- *Corresponding Author:
- Swathi Putta
Raghavendra Institute of Pharmaceutical Education and Research (RIPER)-Autonomous, Ananthapuramu, Andhra Pradesh 515721, India
E-mail: swathidbmp@gmail.com
| Date of Received | 03 July 2023 |
| Date of Revision | 11 March 2024 |
| Date of Acceptance | 14 October 2024 |
| Indian J Pharm Sci 2024;86(5):1601-1610 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Heavy metals, similar to other toxic chemicals from natural or industrial sources can pose a serious threat to human life. Cadmium toxicity is one of the global health problems that affect many functional systems and can cause death in some cases. The sources of cadmium exposure were through air, water, soil, and food. Cadmium has a variety of toxic effects including nephrotoxicity, carcinogenicity, teratogenicity, and endocrine and reproductive toxicity. The review focused on the effect of cadmium on the body's antioxidant defense system according to dose and duration of exposure with evidence. Various mechanisms of cadmium-induced organ toxicity by oxidative stress, cell damage, deoxyribonucleic acid methylation, activation of pro-oncogenes, autophagy, affects cell proliferation, differentiation and apoptosis, inhibits cellular respiration, etc., are further explained. Toxicity leads to organ toxicity, e.g., heart, brain, kidney, liver, pancreas, liver, eye, lung, and reproductive organs. Cadmium toxicity requires gastrointestinal lavages, supportive care, and chemical decontamination of traditional chelation therapy with appropriate new nanoparticle-based chelating agents and antidotes.
Keywords
Cadmium toxicity, global health, central nervous system
In the periodic table of elements, the heavy metal cadmium belongs to group IIb and is found in soils, sediments, air and water. Unlike other metals, cadmium began to be used on a large scale only recently, since the 1940s[1]. Its main uses are in the production of nickel-cadmium batteries, pigments and plastic stabilizers, while alloy, solder and electroplating applications show a downward trend. The environment is exposed to cadmium through anthropogenic sources such as the refining use and smelting of copper and nickel (which is an extractive metallurgy process to create the metal from the ore) and the burning of fossil fuels[2]. Other reasons for concern include the use of contaminated sewage sludge as a soil amendment and phosphate fertilizers, which, depending on the source rock, can contain significant amounts of cadmium[3]. Cigarette smoke, which has relatively high concentrations of this element, is the main source of non-occupational exposure to cadmium for non-smokers who are not exposed at work. An extremely dangerous non-essential heavy metal known to have harmful effects on cellular enzyme systems, produce oxidative stress and deprive plants of needed nutrients is cadmium (Table 1)[4].
| Enzyme | Exposure duration | Effects on activity | Model | Cd dose or concentration | Reference |
|---|---|---|---|---|---|
| SOD | 2 h | - | V79 CELLS | 5×10-5 M | [6] |
| 2 h to 10 d | ↑↓ | RATS | 0.4 mg/kg | [7] | |
| 30 d | ↓ | RATS | 0.5 mg/kg | [8] | |
| 30 to 90 d | ↑↓ | RATS | 10 mg/kg | [9] | |
| 30 d | ↑ | RATS | 10 mg/kg | [10] | |
| CAT | 2 h | - | V79 CELLS | 5×10-5 M | [6] |
| 4 h | ↓ | CHO-K1 CELLS | 4 µM | [11] | |
| 3 h to 10 d | ↑↓ | RATS | 0.4 mg/kg | [7] | |
| 45 d | ↓ | RATS | 0.4 mg/kg | [12] | |
| 30 d | ↓ | RATS | 0.5 mg/kg | [9] | |
| 6 h to 30 d | ↓ | RATS | 2.5 mg/kg | [13] | |
| 30 to 90 d | ↑↓ | RATS | 10 mg/kg | [12] | |
| 30 d | ↑ | RATS | 15 mg/kg | [10] | |
| Cu/Zn SOD | 6 h to 30 d | ↑↓ | RATS | 2.5 mg/kg | [13] |
| Mn-SOD | 6 h to 30 days | ↓ | RATS | 2.5 mg/kg | [13] |
| GST | 3 h to 10 d | ↑ | RATS | 0.4 mg/kg | [7] |
| GSH-Px | 24 h | ↓ | Pneumocyte II | 10-5 M | [14] |
| 2 h | - | V79 CELLS | 5×10-5 M | [6] | |
| 4 h | ↓ | CHO-K1 CELLS | 4 µM | [11] | |
| 45 d | ↓ | RATS | 0.4 mg/kg | [12] | |
| 30 d | ↑ | RATS | 0.5 mg/kg | [9] | |
| 30-90 d | ↑↓ | RATS | 10 mg/kg | [12] | |
| 30 d | ↑ | RATS | 15 mg/kg | [15] | |
| GSSG-R | 2 h | - | V79 CELLS | 5×10-5 M | [6] |
| 24 h | ↓ | Pneumocyte II | 1 µM | [14] | |
| 4 h | ↓ | CHO-K1 CELLS | µM | [11] | |
| 30-90 d | ↓ | RATS | 10 mg/kg | [12] |
Note: (↓): Decreased and (↑): Increased
Table 1: Synopsis of cadmium effects on the activity of enzymes of the antioxidant system.
Exposure
Cadmium is a substance that has significantly increased the number of environmental media that can potentially harm human health. It can travel long distances through atmospheric transport from the emission source. Cadmium can be easily acquired by many different animals, especially molluscs and crustaceans. Cereals, starchy roots and vegetables have lower concentrations. The main sources of human exposure to cadmium are food contamination, inhalation of tobacco smoke by active and passive smokers and inhalation by workers in various industries[5].
