- *Corresponding Author:
- Mao Ming
Department of Infectious Disease, Southern district of Guang’anmen Hosptital, China Academy of Chinese Medical Science, Beijing 100053, China
E-mail: maoming345@gmail.com
| Date of Received | 27 March 2021 |
| Date of Revision | 14 May 2022 |
| Date of Acceptance | 15 September 2022 |
| Indian J Pharm Sci 2022;84(5):1288-1296 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Capillarisin is a primary bioactive compound derived from Artemisia capillaris is known to possess antioxidative and anti-inflammatory responses. Although, studies on the protecting effect of capillarisin against heart attack are still not clear. Therefore, in the current investigation, the experimental model of myocardial infarction was induced using isoproterenol in rats. Male Wistar rats were separated into 4 clusters as control, isoproterenol-induced myocardial infarction, capillarisin pretreated (10 mg/kg/d) before isoproterenol induction, capillarisin drug control for 4 w. Finally, infarct size, cardiac markers, such as creatinine kinase, lactate dehydrogenase, matrix proteins and microRNA signatures were revealed by reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay. Rats induced with myocardial infarction demonstrated the abnormality in echocardiographic measurements with increased biomarkers, for example, heart type fatty acid binding protein, creatinine kinase and troponin-I compared to control. While capillarisin pre-treated rats banned myocardial infarction considerably (p<0.001) with restored cardiac functions. Besides, capillarisin pre-treatment repealed the fibrosis onset with restored matrix proteins. In addition, cardiac biomarkers, for example, atrial natriuretic peptide, erythroid transcription factor, nuclear factor of activated T cells were found increased with reduced sarco(endo)plasmic reticulum calcium-ATPase 2 in rats suffering from myocardial infarction. Additionally, the messenger ribonucleic acid of matrix signaling pathways were also found reduced obviously in capillarisin treatment which was disturbed in myocardial infarction induced rats. Capillarisin protected the hearts from failing induced by isoproterenol in rats and provides cardioprotective effect, through restoring the matrix signaling, antiinflammatory, antioxidant effect suggested possible drug candidate for the therapy of heart attack.
Keywords
Cardioprotective, isoproterenol, myocardial infarction, fibrosis, capillarisin
Cardiovascular Disease (CVD) is a major cause of global deaths worldwide and it affects all classes of society irrespective of their economic background[1,2]. When there is an occlusion in the coronary arteries, a Myocardial Infarction (MI) occurs, to cause cardiac cell death if left untreated and may get complicated into heart failure that leads to heart transplantation at the end-stage. Although heart transplantation is the most effective therapy available today for end-stage cardiac infarction, the availability of heart donors that are compatible with the patients is a severe shortcoming in the treatment of this disease.
When MI is treated with normal blood circulation or reperfusion may cause injury known as ischemia-reperfusion injury[3,4]. It may also lead to improved infarct size and myocardial cell death and treatments aimed at MI must also preserve heart tissues that are injured during the process and must protect the alterations in the cell structure referred as cardiac remodeling and preserving heart function in patients[5].
Excessive cardiac remodeling includes over-accumulation of Extracellular Matrix (ECM)[6,7] in the heart that obstructs the mechanical and electrical signal transduction between the cellular and noncellular constituents prominent to heart dysfunction[8]. Various stimuli aid the transformation of cardiac fibroblasts into myofibroblasts in the diseased heart that leads to collagen accumulation and progressive heart fibrosis[9,10].
Transforming Growth Factor-Beta (TGF-β) has been known to be an essential mediator in the progression of tissue remodeling and fibrosis of the heart. Animal models have revealed that excessive TGF-β binds to its receptor TGF-β RI[11,12] and promotes the cardiac fibrosis through the activation of Suppressor of Mothers Against Decapentaplegic (SMAD)-2/3 pathway[13,14]. Even though various treatments are available for the deterrence or reversal of cardiac fibrosis, problems related to side effects leaving them unattractive. Hence, there is always a need for drug molecules that is compatible and effective in treating the disease condition. Because of these specifications, the traditional use of plant-based molecules fulfils the demand, which is more attractive in the present scientific elucidation. Amongst, the importance of using Capillarisin (CAP) from Artemisia capillaris has been reported earlier with potential beneficial effects such as anti-inflammatory, analgesic, antibiotic activities. In addition, its pharmacological properties of inhibiting liver fibrosis by inhibition of TGF-β is known[15]. Hence, in the current investigation, we have tested the anti-fibrotic property of CAP via TGF-β inhibition and thereby fibrosis prevention in the heart by inducing heart failure in rats using Isoproterenol (ISO) induced MI and subsequently treated with CAP to observe for the cardiac markers, matrix components of the heart and cardiac remodeling.
