- Corresponding Author:
- M. D. Karvekar
Department of Pharmaceutical Chemistry, Krupanidhi College of Pharmacy, No. 5, Sarjapur Road, Koramangala, Bangalore - 560 034, India.
E-mail: ch_pharmacy@yahoo.co.in
| Date of Submission | 17 September 2005 |
| Date of Revision | 12 February 2007 |
| Date of Acceptance | 5 May 2007 |
| Indian J. Pharm. Sci., 2007, 69 (3): 344-351 |
Abstract
Selenium, a nonmetallic element, has structural and enzymatic roles. Selenium influences a number of endocrine processes; selenium supplementation has recently shown some of the important pharmacological intervention, especially in cancer chemoprevention. During the last few years, a tremendous effort has been directed toward the synthesis of stable organoselenium compounds. The biochemistry and pharmacology of selenium-based compounds are subjects of intense current interest, especially from the point of view of public heath. The purpose of this review is to discuss the recent pharmacological applications of organoselenium compounds as therapeutic agents in the treatment of several diseases.
Keywords
Antioxidants, glutathione peroxidase, organoselenium compounds, selenocysteine
The element selenium is considered to be highly potent and a well established nutritional requirement for both animal and human beings. Selenium is an essential trace element and its deficiency in the diet leads to white muscle disease and cardiaomyopathy [1]. It has been investigated that several animals and human enzymes are selenium dependent and involves selenium containing amino acids, which are analogues of selenocyste [2].Selenocyste has been identified in dehydrogenases, mammalian glutathione peroxidases, glycine reductase, hydrogenase [3,4],thioredoxin reductase [5].
In general, organoselenium compounds have substantially greater bioavailability than that of inorganic selenium [6] . More importantly, organic selenium is usually found to be less toxic than inorganic forms [7-10]. Lowig prepared the first organoselenium compound, diethyl selenide in 1836 [11]. Encouraged by these reports a number of novel selenium-based pharmaceutical agents are under development for therapeutic use as anticancer, antioxidant and antimicrobial agents. In the initial period, only simple organoselenium compounds such as selenols, selenides and diselenides were synthesized, which are unstable and difficult to purify. During the past decade, a lot of work has been concentrated on development of stable organoselenium compounds having therapeutic potential toward a variety of human diseases. Several excellent books and reviews appeared in literature describing the biological function of organoselenium compounds [12-15]. This review attempts to provide useful perspective for the future drug development of stable organoselenium compounds and its therapeutic importance.
Organoselenium compounds as antioxidant agents
Biological systems use dehydrogenases, superoxide dismutase, glutathione peroxidase and other enzymes as antioxidant systems to prevent oxidative stress. Any functional changes in these enzymes may lead to cancer, cardiovascular disease and inflammation [16,17]. During the metabolism of oxygen, oxygen species are normally produced within the cells, primarily from the mitochondrial respiratory chain where excess electrons are donated to molecular oxygen to generate peroxide anion and superoxide anion and they are reduced by the superoxide dismutase to hydrogen peroxide and hydrogen peroxide is reduced to water by the enzymes catalase and by glutathione peroxidase (GPx), located in the mitochondria and cytosol. These oxygen species (H2O2, .OH) can damage biomacromolecules.
