 3. Haemoglobin concentration and PCV percent of toxicant treated group (Group II) were significantly decreased as compared to other groups. Total Erythrocytes Count showed significant (p≤0.05) decrease in Group II rats, whereas, it was increased in Group IV as well as Group VI rats. Total leucocyte count was significantly (p≤0.05) increased in Group II as compared to control and other treatment groups, but it was under normal range. There was no significant (p≤0.05) difference recorded in MCV, MCH, MCHC and DLC among different treatment groups.
| Group | I | II | III | IV | V | VI | Level of significance |
|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | CCl4 (1.0 ml/kg) | CCl4 (1.0 ml/kg)+ Silymarine 50 mg/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg | CCl4 (1.0 ml/kg)+CUD 5 ml/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg+CUD: 5 ml/kg | |
| Hb (g/dl) | 13.894±0.167a | 12.605±0.397b | 13.849±0.236a | 14.394±0.360a | 13.905±0.190a | 14.549±0.272a | * |
| PCV (%) | 36.833±1.014a | 34.167±0.477b | 37.333±0.919a | 39.167±0.872a | 38.500±1.088a | 39.000±0.730a | * |
| TEC (millions/?l of blood) | 8.305±0.309ab | 7.613±0.161b | 8.318±0.315ab | 8.505±0.186a | 8.248±0.191ab | 8.601±0.120a | * |
| TLC (thousand/?l of blood) | 5.150±0.268b | 5.783±0.114a | 5.083±0.111b | 5.183±0.168b | 5.192±0.236b | 5.117±0.158b | * |
| MCV (fl) | 44.682± 2.120 | 44.953±0.928 | 45.222±2.124 | 46.139±1.277 | 46.918±2.245 | 45.352±0.661 | NS |
| MCH (pg/dl) | 16.841±0.631 | 16.592±0.620 | 16.778±0.749 | 16.951±0.471 | 16.911±0.503 | 16.933±0.391 | NS |
| MCHC (g/dl) | 37.821±0.807 | 36.882±0.961 | 37.189±0.985 | 36.792±0.920 | 36.260±1.102 | 37.372±0.982 | NS |
| Neutrophils (%) | 30.000±1.897 | 29.500±1.384 | 28.167±1.078 | 26.333±1.382 | 26.833±1.400 | 29.333±1.626 | NS |
| Eosinophils (%) | 0.833±0.307 | 0.500±0.342 | 0.667±0.333 | 0.667±0.333 | 0.500±0.224 | 0.667±0.333 | NS |
| Basophils (%) | 0.500±0.224 | 0.333±0.333 | 0.333±0.211 | 0.500±0.224 | 0.333±0.211 | 0.333±0.211 | NS |
| Monocytes (%) | 1.333±0.211 | 0.833±0.167 | 0.667±0.333 | 0.500±0.224 | 0.667±0.333 | 0.500±0.342 | NS |
| Lymphocytes (%) | 68.500±1.232 | 68.833±2.007 | 70.333±1.282 | 72.500±1.335 | 69.667±0.760 | 69.167±1.833 | NS |
Note: The values are Mean±Standard error of mean of n=6 animals. Different superscripts show significant difference (*p<0.05) in a row. NS: Non significant
Table 2: Effect of H. Spinosa hydro-alcoholic extract, CUD and their combination on hematological parameters of rats with CCl4 induced hepatotoxicity
| Group | I | II | III | IV | V | VI | Level of Significance |
|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | CCl4 (1.0 ml/kg) | CCl4 (1.0 ml/kg)+Silymarine 50 mg/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg | CCl4 (1.0 ml/kg)+CUD 5 ml/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg+CUD: 5 ml/kg | |
| AST (IU/l) | 134.167±2.971c | 231.333±2.603a | 140.833±0.477b | 143.667±1.022b | 145.667±1.498b | 142.833±1.641b | * |
| ALT(IU/l) | 76.500±2.487c | 146.500±2.540a | 84.500±1.544b | 88.500±1.335b | 89.500±1.147b | 85.500±1.839b | * |
| ALP(IU/l) | 99.278±1.056c | 174.425±1.435a | 103.282±1.429bc | 107.177±1.630b | 108.525±3.314b | 106.180±1.441b | * |