Sources of exposure to cadmium:
Cadmium can enter the environment in a variety of ways, including natural processes such as weathering and erosion, river transport and volcanic activity. It can also result from human activities such as tobacco use, mining, smelting and refining of non-ferrous metals, burning of fossil fuels, burning of municipal waste (especially plastics and batteries containing cadmium), production of phosphate fertilizers and recycling of cadmium-plated steel scrap and electronic waste. In addition, cadmium can be released into the environment through historic sources, such as drainage waters contaminated from wastes or metal mines that enter waterways. Finally, cadmium emissions can be transported long distances by air and stored in locations remote from emission sources.
Studies have shown that cadmium, a type of metal, can be carcinogenic due to its ability to create Reactive Oxygen Species (ROS). When present, cadmium can produce harmful molecules such as hydroxyl radicals, superoxide anions, nitric oxide and hydrogen peroxide. In addition, the research found that rats exposed to cadmium had higher levels of lipid peroxidation, which is the breakdown of fats in the liver and liver mitochondria. These findings support the idea that cadmium-induced production of ROS may lead to negative health effects[16].
Toxicology
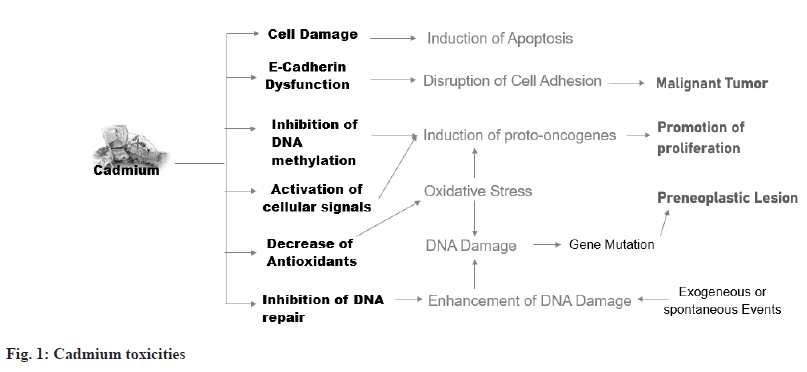
A multistep model of carcinogenesis suggests that cadmium can cause transformation by increasing oxidative stress and producing a small number of critical mutations directly, as well as by interfering with Deoxyribonucleic Acid (DNA) repair and enhancing the number of naturally occurring or exogenously produced mutations. By stimulating immediate early genes and suppressing DNA methylation, cadmium can stimulate tumor growth[17]. Inhibition of DNA methylation, a biological tool for large-scale gene repression, is a unique epigenetic mechanism of proto-oncogene gene activation by cadmium and hypomethylation is associated with overexpression of proto-oncogenes critical to carcinogenesis (fig. 1)[18].
Effects of Cadmium on Different Functional Organs
Effect of cadmium on the brain:
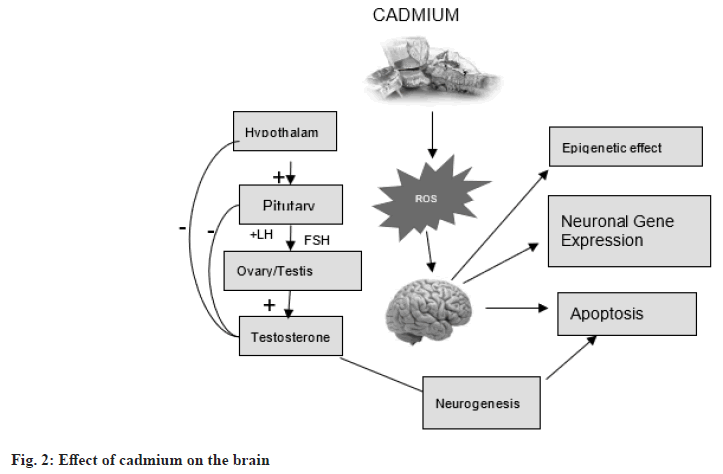
Exposure to ROS and cadmium (Cd) can lead to neuronal cell death by affecting calcium (Ca2+)-mitochondrial signaling and Ca2+ membrane channels. Cadmium accumulation in the brain can alter gene expression and have an epigenetic impact, leading to disruption of neurogenesis. The Hypothalamic-Pituitary-Gonadal (HPG) axis is a complex system that includes stimulation, inhibition and negative feedback control. Cadmium has an estrogen-like effect that can disrupt the endocrine system in various ways by affecting the HPG axis (fig. 2)[19].