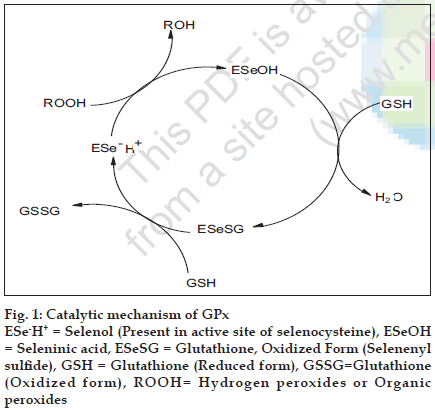
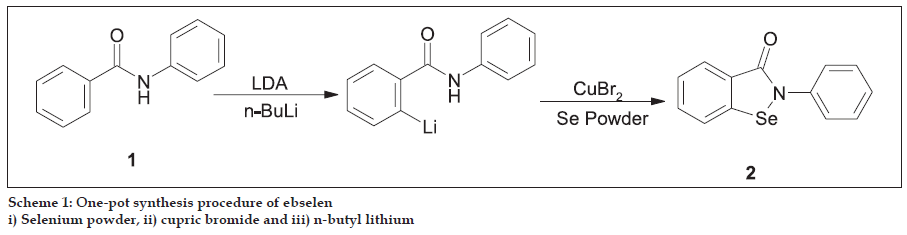
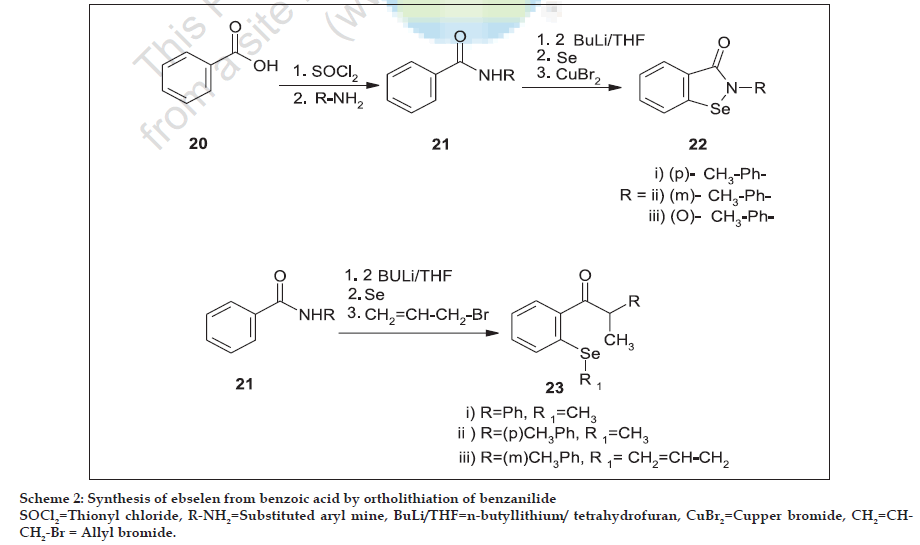
Mills first discovered glutathione peroxidase in animal tissues in 1957 [18]. Glutathione peroxidase (GPx) contains four identical protein subunits each of which contains one selenium (Se) atom at its active site. There are at least four types of Se containing glutathione peroxidases: 1. cytosolic GPx, 2. gastrointestinal (GI) GPx, 3. plasma GPx and 4. phospholipid hydroperoxide GPx. All the above show similarities in their structures. Finally, Flohe et al. reported that GPx are selenoenzymes, which contain selenium in the active site of GPx, and act as antioxidant by catalyzing the reduction of hydrogen peroxide19. The catalytic mechanism of GPx is explained in fig. 1, where the dissociated selenol (ESeH) of the selenocysteine in the active site of GPx undergoes a redox cycle involving the selenolate anion as the active form that reduces hydrogen peroxides and organic peroxides. The selenolate, which is oxidized to selenic acid, reacts with reduced glutathione (GSH) to form a selenosulfide adduct (ESeSG). A second glutathione then regenerates the active form of the enzyme by attacking the selenosulfide to form oxidized glutathione (GSSG). Thus, two equivalents of glutathione are oxidized to the disulfide and water, while the hydroperoxide is reduced to the corresponding alcohol. Based on these studies, several organoselenium compounds have been developed for the destruction of peroxides. Certain features make Se compounds valuable when compared to other (sulfur) compounds. Among them the most promising drug was ebselen (2-phenyl-1,2- benzisoselenazol-3(2H)-one) (2) that mimics the GPx activity in vitro and function as an antioxidant [20]. Ebselen was first prepared by Lesser and Weiss [21] in 1924, later in 1989, Engman et al. reported one-pot procedure in which benzanilide (1) is ortho lithiated by using n-BuLi [22] (Scheme 1) and then converted to ebselen.