Materials and Methods
Chemicals:
ISO was obtained from Sigma Aldrich, United States of America (USA). CAP was purchased from Wako (Osaka, Japan), Ribonucleic Acid (RNA) isolation kits, complementary Deoxyribonucleic Acid (cDNA) synthesis and SYBR® master mix were from Qiagen, USA. Primer sequences for Polymerase Chain Reaction (PCR) were attained from Eurofins MWG (Operon). Assay kits for cardiac troponin-I, heart-type Fatty Acid-Binding Protein (h-FABP), Creatinine Kinase (CK), Lactate Dehydrogenase (LDH) were from Abcam Inc, USA. All other chemical reagents used were analytical grade.
Experimental model of heart attack:
Male Wistar rats (110±15) g were maintained in cages photoperiod at 12 h light-dark cycle and 65 %±5 %, respectively at 25°±2°. Rats have been provided free access to daily standard rat chow and reverse osmosis water. The experimental animal procedure was followed after getting prior permission from the Institutional Animal Ethical Committee and the experiments were approved strictly following the institutional guidelines and suggestions by the ethical committee, China. For the experimentation, six rats per clusters were divided randomly and allocated to four sets as vehicle-control set; ISO-induced group (100 mg/kg. b.wt, intraperitoneally); CAP (dissolved in olive oil, 10 mg/kg, orally) started a week before ISO and CAP alone as drug control. All the experimental animals were maintained for 8 w. During the testing period, the animals were evaluated for echocardiographic indices as defined formerly. The animals were then killed, cardiac tissue was separated out, heart mass to body mass ratio was calculated. For the histopathological analysis, a portion of heart tissue cut-off and stained with Hematoxylin and Eosin (H&E) for histopathological study and Masson’s trichrome staining for fibrosis analysis.
Assessment of cardiac marker enzymes:
The colourimetric assay kits were used for the estimation of CK, LDH (units/l), troponin-I (ng/ml), h-FABP (ng/ml) using the marketable assay kits as per the company’s instruction.
Assessment of gelatinase and fibrotic markers:
The levels of serum Matrix Metalloproteinase (MMP)-1, MMP-2, Tissue Inhibitor of MMPs (TIMP)-1, TIMP-2, TGF-β and Plasminogen Activator Inhibitor type 1 (PAI‐1) in the heart tissue homogenate were elucidated using commercial assay kits obtained from Abcam Scientific Inc, USA, as per the company’s procedure.
Reverse Transcription-PCR (RT-PCR):
To explain the mRNA expression of fibrotic markers, a known amount of RNA was transcribed to cDNA, and the real-time PCR was achieved for exact genes using SYBR® Green/ROX master mix (Thermo Fisher Scientific, Waltham, MA) using Glyceraldehyde 3-Phosphate Dehydrogenase (GADPH) as the house-keeping gene for the gene expression fold increase calculation. To assess the expression of messenger RNA (mRNA) in the current investigation, quantitative RT-PCR was achieved with micro RNA (miR)-specific primers. RNA was extracted from the heart tissues using Trizol reagent and quantified. For quantitative detection of miR-21, miR-34a, miR-328, miR-30a and miR-26a, TaqMan miR reverse transcription kit, TaqMan miR specific assays and small nucleolar RNA (snoRNA) assays were used according to the company’s instructions. SnoRNA was used as a housekeeping control for normalization. The gene-specific primers used in the Table 1. The obtained Ct values were used for the calculation of gene expressions by the comparative Ct method (ΔΔCT).