Effect of cadmium on the kidney:
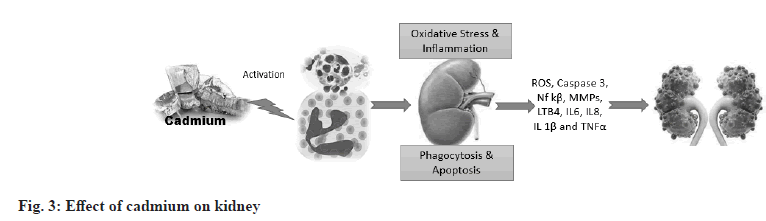
Cadmium is a toxic substance that accumulates in kidney cells, leading to the death of renal epithelial cells through necrotic or apoptotic mechanisms. Improved biomarkers such as Kidney injury molecule-1 (Kim-1) can detect early stages of cadmium toxicity[20]. Studies in mice have shown that the blood transports the Cadmium-Metallothionein (CdMT) complex from the affected organs, especially the liver, to the kidneys. It is believed that the CdMT complex formed and released from the cadmium-damaged liver is what mainly causes cadmium-nephrotoxicity. Circulating CdMT is filtered in the glomerulus and absorbed by the epithelium of the proximal tubule via megalin-mediated transport[21]. Cadmium can enter renal tubular cells through various channels and transporters for ions such as Ca2+, Fe2+ and Zn2+. Cadmium accumulates in the kidney due to its preferential uptake by receptor-mediated endocytosis of freely filtered and metallothionein-bound Cd (Cd-MT) in the renal proximal tubule. Internalized Cd-MT is degraded in endosomes and lysosomes, releasing free Cd2+ into the cytosol where it can generate ROS and activate cell death pathways. An early and sensitive manifestation of chronic cadmium renal toxicity that may be useful in individual and population screening is impaired reabsorption of Low Molecular Weight Proteins (LMWPs) such as Retinol-Binding Protein (RBP) (fig. 3)[22].
Effect of cadmium on the liver:
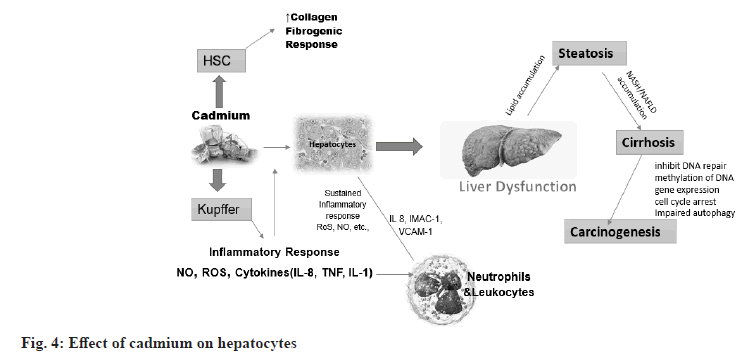
Cadmium accumulation increases liver toxicity and induces liver damage. Cadmium accumulation increases the expression of alanine transaminase, aspartate transaminase, Gamma-glutamyltransferase, malondialdehyde and peroxidase activities, which is an important marker of liver damage. Absorption of cadmium in the liver of women is 10 %-20 % higher than in men. The female liver is more susceptible to cadmium toxicity[23]. This difference may be related to progesterone-activated receptor-dependent calcium channels, channels involved in hepatic cadmium uptake and accumulation. This phenomenon may also be related to a lack of iron in the female body. Iron deficiency promotes the expression of Divalent Metal Transporter 1 (DMT1) in cells, thereby increasing the transport of divalent metal ions into cells. The mechanisms of cadmium-induced acute liver injury mainly involve two pathways. First, cadmium directly binds to sulfhydryl groups on key molecules including Glutathione (GSH) and protein sulfhydryl groups, causing oxidative damage. Second, although cadmium is not a redox-reactive metal, it can cause inflammatory damage, an event associated with oxidative stress (fig. 4)[24].
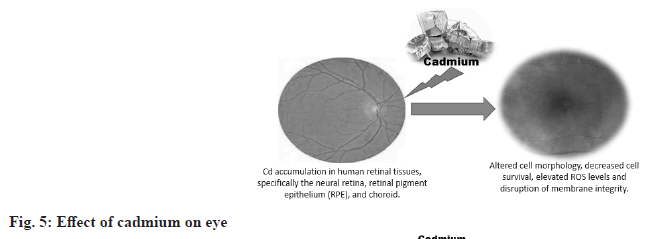
Effect of cadmium on the eye:
Mulak et al.[25], conducted a study of 120 copper factory workers before performing a series of tests, including Free Erythrocyte Protoporphyrin (FEP), whole blood lead and cadmium levels and pupil dilation. While the FEP was accurate, both lead levels were elevated. After episodes of other ocular sensations, such as eye redness and even changes in visual fields, ocular evaluation revealed lenticular changes in 21.7 %, fundus changes in 22 % and episodes of other ocular sensations. This also applies to plant workers who do not know enough about safety procedures and exposure to metals[26]. Human and animal studies have revealed some fascinating information. Research on rabbits fed high doses of cadmium has shown that this concentration of the metal in the eye causes retinopathy and can even destroy the cornea (fig. 5)[27].
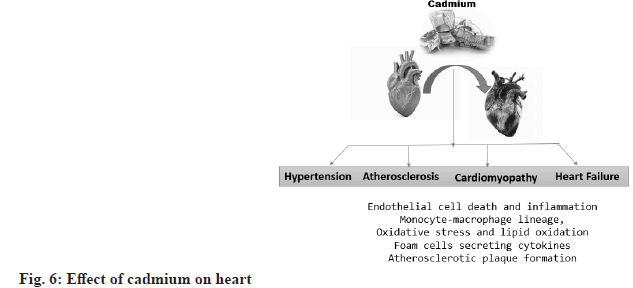
Effect of cadmium on the heart:
Chronic exposure to cadmium is associated with hypertension, atherosclerosis and heart failure. Several cellular transporters and ion channels, including calcium channels, have been identified as transporters of cadmium. Cadmium can also infiltrate vessel walls via cadmium-saturated monocytes[28]. Monocytes and macrophages play a key role in foam cell transdifferentiation and foam cell necrotic death in endothelial dysfunction, thereby promoting atherosclerosis. Cadmium can disrupt endothelial integrity and cause cadmium-mediated endothelial cell death. The formation of gaps between endothelial cells may allow cadmium to diffuse from the bloodstream into the media layer[29]. After transport by endothelial cells, cadmium is mainly retained in smooth muscle cells. Effects of cadmium on smooth muscle cells include interactions with ion homeostasis and calcium ion flux, cytotoxic effects and stimulation of smooth muscle cell proliferation at low cadmium concentrations. This enables the subsequent accumulation of lipids in the vessel walls and the modification of lipid profiles towards an atherogenic state. Cadmium-induced cell death is believed to be a fundamental process by which cadmium promotes atherosclerosis, impairs the integrity of the vascular endothelium and thereby contributes to vascular inflammation (fig. 6)[30].