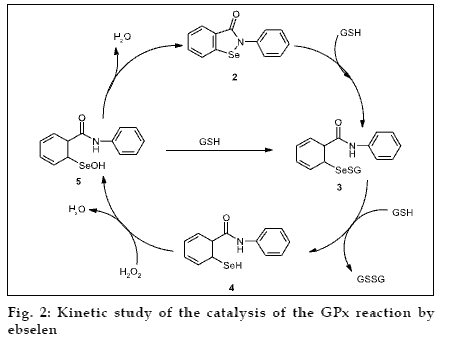
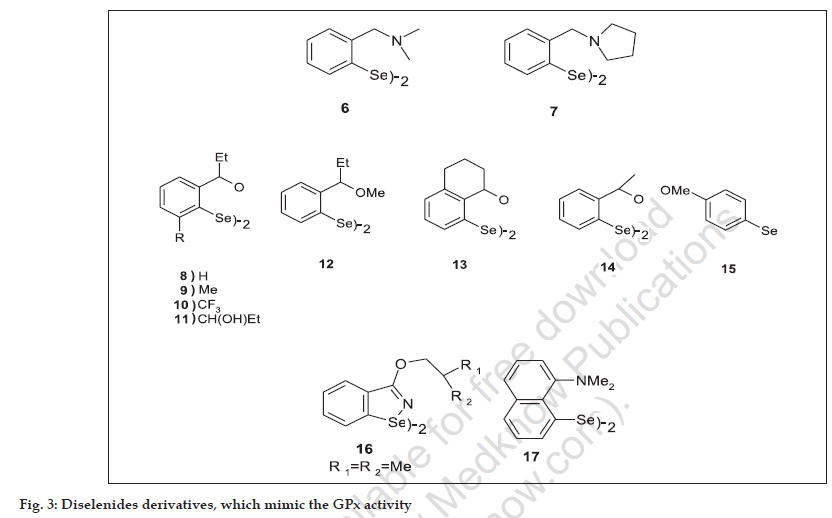
Maiorino et al. performed a kinetic study of the catalysis of the GPx reaction by ebselen and showed that selenol is responsible for the GPx activity of ebselen23 (fig. 2). Further developments in search of new series of organoselenium compound were begun. The first interesting result came from Wilson et al. who reported diselenides (6,7), which possess basic amino groups near the selenium and which exhibit strong antioxidant activity [24] (GPx activity). This study it revealed that the presence of amino group nitrogen activates the Se-Se bond towards an oxidative cleavage and stabilizes the selenenic acid form of the catalyst against further oxidation.
Later, Iwaoka and Tomoda performed studies with different models in order to find out mechanistic roles of the amino groups located at active center of GPx and selenium-containing antioxidant enzyme25. Recently, a new series of diselenides containing an oxygen atom in proximity to selenium were synthesized (8-15) and all diselenides investigated showed GPx-like activities [26] (fig. 3, Table 1). The most active compounds were molecules (8,13), and (15). All bis-ortho substituted diselenides (9-11) showed significantly lower activity. The opposite effect was obtained in compound (15), which shows the highest activity of this series. Interestingly, diselenide (12) with a methoxy group instead of a hydroxy group exhibited only 50% of the activity of compound (8).
| Diselenide | GPx activities [nmol of NADPH min-1] (20μM Se-Equivalents) 100 μM H2O2 |
GPx activities[nmol of NADPH min -1](20μM Se-Equivalents)200 μM t-BuOOH |
|---|---|---|
| 8 | 26.6 | 13.9 |
| 9 | 20.2 | 13 |
| 10 | 8.7 | 4.7 |
| 11 | 18.8 | 9.5 |
| 12 | 14.5 | 7.8 |
| 13 | 27.5 | 14.5 |
| 14 | 17.9 | 11.5 |
| 15 | 30.2 | 17.7 |
Table 1: Gpx Activity of Diselenides
Mugesh et al. worked extensively on intra molecularly co-ordinated organochalcogens and reported a series of diaryl diselenides having intramolecular Se-N interactions showed less GPx activity compared with the diselenides (16,17), which has basic amino groups, but not have Se-N interactions [27].
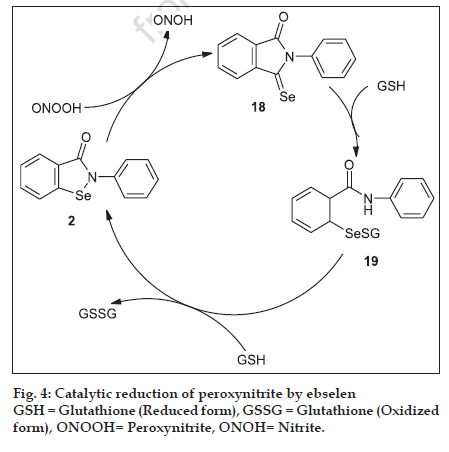
Peroxynitrite is considered as a strong biological oxidant, which inactivates various enzymes by oxidation. Ebselen acts as a glutathione peroxidase mimic and is an excellent scavenger of peroxynitrite with a rate constant of 2×10-6 M [28] . As described in fig. 4. ebselen, reducing peroxynitrite to nitrite in the first step, forming selenoxide(2-phenyl-1,2- benzisoselenazole-3(2H)-one-1-oxide) (18) followed by the reduction of this selenoxide back to ebselen in two consecutive one electron reduction steps via the selenodisulfide (19), utilizing reducing equivalents in the form of glutathione. Chang et al. prepared ebselen and acyclic ebselen derivatives (22,23) and screened for their GPx like activity and for the activity against 1,1-diphenyl-2-pycryl-hydrazyl (DPPH) and peroxynitrite radical [29] (Scheme 2). These compounds mimic GPx-like activity and are slightly higher than that of ebselen. Even, peroxynitrite screening activity showed that the acyclic selenium derivative were more potent than ebselen.