| Gene | Primer | Sequence | Annealing |
|---|---|---|---|
| c-Myc | F | CTATCCAGCTGCCAAGAGGG | 58 |
| R | AAGCTACGCTTCAGCTCGTT | ||
| NFAT | F | AGACCTAGCAGACGTTAGGAAA | 57 |
| R | CAACGAACAGAAGCCACCAC | ||
| GATA4 | F | CCCCAATCTCGATATGTTTGATGA | 57 |
| R | GGGCCGGTTGATACCATTCA | ||
| ANP | F | CCTGGACTGGGGAAGTCAAC | 58 |
| R | GTCAATCCTACCCCCGAAGC | ||
| Smad-2 | F | GCCGCCCGAAGGGTAGAT | 59 |
| R | TTCTGTTCTCCACCACCTGC | ||
| Smad-3 | F | AGTTAAAAGCGAAGTTCGGGC | 57 |
| R | CAAGCTCTTGACCGCCTTCT | ||
| SERCA2 | F | TCACACCGCTGAATCTGACC | 59 |
| R | GCACAAAGGGCCAGGAAATG | ||
| ATF3 | F | CCAGAACAAGCACCTTTGCC | 58 |
| R | CGGCATTCACACTCTCCAGT | ||
| GAPDH | F | AGTGCCAGCCTCGTCTCATA | 57 |
| R | TTCTCAGCCTTGACTGTGCC |
Table 1: List of Primers used in our study.
Statistical examination:
Statistical significance was assessed with student t-test method. Statistics were identified as the mean±Standard Error (SE) of the mean and Graph Pad Prism version 7.0 was used. Differences with p<0.05, were considered statistically significant.
Results and Discussion
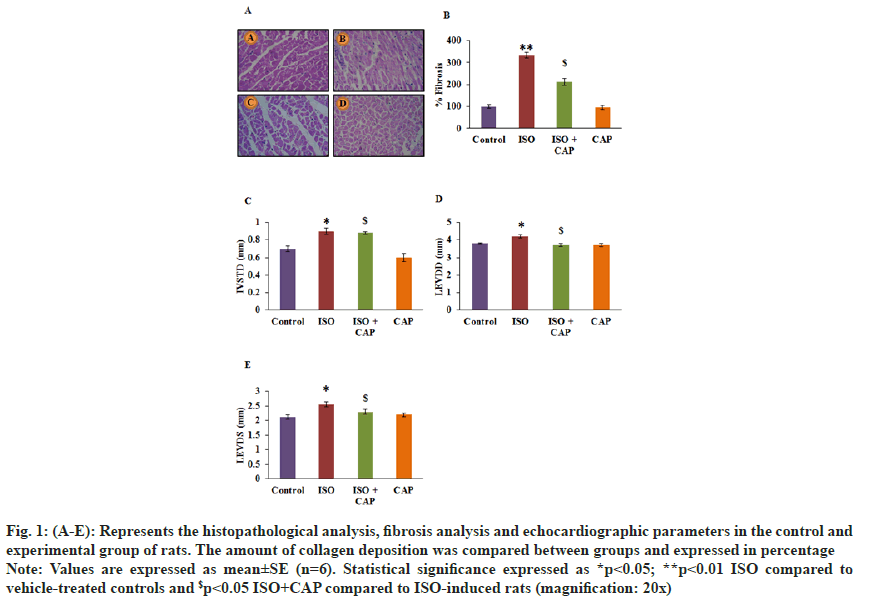
The current investigation aimed at assessing the cardioprotective mechanism of CAP in the experimental MI model and the results presented as follows. Fig. 1 represents the histopathological and fibrosis analysis in the heart tissue sections of experimental animals. Animals induced with MI exhibited increased fibrosis (3.2-fold) with evidenced damaged in the tissue myocytes fibrils in animals suffering from MI. In addition, the echocardiographic analysis revealed a disarrangement in the chamber functions in MI induced rats. Whereas rats received CAP treatment resulted in a marked drop of collagen deposition and normal architecture in the heart tissues with restored echocardiographic indices (fig. 1).
Fig. 1: (A-E): Represents the histopathological analysis, fibrosis analysis and echocardiographic parameters in the control and experimental group of rats. The amount of collagen deposition was compared between groups and expressed in percentage.
Note: Values are expressed as mean±SE (n=6). Statistical significance expressed as *p<0.05; **p<0.01 ISO compared to vehicle-treated controls and $p<0.05 ISO+CAP compared to ISO-induced rats (magnification: 20x).