Cadmium accumulation increases several important pro-inflammatory cytokines, such as Interleukin (IL)-6, IL-8, IL-1β and Tumor Necrosis Factor-Alpha (TNF-α), which may influence inflammation in atherosclerosis. In addition, a study in atherosclerosis-prone apolipoprotein E-deficient mice showed that chronic exposure to cadmium increases total cholesterol and decreases acetylcholine relaxation, thereby reducing the bioavailability of nitric oxide[31]. A study using information from the United States National Health and Nutrition Examination Surveys (NHANES) found an association between high blood pressure and serum cadmium concentrations[32].
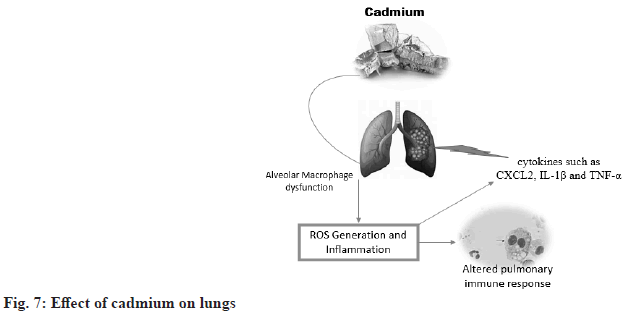
Effect of cadmium on the lungs:
There is evidence linking cadmium exposure to smoking-related lung diseases such as chronic obstructive pulmonary disease. Interestingly, studies show that alveolar macrophages in cigarette smokers can accumulate significant amounts of cadmium without increasing metallothionein content[33]. Metallothioneins are small proteins that contain cysteine ??and can bind to heavy metals such as cadmium, copper and zinc. However, when metallothioneins are saturated with heavy metals, they can impair the ability of mammalian organs to scavenge ROS, leading to redox imbalance, disruption of extracellular matrix homeostasis, and impaired immune function of alveolar macrophages. These mechanisms may become important in lung diseases caused by cadmium exposure (fig. 7)[34].
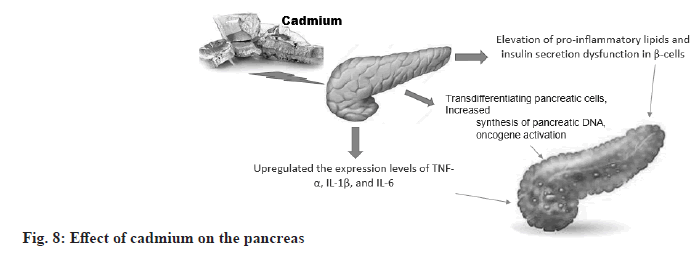
Effect of cadmium on the pancreas:
According to a schematic model, cadmium exposure leads to pancreatic inflammation and induces pancreatic β-cell dysfunction, which in turn accelerates diabetes mellitus. Cadmium interferes with lipid metabolism leading to up-regulation of lipogenesis-related genes glycerol-3-phosphate acyltransferase 4 and perilipin-3 and down-regulation of lipolysis-related genes adipose triglyceride lipase and lipase in pancreatic β-cells leading to lipid accumulation. Cadmium exposure also causes upregulation of pro-inflammatory cytokine expression and increases levels of inflammation-associated lipids such as lysophosphatidylcholines and ceres. These toxic effects contribute to pancreatic damage and insulin secretion dysfunction, thus accelerating the development of diabetes (fig. 8).
Effect of cadmium on the endocrine glands:
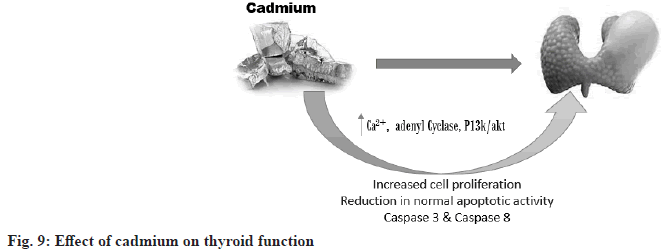
Cadmium Effects on Thyroid Function: Endocrine toxicity can result in hyperfunction or hypofunction of the gland, meaning lower and higher secretion of the gland. Chemically induced thyrotoxicity is reflected in unbalanced plasma levels of thyroxine and/or triiodothyronine and/or thyroid stimulating hormone, which are commonly used as reliable indicators of thyroid function in humans and experimental animals[35]. Changes in serum hormones and disorders of their glandular secretion as well as disorders of extrathyroidal metabolism may occur. The reactions necessary for the formation of T3 and T4 in the follicular cells of the thyroid gland are influenced and controlled by pituitary TSH, while its secretion is controlled by the negative feedback of circulating T4 and T3 levels and also by the influence of the hypothalamic THR[36]. The method of quantifying cadmium in urine or plasma would have a profound effect on determining the relationships between cadmium and thyroid hormone levels[37]. Endocrine toxicity consists of two types i.e. primary endocrine toxicity and secondary endocrine toxicity (fig. 9).