Organoselenium compounds as antitumor agents (fig. 5)
Research over last 20 years has shown that dietary selenium can prevent or reduce the incidence of naturally occurring and both chemically and virally induced cancer. In humans, supplemental levels of 200-µg selenium/day have been reported to exhibit carcinostatic activity, which is 2-3 times of the amount of normal dietary levels. All inorganic selenium compounds that express carcinostatic activity against cancer cells in vivo do so by interaction with thiol compounds and generation of free radical species [30].
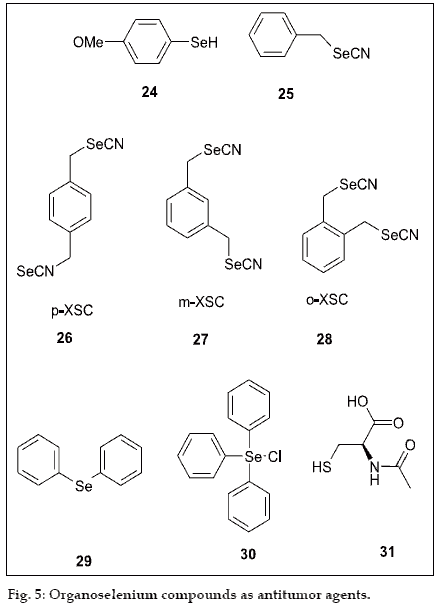
The antitumor activity of organoselenium compound was started with dietary P-methoxybenzeneselenol (24), a synthetic organoselenium compound which was found to inhibit azoxymethane-induced hepatocarcinogenesis in female F344 rats without clinical signs of toxicity [31], and was an effective inhibitor of benzo(α)pirene-induced forestomach tumors in mice [32] and colon carcinogenesis in rats [33].Subsequently, Reddy et al. reported chemopreventive effect of 1,4-phenylenebis(methylene)selenocyanate (p-XSC) on azoxymethane-induced colon tumor [34,35] (25), and 7,12-dimethyl-benz(α)anthracene-induced mammary tumors during the initiation phase of carcinogenesis in rats36. Although, compound (25) was more effective and less toxic, the rats suffered significant growth depression. Because compound (25) has a very strong odor similar to that of burnt rubber, the rats avoided food. To reduce the volatility of compound (25), a second methyleneselenocyanate group was added in the para-position to obtain 1,4-phenylenebis (methylene)selenocyanate (26). Compound (26) is commonly called pxylylselenocyanate or p-XSC. The effect of (26) at the molecular level that accounts for mammary cancer chemoprevention in vivo in the rat was performed by EI-Bayoumay et al.[37,38], who employed cDNA microarray method for analysis of mammary cancer prevention by organoselenium (XSC).
The effects of (26) and its o- and m-isomers (27,28) was also studied on xenobiotic metabolizing enzymes in vivo by using female rat CD rats [39,40]. The study revealed that the o-XSC was more potent enzyme induced than m- or p-XSC because, in hepatic microsomes, o-XSC significantly induces CYP2E1 due to increased N-nitrosodimethylamineN-demethylase activity without effecting the activity of CYP2E1 (ethoxy resorufin-o-dealkylase).
The recent study by Rao et al. showed that p-phenyl enebis(methylene)selenocyanate [41] is a superior cancer chemopreventive agent and less toxic than selenite or certain naturally occurring selenoamino acid. It was also more effective against colon carcinoegensis when administrated during the post initiation stage and inhibits cyclooxygenase activity. During this pharmacological study of various organoselenium, ebselen has been studied for antitumor activity and conclude that ebselen inhibits cell growth in human, including breast and colon cancer cell and also induces apoptosis in the human hepatoma cell line HepG2 [42,43]. Another synthetic selenium compound, triphenylselenonium chloride (29), which exhibited superior cancer chemopreventive than diphenyl selenide (30) by inhibiting tumor development during study [44,45]. Compound (29) has appreciable lipophilic character, which would appear to be the basis for relatively slow release of selenium from these types of compounds. However, compound (29) does not release inorganic selenium for selenoprotein synthesis, which is responsible for the cancer activity.