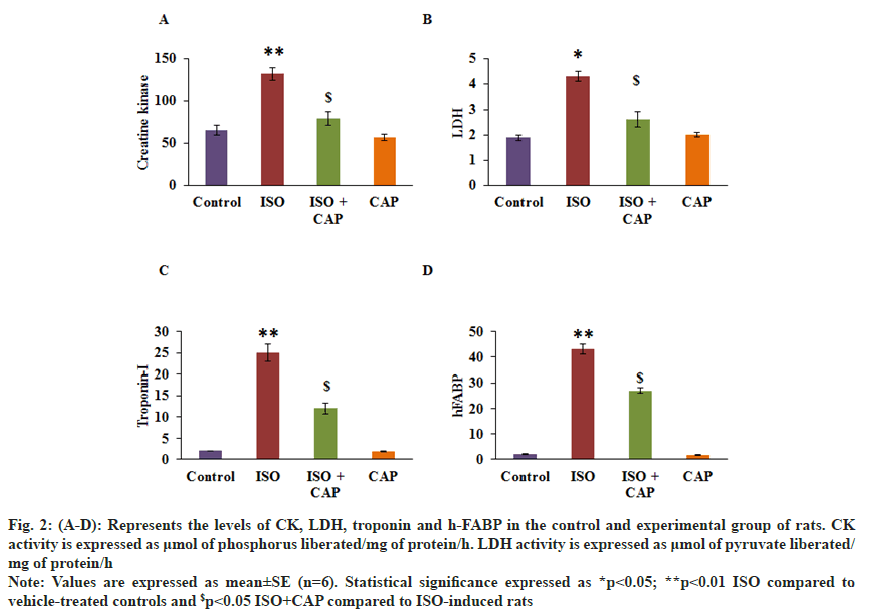
Fig. 2 shows the levels of CK, LDH, troponin I and h-FABP levels in the serum of rats. As obvious, rats with ISO attested a significant increase in the cells stress markers of heart tissue compared to control. On the other hand, rats pre-treated with CAP displayed restored cardiac markers such as CK, LDH, troponin I and h-FABP compared to ISO induced rats (fig. 2). FABP levels in the serum of rats. As obvious, rats with ISO attested a significant increase in the cells stress markers of heart tissue compared to control. On the other hand, rats pre-treated with CAP displayed restored cardiac markers such as CK, LDH, troponin I and h-FABP compared to ISO induced rats (fig. 2).
Fig. 2: (A-D): Represents the levels of CK, LDH, troponin and h-FABP in the control and experimental group of rats. CK activity is expressed as μmol of phosphorus liberated/mg of protein/h. LDH activity is expressed as μmol of pyruvate liberated/mg of protein/h.
Note: Values are expressed as mean±SE (n=6). Statistical significance expressed as *p<0.05; **p<0.01 ISO compared to vehicle-treated controls and $p<0.05 ISO+CAP compared to ISO-induced rats.
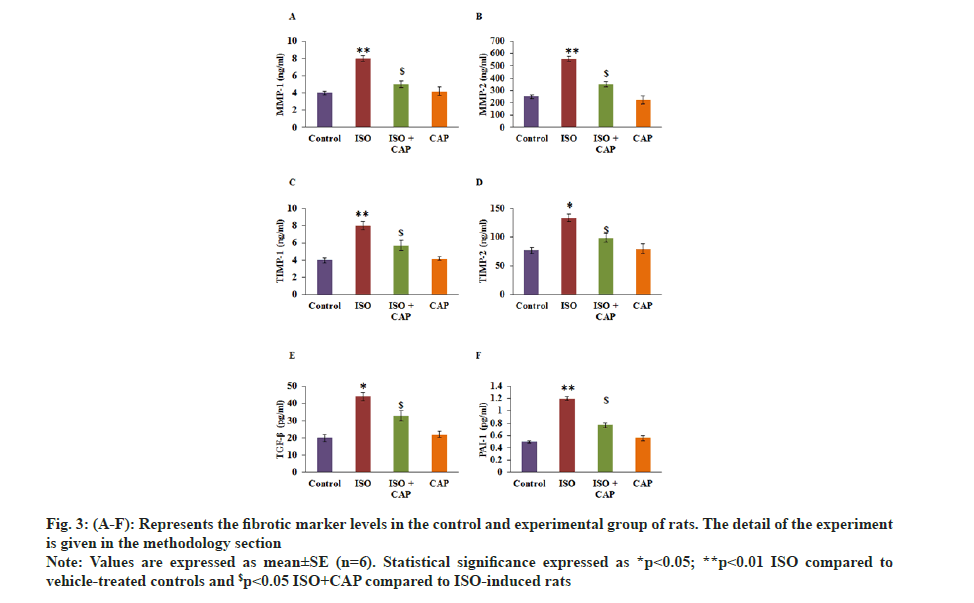
Furthermore, the fibrotic markers level analysis proved that the improved levels of MMP-2 (p<0.01), MMP-1 (p<0.05), TIMP-1 (p<0.05), TIMP-2 (p<0.05), (p<0.05) TGF-β (p<0.01) and PAI-1 (p<0.01) in MI induced rats than control. While these fibrotic markers were diminished in the CAP pre-treatment with reduced collagen accumulation as stated in staining analysis mentioned above (p<0.01) point out that the protective role of CAP is through abstaining the pestilent fibrotic signaling from being serious in the heart tissues (fig. 3).