Cadmium effects on reproductive organs & steroid synthesis:
Male reproductive organs: Cadmium is dangerous for testicular function. At high concentrations, cadmium causes testicular necrosis[38]. Cadmium-induced testicular necrosis occurs even when very little cadmium accumulates in the testes[39]. The target of cadmium in the testis is thought to be the vasculature, as similar damage can be caused by ligation of testicular blood vessels[40]. After several weeks of exposure to cadmium, the testis weight decreases and the testis become atrophic and calcified[41]. Also, a low dose of cadmium, which does not induce overt testicular atrophy, significantly reduces gonadotropin-stimulated serum testosterone levels. STAR plays a role in the negative regulation of progesterone. Production in the corpus luteum via activation of phospholipase C (PLC)/PKC, which can reduce cholesterol transport from the outer to the inner mitochondrial membrane[42].
Female reproductive organs: Acute exposure to cadmium in vivo decreased serum concentrations of progesterone and estradiol in rats in a reproductive stage-dependent manner[43]. Cadmium exposure induced a delay in the increase in ovarian progesterone secretion during 1-6 d of pregnancy. Blood progesterone levels also did not increase until 4th d of pregnancy in cadmium-exposed rats. An increase in the concentration of cadmium and a decrease in the level of progesterone were found in the placenta of smokers.
Cadmium steroid hormone actions (on estrogen receptor): Cadmium has potent endocrine-disrupting activity (which mimics the effect of estrogen) in in vivo experiments in female rats. Exposure of ovariectomized rats to cadmium increased uterine weight and promoted mammary gland growth and development. In the case of the uterus, weight gain was accompanied by endometrial proliferation, induction of progesterone receptors and increased expression of the complement component gene C3. Cadmium increased epithelial density and induced milk protein production in mammary glands. These effects were similarly observed with estradiol exposure, and the effects of cadmium were suppressed in the antiestrogen ICI-182780[44]. Cadmium showed estrogenic activity through the estrogen receptor in MCF-7 breast cancer cells. Cadmium-induced an increase in progesterone receptor (PgR) protein and expression of pS2, an estrogen-inducible gene, in MCF-7 cells, and these effects were completely blocked by an antiestrogen. Cadmium treatment of an ER-negative breast cancer cell line transfected with the ER gene caused an increase in PgR and pS2 mRNA levels.
Reports on drugs available for cadmium intoxication:
Removal of heavy metals from contaminated soil includes; washing, leaching and flushing with chemical means, addition of some non-toxic materials to reduce the solubility of heavy metals, electromigration, overlaying the original pollutants with clean materials, mixing polluted materials with clean materials in the surface and subsurface to reduce the concentration of heavy metals an iphytoremediation by plants[45].
Cadmium accumulates in the body for a long time and its concentration can gradually increase several years after exposure[46]. Cadmium exposure causes skeletal demineralization and can directly interact with bone cells, reducing mineralization as well as inhibiting procollagen C-proteinase and collagen production[47]. Saliva analysis can be an excellent method for the long-term detection of heavy metal contamination. The average level of cadmium in saliva with tolerable standard limits in the human body is less than 0.55 µg/l[48]. The long half-life of cadmium (30 y) may be due to long-term accumulation of cadmium in the body, but the short half-life of cadmium in the blood (3-4 mo) may have resulted from recent exposure. The detection limit for cadmium concentration in blood is 0.3 µg/l[49].
Chelating agents:
Penicillamine used to reduce toxic mercury concentrations and lead exposure is not effective in cadmium overdose. Ethylenediaminetetraacetate (EDTA) significantly increased urinary cadmium excretion. One important point is that EDTA can increase the content of cadmium in the kidney and can increase the risk of renal dysfunction[50]. The normal dose of EDTA is 500 mg Ca2+ EDTA combined with 50 mg/kg Glutathione (GSH) via intravenous infusion over the next 24 h and repeated for 12 consecutive days[51]. Renal dysfunction can be reversed if the initial urinary cadmium concentration is <10 µg/g creatinine. Urinary cadmium concentration higher than 10 µg/gr creatinine can cause irreversible kidney damage[50].
Dimercaprol (British Anti-Lewisite (BAL)) is an effective antidote for heavy metal poisoning[52]. BAL and their analogs meso-2,3-dimercaptosuccinic acid (DMSA) and 2,3-dimercapto-1-propanesulfonic acid (DMPS) are used as antidotes in the therapy of heavy metal poisoning. BAL must be administered within the first 4 h after poisoning. A deep intramuscular injection of 3-4 mg/kg into the gluteal muscle is recommended. It is given every 4 h for the first 2 d and twice a day for the next 10 d[53]. It has been reported that the cadmium-BAL complex has more nephrotoxic effects than cadmium alone and that the combination is not useful and it is recommended to treat or manage actual exposure to the poison with other treatments. BAL therapy may increase the risk of nephrotoxicity[54]. BAL increases renal and hepatic cadmium burden may decrease survival and increases nephrotoxicity. For these reasons, it is not administered in case of cadmium intoxication.