Sridharan et al. carried out in vitro cytotoxic effect of N-acetylcysteine (a precursor of intracellular reduced glutathione) on oral epidermoid carcinoma cell (KB) and reported that the N-acetylcysteine (31) shows significant cytotoxic effect than the culture cell by decreasing cell validity and lactate dehydrogenase (LDH), gamma glutamyl trasferase (γ-GT) and 5’- nucleotidase [46]. Later Short et al. observed promising results with selenazolidine carboxylic acid derivative, a prodrug of selenocysteine that exhibits reduced toxicity to V79 cells [47] and has been characterized as cancer chemoprevention agent. A new series of 7-arylseleno-7-deoxydaunomycinone derivatives were synthesized, which show antitumor activity against human stomach cancer SGC-7901 and human leukaemia HL60 [48].
Recently, using cultured rat bone marrow cells performed in vitro cytogentic testing of an organoselenium. Fluorescence-Plus-Giemsa (FPG) technique was used to visualize chromosomes for induced sister chormatid exchange (SCE) analysis and cell division expressed as mitotic index determination [49]. Das et al. observed the inhibitions of induced 7,12-dimethylbenz(α)anthracene (DMBA)/croton oil-induced two-stage mouse skin carcinogenesis by diphenyl methyl selenocyanate due to inhibition of thiobarbituric acid that resulted in reduction of papillomo formation during chemically induced carcinogenesis [50]. However, selenium (Se) supplementation influences oxidative stress (malondialdehyde) and the GPx system in patients with ovarian cancer undergoing chemotherapy [51]. The patients with ovarian cancer undergoing chemotherapy and receiving Se showed a significant increase in the activity of GSH-Px in erythrocytes and an increase of the concentration of malondialdehyde (MDA) following the administration of Se even after 2-3 mo were found to be significant. Methylselenocysteine (MSC) is under clinical trials, involving prostate, lung and colon carcinoma. And it was found that methioninase-activated MSC potentiates 7-ethyl-10- hydroxycamptothecin (SN-38)-induced cell lethality in vitro in the p53-defective human head and neck carcinoma A253 cells [52].
Organoselenium compounds as antiinfective agents
A series of heterocyclic compounds containing sulphur or selenium, were tested for cytotoxicity, antiviral and antimicrobial activities (fig. 6). Generally, seleniumcontaining compounds were more toxic than the corresponding sulphur-containing ones (30-75 times more toxic) and, furthermore, the biological activity against some microorganisms was also enhanced. In particular, some selenium-containing derivatives exerted an inhibitory activity on plant pathogenic fungi at doses markedly lower than the toxic ones, showing an interesting selectivity of action.
Organoselenium compounds as antiviral agents
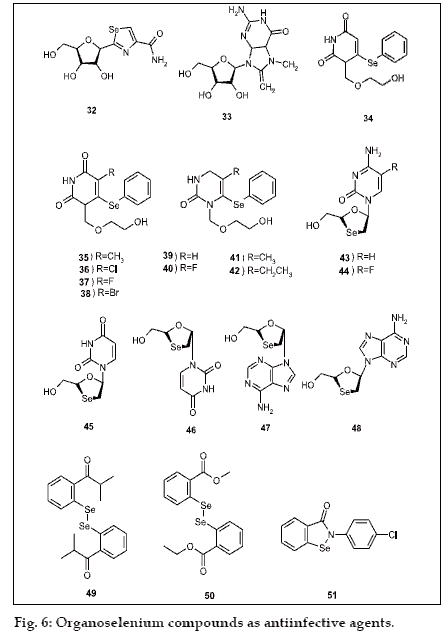
Almost all synthesized organoselenium compounds exhibit an antiviral activity one such is selenazofurin (32) against Type 1 herpes simplex virus, Type 3 parainfluenza virus, and Type 13 rhinovirus was associated with the inhibition of guanine nucleotide biosynthesis [53] but no antiviral activity against Pichinde virus (PCV) in infected hamsters was found [54]. The purine analog, 7-methyl-8-selenoguanosine (33) was another good example for antiviral activity against Semliki Forest virus (SFV) infection in a mouse model.