| Total protein (g/dl) | 6.787 ±0.312a | 4.895±0.153b | 6.605±0.133a | 6.637±0.105a | 6.430±0.182a | 6.662±0.236a | * |
| Albumin (g/dl) | 4.818±0.270a | 3.825±0.225b | 4.832±0.188a | 4.840±0.335a | 4.790±0.213a | 4.815±0.386a | * |
| A: G ratio | 1.297±0.171 | 1.414±0.191 | 1.303±0.104 | 1.346±0.171 | 1.357±0.137 | 1.288±0.228 | - |
| Bilirubin total (mg/dl) | 0.910±0.124b | 1.691±0.031a | 0.966±0.078b | 0.998±0.118b | 1.030±0.076b | 0.931±0.123b | * |
| Bilirubin conjugated (mg/dl) | 0.350±0.066b | 0.758 ±0.087a | 0.370 ±0.090b | 0.393 ±0.031b | 0.407 ±0.026b | 0.352 ±0.022b | * |
| Cholesterol (mg %) | 31.430±0.890c | 52.527 ±0.844a | 34.848 ±0.777b | 35.250 ±0.651b | 31.977±0.684c | 32.035±1.150c | * |
| BUN (mg/dl) | 11.667±0.803b | 23.333 ±0.919a | 13.667±1.282b | 14.333±1.358b | 14.500±1.057b | 12.667±0.667b | * |
| Creatinine (mg/dl) | 0.422±0.015b | 0.748±0.045a | 0.430±0.021b | 0.425±0.010b | 0.442±0.034b | 0.418±0.029b | * |
| Calcium(mg/dl) | 9.318±0.390a | 7.398±0.661b | 9.270±0.418a | 9.117±0.285a | 9.175±0.471a | 9.263±0.336a | * |
Note: The values are Mean±Standard error of mean of n=6 animals. Different superscripts show significant difference (*p<0.05) in a row. NS: Non significant
Table 3: Effect of H. Spinosa hydro-alcoholic extract, CUD and their combination on biochemical parameters in rats with CCl4 induced hepatotoxicity
Acephate induced toxicity caused significant reduction in the level of TEC, Thin Layer Chromatograph (TLC), Hb, PCV and increase in MCV and neutrophil percent [15]. Immunomodulatory effect of CUD in healthy and cyclophosphamide induced immunosuppressive mice revealed significant decrease in TLC and Lymphocyte count in cyclophosphamide induced immunosuppressed mice as compared to control group. Treatment with CUD (2 ml/kg, 4 ml/kg and 6 ml/kg) significantly increased TLC and lymphocyte count in immunosuppressed mice treated in dose dependent manner [16].
Induction of hepatotoxicity significantly (p≤0.05) increased the activity of liver function marker enzymes viz., Aspartate Amino Transferase (AST), Alanine Amino Transferase (ALT) and Alkaline phosphatase (ALP) in rats as compared to the control group rats. Activity of theses enzymes revealed significant (p≤0.05) recovery in animals of Group III, IV, V and VI. ALT and AST are clinically significant aminotransferases, which are linked with amino acid metabolism. Moreover, Phosphatases (ALP and ACP) are membrane bound enzymes and alteration in activity of these indicates membrane disintegration affecting membrane permeability and transport of metabolites. These enzymes are considered as important liver injury markers as liver is the major site of metabolism. CCl4 induced hepatocellular injury, leakage of these enzymes into blood and elevated their level. Decrease in serum biomarker enzymes by treatment with Sylimarin, HSE, CUD and their combination indicated prevention of cellular damage and leakage of these enzyme and maintenance of cellular integrity showing hepatoprotective activity. Significant decrease in activity of liver marker enzymes AST, ALT and ALP by H. auriculata in mercuric chloride induced toxicity in rats were explored [17]. Hepatoprotective activity of H. auriculata (100 mg/kg) against Isoniazid 100 mg/kg) and Rifampicin (100 mg/kg) induced hepatotoxicity in rats was also unveiled [14].