Dithiocarbamate derivatives are used in many fields such as agriculture, manufacturing and medicine. N-tetramethylenedithiocarbamate (ATC) belongs to dithiocarbamate derivatives with a chelating effect. It increases the excretion of cadmium in urine and bile, it also reduces side effects and general symptoms of poisoning. It may be useful for primary diagnostic evaluation of the effectiveness of chelating agents[55]. The effectiveness of dithiocarbamates in reducing cadmium toxicity has been confirmed in animal studies.
Succimer, DMSA is a water-soluble analog of BAL with the chemical formula C4H6O4S2[56]. The tolerated dose of DMSA is 10 mg/kg three times a day, but it is not an intracellular chelator. Cadmium binds tightly to metallothionein and is deposited in the liver and kidneys. Consequently, it appears that DMSA cannot be the drug of choice for cadmium poisoning[57].
Unithiol, DMPS is a water-soluble analog of BAL with the chemical formula C3H7O3S3Na. It is available in various dosage forms such as oral, intravenous, rectal, or topical[56]. DMPS is transported into the intracellular space. It did not show major adverse effects[58]. DMPS is oxidized to the disulfide form. At least 80 % of DMPS is oxidized within the first 30 min and the kidneys excrete 84 % of total DMPS within 96 h[59]. Dose of 5 mg/kg IV every 4 h for 24 h and may be increased to 100 mg twice daily if needed.
New analogs of DMSA mono and diesters are more effective and safer antibodies against heavy metal poisoning compared to DMSA alone[58]. Among these monoesters, monoisoamyl DMSA (MiADMSA), a C5-branched alkyl monoester, is effective in lead, cadmium, mercury and gallium arsenide overdoses[60]. MiADMSA is a water-soluble lipophilic chelating agent. It can enter intracellularly and access various endogenous ligands. Consequently, MiADMSA is preferred over its parent compound.
Conclusion
Cadmium toxicity remains one of the major global health problems due to its ubiquitous nature in the environment and persistent accumulation in biological systems. Exposure through air, water, soil and food allows cadmium to penetrate human tissues, imposing a wide range of toxic effects across multiple organ systems. Mechanisms are numerous regarding cadmium toxicity, the central being its generation of oxidative stress causing cellular damage that ranges from interference in the defense mechanism against oxidation. In so doing, it would have interfered with DNA methylation on the pathway of cells; it leads to an oncogene activation state with impairment in cellular respiration. With these impacts in the pathways, therefore, the chemical results in carcinogenic, teratogenic, and endocrine-disrupting effects. This affects organs damaged consequently, among which are disorders in kidneys, livers, the brain, the heart, lung disorders and reproduction disorders.
While traditional chelation therapies have demonstrated a certain efficacy, they suffer from a lot of problems with toxicity, poor targeting, and side effects; thus, safer and more effective treatments are required. Nanoparticle-based chelating agents and other advanced therapeutic approaches promise better avenues for cadmium detoxification with enhanced organ function protection and precision targeting. Future research should continue the exploration of these innovative therapeutic strategies and further clarification of cadmium toxicity mechanisms, still emphasizing preventive measures and better environmental regulations to reduce exposure to cadmium. It is now necessary to have a comprehensive approach that integrates clinical care, the safety of the environment, and molecular research to mitigate the health risks of cadmium and protect public health.
Conflict of interest:
The authors declared no conflict of interests.
References
- Stoeppler M. Metals and Their Compounds in the Environment. Occurrence, Analysis, and Biological Relevance. FW Jr (Ed.), VCH, Weinheim. 1991:803-51.
- Roy JI. Environmental Contaminants Encyclopedia Chromium VI (Hexavalent Chromium) Entry. Colorado: National Park Service;1997.
- Nunes N, Ragonezi C, Gouveia CS, Pinheiro de Carvalho MÂ. Review of sewage sludge as a soil amendment in relation to current international guidelines: A heavy metal perspective. Sustainability 2021;13(4):2317.
- Irfan M, Hayat S, Ahmad A, Alyemeni MN. Soil cadmium enrichment: Allocation and plant physiological manifestations. Saudi J Biol Sci 2013;20(1):1-10.
[Crossref] [Google Scholar] [PubMed]
- Leonard SS, Bower JJ, Shi X. Metal-induced toxicity, carcinogenesis, mechanisms and cellular responses. Mol Cellular Biochem 2004;255:3-10.
[Crossref] [Google Scholar] [PubMed]
- Ochi K. A rel mutation abolishes the enzyme induction needed for actinomycin synthesis by Streptomyces antibioticus. Agri Biol Chem 1987;51(3):829-35.
- Sarkar S, Yadav P, Bhatnagar D. Lipid peroxidative damage on cadmium exposure and alterations in antioxidantsystem in rat erythrocytes: A study with relation to time. Biometals 1998;11(2):153-7.
[Crossref] [Google Scholar] [PubMed]
- Hussain T, Shukla GS, Chandra SV. Effects of cadmium on superoxide dismutase and lipid peroxidation in liver and kidney of growing rats: In vivo and in vitro studies. Pharmacol Toxicol 1987;60(5):355-8.
[Crossref] [Google Scholar] [PubMed]
- Salovsky PT, Shopova VL. Early biological effects of whole body irradiation on rat lungs. Radiat Environ Biophys 1992;31(4):333-41.
[Crossref] [Google Scholar] [PubMed]
- Kosti? MM, Ognjanovi? B, Dimitrijevi? S, Ziki? RV, ZSCtajn A, Rosi? GL, et al. Cadmium?induced changes of antioxidant and metabolic status in red blood cells of rats: In vivo effects. Eur J Haematol 1993;51(2):86-92.