Several selenium-substituted acyclouridine derivatives (34-38) were studied for their antiviral activity in human peripheral blood mononuclear (PBM) cells infected with HIV-1 (strain LAV) [55]. Compounds (34) and (35) are the least and more effective compounds against HIV virus, respectively. However, when these compounds were tested in human PBM cells infected with HIV-2 (strain ROD-2), compound (35) was about 25-fold less active and (38) was the most active compound. The chemical modifications of the acyclic side chain produced compounds (39-42). In which compound (42) is the most potent antiviral against HIV-1 as compared to the hydroxyl and methyl analogs [56]. During the past decade, 2´,3´- dideoxynucleoside analogues have been intensively studied as potential antiviral agents. The use of these analogues in combination with protease inhibitors has been responsible for the reduction of infections and mortality in HIV patients [57]. Due to the adverse effects of certain antiviral agents and the drug-resistant viral strains, new antiHIV agents are needed. Some 3’-Sesubstituted dideoxynucleosides have been synthesized and exhibited potent antiHIV and antiHBV activities. Recently several R- and S-anomers of oxaselenolane nucleosides have been studied for HIV and hepatitis B viruses [58,59]. The racemic forms of cytosine and 5- fluorocytosine analogues (43 and 44) showed potent anti-HIV and antiHBV activities in various cell lines (PBM, CEM, and Vero). The racemic form of the R-isomer also exhibited moderately potent antiviral activity against HIV [58]. The racemic R- and S-thymine, guanine, and adenine derivatives (45-48) also exhibited significant anti-HIV and anti-HBV activities [59]. The antiHIV activity of the corresponding resolved R- and S-enantiomers has also been evaluated. It was found that the (-) enantiomers are more potent than their (+) counterparts. The enantiomerically pure compounds showed much higher activities compared with the racemic compounds.
Organoselenium compounds as antibacterial and antifungal agents
Many organoselenium compounds were synthesized and their antimicrobial activity was studied [53,60,61]. The most effective one is ebselen (2-phenyl-1,2- benzisoselenazol-3-(2H)-one)2, which possess antiinflammatory, antiatherosclerotic, cytoprotective, and antimicrobial activity against Staphylococcus aureus [62]. The compound (7) and its analog (49) exhibited strong inhibitory activity against the growth of Saccharomyces cerevisiae 127 strains. The compound (49) also inhibited the growth of Candida albicans 258 strains. But, the diaryl diselenides compounds (50), or carboxy-alkyl (51) had no inhibitory activity on fungi growth. However, the benzisoselenazolones shows antibacterial activities against gram-negative E. coli K-12 Row and grampositive S. aureus 209P bacteria strains. Even 4H- 5,6-dihydro-1,3- selenazine derivatives also shows antibacterial activity against E. coli and S. aureus [63]. Recently, selenoxanthenes and selenopyrylium salts have been synthesized, and studied for antibacterial activity [64]. These compounds have more pronounced activity with respect to S. aureus, rather than E. coli species.
Organoselenium compounds as antiinflammatory agents
The reactive oxygen species are known for tissue damage and inflammation will be the important factors in hydroperoxide-related tissue damage. Based on these studies several organoselenium compounds were synthesized, which were found effective in the treatment of inflammatory diseases. Recent data on ebselen (2-phenyl-1,2-benzisoelenazol-3(2H)-one), has suggested that this compound possessed antioxidative as well as antiinflammatory properties. Belvisi et al.[65] proved the antiinflammatory properties of ebselen in a model of sephadex-induced lung inflammation, where ebselen showed significant inhibition of lung oedema. Later Shen et al.[66] synthesized new diselenide compounds, bis-(2-hydroxyphenyl) diselenide, bis-(3- hydroxyphenyl) diselenide and bis-(4-hydroxyphenyl) diselenide, which were found to have strong in vitro antiinflammatory activity.
Ebselen was also found to inhibit the production of reactive oxygen species (ROS) by zymosanstimulated mouse macrophages [67], endothelial isoform of nitric oxide synthase (NOS) in the rabbit and bovine aorta [68] and polymorphonuclear leucocytes (PMNL) adhesion to vascular endothelium and transendothelial migration [69]. Recently, Joszef and Filep described a novel mechanism by which ebselen, methylselenocysteine, and selenocysteine might affect the inflammatory response, which resulted in the reduction of peroxynitrite-mediated nuclear accumulation of transcription factors NF-kB and activator protein (AP-1) and suppressed IL-8 gene expression in human leukocytes [70]. Wang et al. reported that ebselen inhibited the production of superoxide anion and nitric oxide in rat Kupfer cells [71].
Conclusions
It is now clear that many organoselenium compounds play important roles in biochemical processes and are used in a variety of conditions ranging as antioxidants, anticancer and antiviral agents. The unique redox properties of selenium influence the catalytic activities of organoselenium compounds. Although the toxicity of many selenium compounds is a limiting factor for their use in pharmacotherapy, recent evidence suggests that the toxicity could be considerably lowered by suitable substitution. We envisage that the progress and perspective described in this review will stimulate further efforts from researchers all across the organoselenium community.
Acknowledgements
The authors wish to sincerely thank the management and staff of Krupanidhi College of Pharmacy, for their dedicated collaboration and devotion.
References
- Bruce,R.K.,In; Encyclopedia of Inorganic Chemistry,2nd Edn.,Vol.,10,John Wiley and Sons,2005,1.