Serum total protein and serum albumin concentration in rats with induced hepatotoxicity were significantly decreased (p≤0.05). Significant (p≤0.05) elevated levels of total and conjugated bilirubin were observed in Group II rats and recovery in level of these biochemical parameters in Group III, IV, V and VI showed the hepatoprotection provided by administration of Silymarin, HSE, CUD and combination of HSE and CUD. Cholesterol level was significantly increased in group II rats, which was significantly decreased by treatment with HSE, CUD and their combination, this recovery was maximum with treatment by CUD and combination of HSE and CUD, and is equivalent to control group rats.
Bilirubin, an important metabolite of heme is a potentially toxic substance. Total bilirubin includes two forms of bilirubin- conjugated and unconjugated form. Bilirubin is conjugated within the hepatocyte to glucuronic acid by Uridine-Diphosphate Glucuronyl-Transferase (UDP-GT), this conjugation is an important detoxification process and promotes its excretion. Unconjugated bilirubin present in body is always in bound form normally with albumin. Increased total bilirubin reflects liver disease, whereas increased conjugated bilirubin indicates hepatocellular dysfunction. Elevation of both ALT and bilirubin is more indicative of serious liver injury than is elevation in ALT alone [18]. Increased total bilirubin and conjugated bilirubin due to hepatocellular injury by CCl4 is revered by treatment of HSE, CUD and their combination.
Liver cells synthesize albumin, fibrinogen, prothrombin, hepatoglobin, transeferin, alpha fetoproteins and acute phase reactant proteins. The blood level of these plasma proteins decreases in extensive liver damage. Hypoproteinaemia is observed in renal diseases, liver diseases, proteinuria etc. and hypoalbuminemia is indicative of chronic hepatic diseases as well as acute nephritis etc. Liver damage due to CCl4 treatment in this experiment was also evidenced by decrease in total protein and albumin. Significant increase in cholesterol by CCl4 treatment in this study is in accordance with fatty liver degeneration by CCl4 [19]. In this study, recovery in liver function markers, total protein, albumin, bilirubin and cholesterol level indicated regeneration of hepatocytes and healing of liver parenchyma in HSE, CUD, their combination and Silymarin treatment groups.
The rats with induced hepatotoxicity had significantly increased (p≤0.05) Blood urea nitrogen and serum creatinine level as compared other group rats (Group I, III, IV, V and VI). Serum Calcium concentration of CCl4 intoxicated group was reduced significantly (p≤0.05). Elevated serum creatinine is observed in severe nephritis and elevated serum urea indicates nephritis and cirrhosis of liver [20]. Significantly decreased calcium and increased serum creatinine and urea in CCl4 intoxicated rats in this study, suggested nephrotoxic profile of CCl4 along with hepatotoxicity. Alteration of these parameters near normal values indicates protection to both organs, liver and kidney by CUD, HSE and their combination in increasing order.
Pre-treatment with H. spinosa methanolic extract p.o significantly (p<0.01) lowered the elevated serum urea and creatinine in comparison to the cisplatin treated nephrotoxic group [21]. Hepatonephrotoxicity using CCl4 at dose of 10 ml/kg body weight (30 % CCl4 in olive oil) on d 0, 7, 14, and 21 [22] significantly (p<0.05) increased the liver enzymes ALT (42.82±2.70 IU/l), AST (92.97±6.34 IU/l) and ALP (421.10±12.89 IU/l) in all the treated rats when compared to control group rats. In the CCl4 and 200 mg and 500 mg Pleurotus tuber-regium (P. tuber-regium) treated group significant reversal of CCl4 effects on the ALT, AST and ALP was observed.