[Crossref] [Google Scholar] [PubMed]
- Yang JL, Chao JI, Lin JG. Reactive oxygen species may participate in the mutagenicity and mutational spectrum of cadmium in Chinese hamster ovary-K1 cells. Chem Res Toxicol 1996;9(8):1360-7.
[Crossref] [Google Scholar] [PubMed]
- Shukla A, Shukla GS, Srimal RC. Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 1996;15(5):400-5.
[Crossref] [Google Scholar] [PubMed]
- Casalino E, Calzaretti G, Sblano C, Landriscina C. Molecular inhibitory mechanisms of antioxidant enzymes in rat liver and kidney by cadmium. Toxicology 2002;179(1-2):37-50.
[Crossref] [Google Scholar] [PubMed]
- Tátrai E, Kováciková Z, Hudák A, Adamis Z, Ungváry G. Comparative in vitro toxicity of cadmium and lead on redox cycling in type II pneumocytes. J Appl Toxicol 2001;21(6):479-83.
[Crossref] [Google Scholar] [PubMed]
- Mondola P, Damiano S, Sasso A, Santillo M. The Cu, Zn superoxide dismutase: not only a dismutase enzyme. Front Physiol 2016;7:594.
[Crossref] [Google Scholar] [PubMed]
- El?Maraghy SA, Gad MZ, Fahim AT, Hamdy MA. Effect of cadmium and aluminum intake on the antioxidant status and lipid peroxidation in rat tissues. J Biochem Mol Toxicol 2001;15(4):207-14.
[Crossref] [Google Scholar] [PubMed]
- Branca JJ, Fiorillo C, Carrino D, Paternostro F, Taddei N, Gulisano M, et al. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020;9(6):492.
[Crossref] [Google Scholar] [PubMed]
- Takiguchi M, Achanzar WE, Qu W, Li G, Waalkes MP. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp Cell Res 2003;286(2):355-65.
[Crossref] [Google Scholar] [PubMed]
- Mead MN. Cadmium confusion: Do consumers need protection? Environ Health Perspect 2010;118(12):a528–a534.
[Crossref] [Google Scholar] [PubMed]
- Yan LJ, Allen DC. Cadmium-induced kidney injury: Oxidative damage as a unifying mechanism. Biomolecules 2021;11(11):1575.
[Crossref] [Google Scholar] [PubMed]
- Nordberg M, Nordberg GF. Metallothionein and cadmium toxicology—Historical review and commentary. Biomolecules 2022;12(3):360.
[Crossref] [Google Scholar] [PubMed]
- Moody EC, Coca SG, Sanders AP. Toxic metals and chronic kidney disease: A systematic review of recent literature. Curr Environ Health Rep 2018;5:453-63.
[Crossref] [Google Scholar] [PubMed]
- Kang MY, Cho SH, Lim YH, Seo JC, Hong YC. Effects of environmental cadmium exposure on liver function in adults. Occup Environ Med 2013;70(4):268-73.
[Crossref] [Google Scholar] [PubMed]
- Salama SA, Arab HH, Hassan MH, Maghrabi IA. Cadmium-induced hepatocellular injury: Modulatory effects of γ-glutamyl cysteine on the biomarkers of inflammation, DNA damage, and apoptotic cell death. J Trace Elem Med Biol 2019;52:74-82.
[Crossref] [Google Scholar] [PubMed]
- Mulak M. The effect of heavy metals (lead, cadmium, and manganese) on the function of the visual system. Med Pr 1998;49(6):603-7.
[Google Scholar] [PubMed]
- Nelke KH, Mulak M, ?uczak K, Pawlak W, Nienartowicz J, Szumny D, et al. Occurrence and exposure to lead and cadmium and their environmental influence on eyesight. Polish J Environ Studies 2015;24(4):1491-6.
- Prost M, Gerkowicz K. Experimental studies on cadmium accumulation in eye structures. Klin Oczna 1986;88:330.
- Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The vascular system as a target of metal toxicity. Toxicol Sci 2008;102(2):207-18.
[Crossref] [Google Scholar] [PubMed]
- Knoflach M, Matosevic B, Rücker M, Furtner M, Mair A, Wille G, et al. Functional recovery after ischemic stroke—a matter of age: Data from the Austrian Stroke Unit Registry. Neurology 2012;78(4):279-85.
[Crossref] [Google Scholar] [PubMed]
- Bonaventura P, Lamboux A, Albarede F, Miossec P. Differential effects of TNF-α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PLoS One 2018;13(5):e0196285.
[Crossref] [Google Scholar] [PubMed]
- Satarug S, Nishijo M, Lasker JM, Edwards RJ, Moore MR. Kidney dysfunction and hypertension: Role for cadmium, p450 and heme oxygenases? Tohoku J Exp Med 2006:208(3):179-202.
[Crossref] [Google Scholar] [PubMed]
- Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 National Health and Nutrition Examination Survey (NHANES). Environ Health Perspect 2008;116(1):51-6.
[Crossref] [Google Scholar] [PubMed]
- Grasseschi RM, Ramaswamy RB, Levine DJ, Klaassen CD, Wesselius LJ. Cadmium accumulation and detoxification by alveolar macrophages of cigarette smokers. Chest 2003;124(5):1924-8.