- Stadtman,T.C.,Annu. Rev. Biochem.,1996,65,83.
- Stadtman,T.C.,Annu. Rev. Biochem.,1990,59,111.
- Kohrle,J.,Flohe,B.R.,Bock,A.,Gartner,R.,Meyer,O. and Fohle,L.,Biol. Chem. J.,2000,381,849.
- Mustacich,D. and Powls,G.,Biol. Chem. J.,2000,246,1.
- Cantor,A.,Scot,M. and Noguch,T.,J. Nutr.,1976,105,96.
- Bendsleve,D.A.,Abdulla,M.,Jepsrn,A. and Pedeson,E.,Trace. Elem. Med.,1988,5,29.
- Khalil,A.M.,Mutat. Res.,1989,224,503.
- Khalil,A.M.,Toxicol. Environ. Chem.,1994,41,147.
- Saito,Y.,Fiji,T.,Honda,M.,Maeda,A.,Seo,H. and Chikuma,M.,In; Proceedings of the seventh International Symposium,Selenium in Biology and Medicine,Venenzia,2000,1.
- Lowig,C.,J. Pogg. Ann.,1836,37,552.
- Garcia,M.S.,Curr. Med. Chem.,2004,11,1669.
- Reily,C.,In; Selenium in Food and Health,1st Edn.,Blackie Academic and Professional,London,1997,22.
- Ihnat,M.,In; Occurrence and Distribution and Selenium,CRC Press,2000,286.
- Craig,P.J.,In; Organometallic Compounds in the Environment,2nd Edn.,John Wiley and Sons,2003,391.
- Huong,N.T.T.,Matsumoto,K.,Kasai,R.,Yamasaki,K. and Watanabe,W.,Bio. Pharm. Bull.,1998,21,978.
- Haraguchi,H.,Ishikawa,H. and Kubo,I.,PlantaMed.,1997,63,213.
- Mills,G.C.,J. Biol. Chem.,1957,229,197.
- Flohe,L.,Gunzler,E.A. and Schock,H.H.,FEBS Lett.,1973,32,132.
- Sies,H.,Free Rad Biol Med.,1993,14,312.
- Lesser,R. and Weiss,R.,Dtsch. Chem. Ges.,1924,57,1077.
- Engman,L. and Hallberg,A.,J. Org. Chem.,1989,54,2964.
- Maiorino,M.,Roveri,A.,Oassin,M. and Ursini,F.,Biochem. Pharmacol.,1988,37,2267.
- Wilson,S.R.,Zucker,P.A.,Huang,R.R.C. and Spector,A.,J. Amer. Chem. Soc.,1989,111,5936.
- Michio,I. and Shuji,T.,J. Amer. Chem. Soc.,1994,116,2557.
- Thomas,W.,Molecules,1998,3,164.
- Mugesh,G.,Panda,A.,Singh,H.B.,Punekar,N.S. and Butcher,R.J.,Chem. Commun.,1998,2227.
- Masumoto,H.,Kissner,R. and Koppenol,W.H.,FEBS Lett.,1996,398,179.
- Chang,T.C.,Huang,M.L.,Lin,W.,Hwang,J.M.and Yih,L.,Chem. Pharm. Bull.,2003,51,1413.
- Spallholz,J.E.,The Bulletin of Selenium-Tellurium Development Association,2001,9,1024.
- Tanaka,T.,Reddy,B. S. and El-Bayoumy,K.,Jpn. J. Cancer Res.,1985,76,462.
- El-Bayoumy,K.,Cancer. Res.,1985,45,3631.
- Reddy,B.S.,Tanaka,T. and Simi,B.,J. Natl. Cancer. Inst.,1985,75,791.
- Reddy,B.S.,Sugie,S.,Maruyama,H.,El-Bayoumy,K. and Marra,P.,Cancer. Res.,1987,47,5901.
- Jibril,I.,AbdAlhadi,E. and Hamadah,Z.,Transition Metal Chem.,2000,25,407.
- Nayini,J.,El-Bayoumy,K.,Sugie,S.,Cohen,L. A. and Reddy,B. S.,Carcinogenesis,1989,10,509.
- El-Bayoumy,K.,Bhagavathi,A.,Narayanan.,Dhimant,H.,Desai.,Narayanan,K.,Brian,P.,Shantu,G.,Schwartz,J. and Daniel,W. N.,Carcinogenesis,2003,9,1505.
- El-Bayoumy,K. and Raghu,S.,Mutat. Res.,2004,13,181.
- Sohn,O.S.,Fiala,E.S.,Upadhyaya,P.,Chae,Y.H. and El-Bayoumy,K.,Carcinogenesis,1999,20,615.