Elevation in the serum levels of Bilirubin (1.21±0.07 mg/dl), Creatinine (0.91±0.04 mg/dl) and Urea (45.76±3.10 mg/dl) by CCl4 was found as compared to control group (0.66±0.07, 0.76±0.05 and 24.48±4.70 mg/dl, respectively). Treatment with P. tuber-regium restored the levels of these markers in a dose dependent manner comparable to the control group.
The Antioxidant status of hepatoprotective study is presented in Table 4. Lipid peroxidation in serum and liver tissue was significantly increased in CCl4 treated rats as compared to control group. Decrease in Lipid Peroxidation (LPO) by treatment with combination of HSE and CUD was comparable to that of Sylimarin. Reduced Glutathione and GPx in CCl4 treatment group were significantly (p≤0.05) decreased. Administration of HSE and CUD combination caused maximum increase in GSH and GPx which were comparable to Sylimarin treated group.
| Group | I | II | III | IV | V | VI | Level of Significance | |
|---|---|---|---|---|---|---|---|---|
| Treatment | Normal saline | CCl4 (1.0 ml/kg) | CCl4 (1.0 ml/kg)+Silymarine 50 mg/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg | CCl4 (1.0 ml/kg)+CUD 5 ml/kg | CCl4 (1.0 ml/kg)+HSE 400 mg/kg+CUD: 5 ml/kg | ||
| Serum antioxidant parameters | LPO (mmol/ mg Hb) | 0.722±0.150b | 1.309±0.173a | 0.772±0.142b | 0.812±0.063b | 0.845±0.075b | 0.799±0.051b | * |
| GSH (mmol/ mg Hb) | 0.094±0.009a | 0.046±0.008b | 0.091±0.004a | 0.078 ±0.006a | 0.070 ±0.012a | 0.091±0.003a | * | |
| GPx (units/ml) | 60.134±6.974a | 28.925±4.133b | 58.240±9.286a | 54.903± 7.745a | 53.674±8.515a | 57.298±7.799a | * | |
| LPO in liver tissue (nmole/g) | 51.624±2.500a | 87.179±3.664a | 52.650±0.951c | 56.068±2.209 bc | 59.316±2.051b | 53.504 ±1.056bc | * | |
Note: The values are Mean±Standard error of mean of n=6 animals. Different superscripts show significant difference (*p<0.05) in a row. NS: Non Significant
Table 4: Effect of H. Spinosa hydro-alcoholic extract, CUD and their combination on antioxidant status of rats with CCl4 induced
Metabolism of CCl4 produce free radicals namely trichloromethyl radicals (CCl3 and CCl3O2) by cytochrome P450 (CYP2E1) [23] are highly hepatotoxic. Their deleterious effects includes enhanced membrane lipid peroxidation, binding with sulfhydryl groups of molecules, ATP depletion, generation of inflammatory cytokines, loss of Ca homeostasis and fatty changes in liver. These are also associated with oxidative stress by binding and inactivation of hepatic antioxidant enzymes including GPx and their binding with sulfhydryl groups such as those in Glutathione (GSH) and the protein thiol [24].
Decreased concentration of GSH increases the sensitivity of organs to oxidative and chemical injury, whereas, decrease in GPx activity enhances lipid peroxidation. Lipid peroxidation is a free radical mediated chain reaction that damage cell membrane and enhanced lipid peroxidation probably was most contributing factor for hepatotoxicity by CCl4 in rats. So, prevention and treatment of CCl4 mediated liver injury requires inhibition of production and activation of free radicals.
There was about 2 fold increase in lipid peroxidation and decrease in Superoxide Dismutase (SOD), Catalase (CAT) and GPx activity in mercuric chloride induced oxidative stress in rat liver, where treatment with H. auriculata resulted in significant decrease in lipid peroxidation and increase in enzymatic antioxidants [17]. Antioxidant property and protective effect of H. spinosa was unveiled against nephrotoxicity in rats, where Cisplatin treated rats showed increase in Malondialdehyde (MDA) (4.06±0.26 U/mg protein) and decrease in GPx (9.373±0.29 U/mg protein) and GSH levels (5.33±0.28 U/mg protein).