[Crossref] [Google Scholar] [PubMed]
- Koustav G, Bettina L, Lena P, Agneta Å, Anders L. Cadmium in tobacco smokers: A neglected link to lung disease? Eur Respir Rev 2018;27(147):1-8.
[Crossref] [Google Scholar] [PubMed]
- Aleksandra B, Vesna M, Biljana A, Zorica B, Marijana C, Elisavet AR, et al. Overview of cadmium thyroid disrupting effects and mechanisms. Int J Mol Sci 2018;19(5):1-19.
[Crossref] [Google Scholar] [PubMed]
- Kelly GS. Peripheral metabolism of thyroid hormones: A review. Altern Med Rev 2000;5(4):306-333.
[Google Scholar] [PubMed]
- Nie X, Chen Y, Chen Y, Chen C, Han B, Li Q, et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ Pollut 2017;230:320-328.
[Crossref] [Google Scholar] [PubMed]
- Shiraishi N, Waalkes MP. Acquired tolerance to Cd-induced toxicity in the rodent testes. Toxic Subst Mech 1996;15:27-42.
- Gunn S, Gould T. Cadmium and other mineral elements. In: Johnson A, Gomes W, Van Demark N, editors. The Testis: Influencing Factors. New York: Academic Press; 1970. p. 377-481.
- Nolan, CV, Shaikh ZA. The vascular endothelium as a target tissue in acute cadmium toxicity. Life Sci 1986 oct 20;39(16):1403–1409.
[Crossref] [Google Scholar] [PubMed]
- Elindaer CG. Other toxic effects. A Toxicological and Epidemiological Appraisal. Vol. I CRC Press 1986. p. 159-204.
- Wang SS, Chen L, Xia SK. Cadmium is acutely toxic for murine hepatocytes: Effects on intracellular free Ca2+ homeostasis. Physiol Res 2007;56(2):193-201.
[Google Scholar] [PubMed]
- Piasek M, Laskey JW. Acute cadmium exposure and ovarian steroidogenesis in cycling and pregnant rats. Reprod Toxicol 1994;8(6):495-507.
[Crossref] [Google Scholar] [PubMed]
- Makoto S, Masashi K, Masafumi M, Seitaro O, Kohei K. First phase of glucose-stimulated insulin secretion from MIN 6 cells does not always require extracellular calcium influx. J Pharmacol Sci 2006;101(4):293-302.
[Crossref] [Google Scholar] [PubMed]
- Wuana RA, Okieimen FE, Imborvungu JA. Removal of heavy metals from contaminated soil using organic chelating acids. Int J Environ Sci Tech 2010;7(3):485-496.
- Rafati RM, Rafati RM, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Caspian J Intern Med 2017;8(3):135-145.
[Crossref] [Google Scholar] [PubMed]
- Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, et al. Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study. Lancet 1999;353(9159):1140-4.
[Crossref] [Google Scholar] [PubMed]
- Ogboko B. Cadmium and lead concentration in saliva of children in ceres district of South Africa. J Basic Appl Sci Res 2011;1:825-31.
- Silver MK, Lozoff B, Meeker JD. Blood cadmium is elevated in iron deficient US children: A cross-sectional study. Environ Health 2013;12(1):1-9.
[Crossref] [Google Scholar] [PubMed]
- Wu X, Su S, Zhai R, Chen K, Jin T, Huang B, et al. Lack of reversal effect of EDTA treatment on cadmium induced renal dysfunction: A fourteen-year follow-up. Biometals 2004;17(4):435-441.
[Crossref] [Google Scholar] [PubMed]
- Gil H, Kang E, Lee K, Yang J, Lee E, Hong S. Effect of glutathione on the cadmium chelation of EDTA in a patient with cadmium intoxication. Hum Exp Toxicol 2011;30(1):79-83.
[Crossref] [Google Scholar] [PubMed]
- Vilensky JA, Redman K. British anti-Lewisite (dimercaprol): An amazing history. Ann Emerg Med 2003;41(3):378-383.
[Crossref] [Google Scholar] [PubMed]
- Karmaker RN. Forensic medicine and toxicology. 3rd ed. Kolkata: Academic Publishers; 2010.
- Lapus RM. Activated charcoal for pediatric poisoning: The universal antidote? Curr Opin Pediatr 2007;19(2)216-222.
[Crossref] [Google Scholar] [PubMed]
- Nabilaldine FS, Solmaz T, Jamilaldine FS, Behnam N. Evaluation of ATC as an orally administered drug in treatment of cadmium toxicity of rat organs. J Chem 2009;6(2):504-510.
- Katzung BG, Masters SB, Trevor AJ. Basic and clinical pharmacology. 12th ed. NewYork: McGraw Hill; 2012.
- Patrick L. Toxic metals and antioxidants: Part II. The role of antioxidants in arsenic and cadmium toxicity. Altern Med Rev. 2003 May;8(2):106-128.
[Google Scholar] [PubMed]
- Flora SJS. Metal poisoning: Threat and management. Al Ameen J Med Sci 2009;2(2):4-26.
- Vamnes JS, Eide R, Isrenn R, Hol PJ, Gjerdet NR. Blood mercury following DMPS administration to subjects with and without dental amalgam. Sci Total Environ 2003;308(1-3):63-71.
[Crossref] [Google Scholar] [PubMed]
- Mehta A, Pant SC, Flora SJ. Monoisoamyl dimercaptosuccinic acid induced changes in pregnant female rats during late gestation and lactation. Reprod Toxicol 2006;21(1):94-103.
[Crossref] [Google Scholar] [PubMed]