- Jacob,L.A.,Matos,B.,Mostafa,C.,Rodriguez,J. and Tillotson,J.K.,Molecules,2004,9,622.
- Rao,C.V. and Wang,C.Q.,Cancer. Res.,2001,61,3647.
- Engman,L.,Cotgreave,I.,Angulo,M.,Taylor,C.W.,Murrieta,G.D.P. and Powis,G.,Anticancer Res.,1997,17,4599.
- Yang,C.F.,Shen,H.M. and Ong,C.N.,Arch. Biochem. Biophys.,2000,374,142.
- Ip,C.,Thompson,H. and Ganther,H.,Carcinogenesis,1994,15,2879.
- Ip,C.,Lisk,D.J.,Ganther,H. and Thompson,H.J.,Anticancer Res.,1997,17,3195.
- Sridharan,S.,Ramamurthy,N. and Devi,S.C.S.,Indian J. Pharmacol.,2001,33,343.
- Xiey.,Short,M.D. and Cassidy,P.B.,Bioorg. Med. Chem. Lett.,2001,11,2911.
- Zhang,S.J.,Jia,Z.P. and Wang,Y.G.,Bioorg. Med. Chem.,2002,10,3899.
- Jacob,J.H.,Khalil,A.M. and Maslat,A.O.,J. Carcinog.,2004,3,5.
- Das,R.K.,Ghosh,S.,Sengupta,A.,Das,S. and Bhattacharya,S.,Eur. J. Cancer Prev.,2004,13,411.
- Yin,M.B.,Li,Z.R.,Cao,S.,Durrani,F.A.,Azrak,R.G.,Frank,C. and Rustum,Y.M.,Mol. Pharmacol.,2004,66,153.
- Sieja,K. and Talerczyk,M.G.,GynecolOncol.,2004,93,2320.
- Parnham,M.J. and Graf,E.,Prog. Drug. Res.,1991,36,9.
- Smee,D.F.,Gilbert,J.,Leonhardt,J.A.,Barnett,B.B.,Huggins,J.H. and Sidwell,R.W.,Antiviral Res.,1993,20,57.
- Goudgaon,N.M. and Schinazi,R.F.,J. Med. Chem.,1991,34,3305.
- Goudgaon,N.M.,McMillan,A. and Schinazi,R.F.,Antiviral Chem. Chemother.,1992,3,263.
- Cohen,J.,Science,1997,276,520.
- Du,J.,Surzhykov,S.,Lin,J. S.,Newton,M. G.,Cheng,Y.C.,Schinazi,R. F. and Chu,C. K.,J. Med. Chem.,1997,40,2991.
- Chu,C.K.,Li,M.,Olgen,S.,Pierra,C.,Du,J.,Gumina,G.,Gullen,E.,Cheng,Y.C. and Schinazi,R.F.,J. Med. Chem.,2000,43,3906.
- Xu,H.,Huang,K.,Xu,H. and Huang,K.,Edn.,Huazhong University of Science and Technology Press,Wuhan,1994.
- Kulkami,Y.D.,Rani,A.,Bishiioi,A.,Shukla,R.L. and Anan,Z.K.,J. Indian Chem. Soc.,1995,72,261.
- Nozawa,R.,Yokota,T. and Fujimoto,T.,Antimicrob. Agents Chemother.,1989,33,1388.
- Koketsu,M.,Ishihara,H. and Hatsu,M.,Res. Commun. Mol. Path. Pharmacol.,1998,101,179.th
- Ratushnaya,E.V.,Kirova,Y.I.,Suchkov,M.A.,Drevko,B.I. and Borodulin,V.B.,Pharm. Chem. J. 2002,36,12,16.
- Belvisi,M.G.,Haddad,E.G.,Battram,C.,Birrell,M.,Foster,M. and Webber,S.,Eur. Respir. J.,2000,15,579.
- Shen,L.,Shin,K.M.,Lee,K.T. and Jeong,J.H.,Arch Pharm. Res.,2004,27,816.
- Parnham,M. J. and Kindt,S.,Biochem. Pharmacol.,1984,33,3247.
- Zembowicz,A.,Hatchett,R.J.,Radziszewski,W. and Gryglewski,R.J.,J. Pharmacol. Exp. Ther.,1993,267,1112.
- Gao,J.X. and Issekutz,A.C.,J. Immunol.,1993,150,307.
- Jozsef,L. and Filep,J.G.,Free Rad. Biol. Med.,2003,35,1018.
- Wang,J.F.,Komarov,P.,Sies,H. and Groot,H.,Hepatology,1992,15,1112.x