Pre-treatment with Sylimarine or H. spinosa at dose of 500 mg/ kg body wt. significantly reduced the MDA level (2.17±0.12 or 2.82±0.22 U/mg protein) and elevated in GSH (11.29±0.22 or 10.52±0.12 U/mg protein) and GPx (17.41±0.76 or 16.72±0.59 U/mg protein) [21]. Significant increase in MDA level, significant decrease in GSH and GPx values in CCl4 treated mice were reverted by Silymarin and Croton bonplandianus leaf extract [25].
Research on in vitro antioxidant activity and antibacterial activity using of cow urine from different altitudes across Nepal using 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) as free radicals and agar disc diffusion method against Escherichia coli (gram-negative) and Staphylococcus aureus (gram-positive) explored antioxidant activity and antibacterial activity of cow urine it was also suggested that but these activities vary in potency according to altitudinal and climatic differences.
Increase in LPO in CCl4 treated rats indicated formation of excessive free radicals by CCl4 metabolism led to exhaustion of antioxidants manifested by oxidative stress. Reduction in GPx, an antioxidant enzymatic defence, attributed to its excessive utilization in inactivation of free radicals generated during CCl4 metabolism. The oxidative stress and liver damage also depleted the non-enzymatic antioxidants like GSH as it inhibits oxidative stress by reducing lipid peroxidation.
Alteration in lipid peroxidation, enzymatic antioxidant (GPx) and non-enzymatic antioxidant (GSH) by CCl4 administration were reversed by treatment of HSE, CUD and their combination similar to Sylimarin treated rats. However, reversal in LPO in liver tissues was maximum with Sylimarin, followed by CUD and HSE combination, HSE and then by CUD. The pattern of recoupment of oxidative stress suggests antioxidant effect of HSE was better than CUD but CUD synergized the antioxidant effect of HSE, which supported its bio enhancing effect. Antioxidant activity of HSE might be due to presence of phenolic and that of CUD might be due to presence of volatile fatty acids.
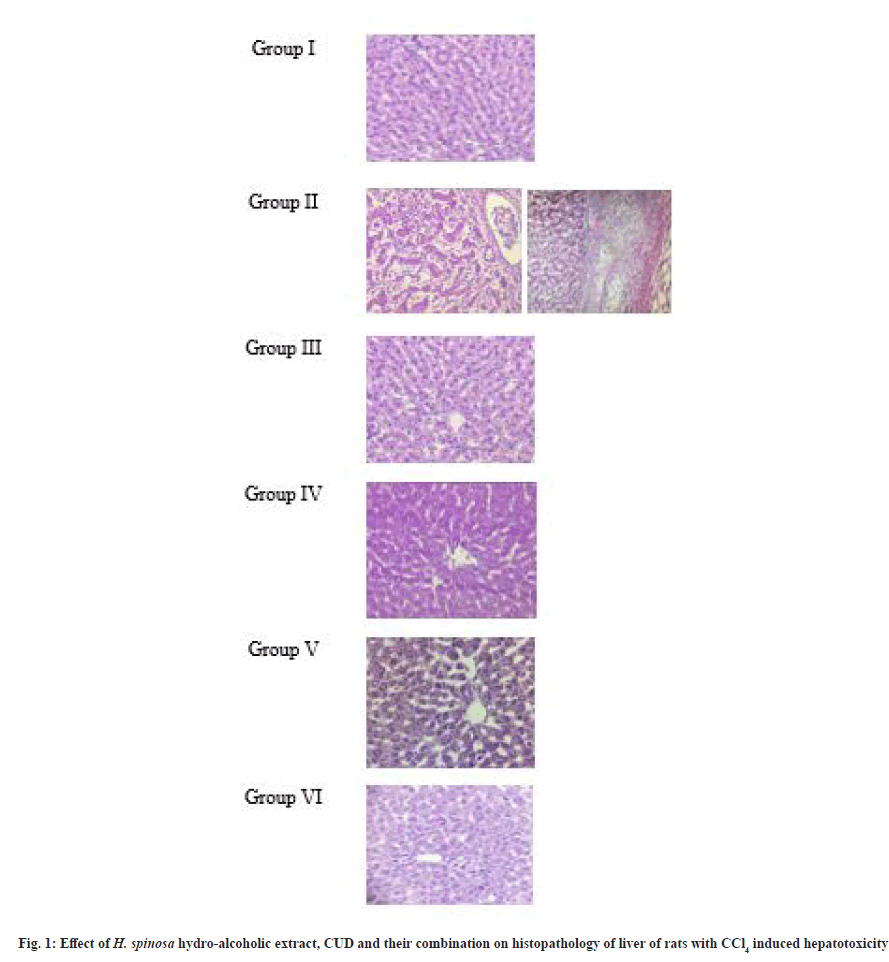
Gross examination of liver of CCl4 induced toxicity rats (Group II) showed development of fibrosis, massive fatty changes which was not observed in other treatment groups. In comparison to liver of control group rats, the fatty changes were moderate in CUD treated group, mild changes in liver of HSE treated rats and liver was apparently normal in rats treated by HSE in combination with CUD, which was similar to Sylimarin treated group rats.
The microscopic examination of liver tissues from control group rats showed normal hepatocytes with well-preserved cytoplasm, prominent nuclei; hepatic cells were arranged properly in hepatic cord. The liver tissue section from Group II rats presented inflammation, degeneration, necrotic changes and massive fatty changes along with lysis of hepatocytes. Hepatic cord was distorted along with infiltration of mononuclear cells. Development of fibrosis was evidenced in liver tissue of this group rats.
Histopathology of Group III rats indicated improvement with mild infiltration of mononuclear cells and architecture approaching normal liver tissues, thus showing recovery from hepatotoxicity. Histological examination of liver of Group IV rats presented mild improvement with reduction in fatty changes and infiltration of mononuclear cells. Microscopic examination of Group V rats indicated moderate recovery with fewer fatty changes and Group VI rats liver sections showed moderate recovery comparable to Sylimarin treated rats.
Hepatotoxicity induced using Isoniazid (INH) and Rifampicin (RIF) 100 mg/kg each, orally for 28 d in Wistar albino rats revealed presence of portal inflammation, ballooning degeneration, severe fatty changes and severe necrosis on histopathological examination. However, groups treated with Silymarin and hydroalcoholic extract of H. auriculata showed a significant reduction in inflammation, ballooning degeneration with absence of fatty changes and necrosis [14].
Evaluation of various parameters in present study hematological, biochemical and antioxidant examination, gross and histopathological findings are related parallelly. So, it is suggested that HSE has hepatoprotective effect. Kosli CUD also provided some degree of hepatoprotection, moreover, CUD showed significant bio enhancing effect in hepatoprotection by HSE against CCl4 intoxication.
Conflict of interests:
The authors declared no conflict of interests.
References
- Almeida MR, Almeida SM. Hygrophila schulli (Hamilt.) species. India Biodiversity Portal. 2020.
- Noor N, Satapathy KB. Phytodiversity of Dhauligiri hill and its adjoining area, Odisha, India: A floristic approach. Plant Arch (09725210). 2020;20(1).
- Gaikwad S, Kothapalli L, Thomas A. Phytochemical and pharmacological aspects of Hygrophila spinosa: An overview. Curr Indian Sci 2023;1(1):e191022210155.
- Nithya V. Indigenous cow ark with Allium sativum-a key to therapeutic and an effective antioxidant to hepatocellular carcinoma. Int J Aquatic Sci 2021;12(2):1631-44.
- Dhama K, Saminathan M, Jacob SS, Singh M, Karthik K, Tiwari R, et al. Effect of immunomodulation and immunomodulatory agents on health with some bioactive principles, modes of action and potent biomedical applications. Int J Pharmacol 2015;11(4):253-90.
- Chawla PC. Risorine-A novel CSIR drug curtails TB treatment, CSIR News. 60:52. 2010.
- Chauhan RS, Garg N. Cow therapy as an alternative to antibiotic. Presented at the Indian Science Congress, Bangalore, Karnataka; 2003.
- Teschke R. Liver injury by carbon tetrachloride intoxication in 16 patients treated with forced ventilation to accelerate toxin removal via the lungs: A clinical report. Toxics 2018;6(2):25.
[Crossref] [Google Scholar] [PubMed]
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci 2015;16(11):26087-124.
- Jain NC. Haematological techniques in Schalms Veterinary Haematology, 4th ed. Lea and Febinger. Philadelphia; 1986; p. 20-86.
- Placer ZA, Cushman L, Johnon B. Estimation of products of lipid peroxidation (malondialdehyde) in biochemical system. Analytical Biochem 1966;16:359-64.
- Prins HK, Loos JA. “Glutathione,” In: JG Yunis ed. Biochemical methods in red cell genetics, Academic Press. New York; 1969. p. 127-9.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Laboratory Clin Med 1967;70(1):158-69.
[Google Scholar] [PubMed]
- Vir DK, Doifode RM. Hepatoprotective activity of hydro-alcoholic extracts of Hygrophila auriculata Heine against isoniazid and rifampicin induced hepatotoxicity in rats. Int J Innov Sci Eng Technol 2019;6(8):2348-7968.
- Mishra CS. Studies on toxicopathology of acephate in Wistar rats and its amelioration with cow and goat urine distillates (Doctoral dissertation, Chhattisgarh Kamdhenu Vishwavidyalaya, Durg); 2014.
- Pancha PG. Studies on immunomodulatory effect of cow urine distillate in healthy and cyclophosphamide induced immunosuppressive mice. Anand agricultural university, Anand; 2015.
- Sridhar MP, Geetha R, Anitha D, Muthukumar R, Ramesh V. Modulation of oxidant: Antioxidant imbalance by Hygrophila auriculata (K. Schum) heine in mercuric chloride induced oxidative stress in rat liver. Intern J Med Health Res 2016;2(6):15-22.
- Gwaltney-Brant SM. "Nutraceuticals in hepatic diseases". Nutraceuticals. Elsevier; 2016. p. 87-99.
- Shailajan S, Chandra N, Sane RT, Menon S. Effect of Asteracantha longifolia Nees. against CCl4 induced liver dysfunction in rat. Indian J Exp Biol 2005;43(1):68-75.
[Google Scholar] [PubMed]
- Chauhan RS, Chandra D. Examination of blood serum for biochemical attributes. In: Veterinary laboratory diagnosis. 2nd ed, International Book Distributing Co., Lucknow; 2007. p. 99-124.
- Ingale KG, Thakurdesai PA, Vyawahare NS. Protective effect of Hygrophila spinosa against cisplatin induced nephrotoxicity in rats. Indian J Pharmacol 2013;45(3):232-6.
[Crossref] [Google Scholar] [PubMed]
- Okolo KO, Siminialayi IM, Orisakwe OE. Carbon tetrachloride induced hepatorenal toxicity in rats: Possible protective effects of wild Pleurotus tuber-regium. Clin Phytosci 2017;3(1):2.
- Fouad D, Badr A, Attia HA. Hepatoprotective activity of raspberry ketone is mediated via inhibition of the NF-κB/TNF-α/caspase axis and mitochondrial apoptosis in chemically induced acute liver injury. Toxicol Res 2019;8(5):663-76.
- Dutta S, Chakraborty AK, Dey P, Kar P, Guha P, Sen S, et al. Amelioration of CCl4 induced liver injury in swiss albino mice by antioxidant rich leaf extract of Croton bonplandianus Baill. PloS One 2018;13(4):e0196411.
[Crossref] [Google Scholar] [PubMed]
- Joshi DR, Aryal P, Chaudhary MK, Kumar BK P, Yadav SP, Rawal P, et al. Study of in vitro antioxidant and antibacterial activity of cow urine from different altitudinal and climatic region of Nepal. Microbiol Res J Int 2019;29(2):1-8.