Fig. 3: (A-F): Represents the fibrotic marker levels in the control and experimental group of rats. The detail of the experiment is given in the methodology section.
Note: Values are expressed as mean±SE (n=6). Statistical significance expressed as *p<0.05; **p<0.01 ISO compared to vehicle-treated controls and $p<0.05 ISO+CAP compared to ISO-induced rats
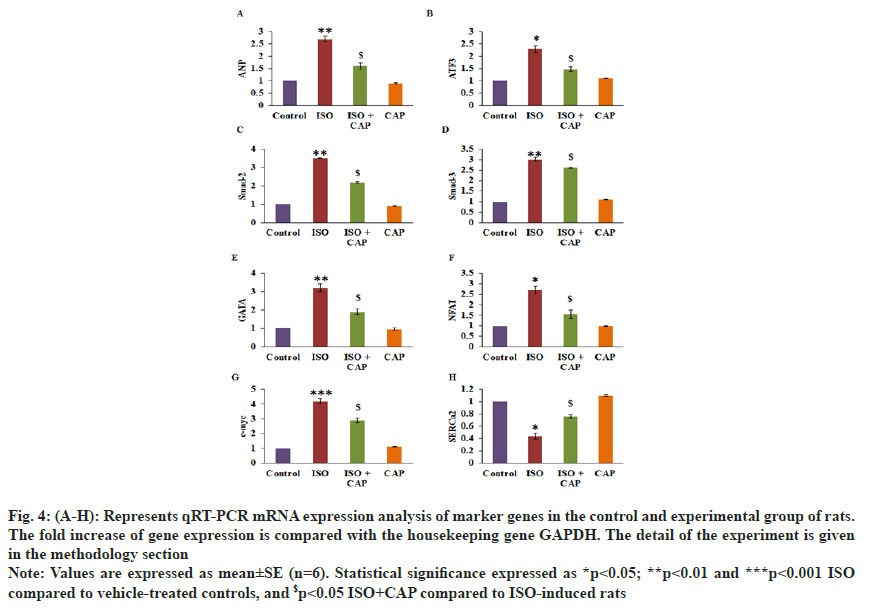
To validate the role of CAP on the modulation of cardiac and fibrotic markers, RT-PCR was achieved and the results are displayed in fig. 4. Rats induced with MI proved a substantial upsurge in fibrotic markers such as SMAD-2 (3.5-fold), SMAD-3 (3-fold), Atrial Natriuretic Peptide (ANP) (2.7-fold) erythroid transcription factor (GATA) (3.2-fold), Nuclear Factor of Activated T cells (NFAT) (2.7-fold), cardiac Myosin-binding protein C (c-Myc) (4.2-fold), Activating Transcription Factor 3 (ATF3) (2.3-fold) with reduced Sarcoplasmic/Endoplasmic Reticulum Calcium ATPase 2 (SERCA2) mRNA than control. Though, the improved levels of these genes were found normalized in CAP administered rats suggest that the drug uses an anti-fibrotic effect in the rat hearts (fig. 4).
Fig. 4: (A-H): Represents qRT-PCR mRNA expression analysis of marker genes in the control and experimental group of rats. The fold increase of gene expression is compared with the housekeeping gene GAPDH. The detail of the experiment is given in the methodology section.
Note: Values are expressed as mean±SE (n=6). Statistical significance expressed as *p<0.05; **p<0.01 and ***p<0.001 ISO compared to vehicle-treated controls, and $p<0.05 ISO+CAP compared to ISO-induced rats.
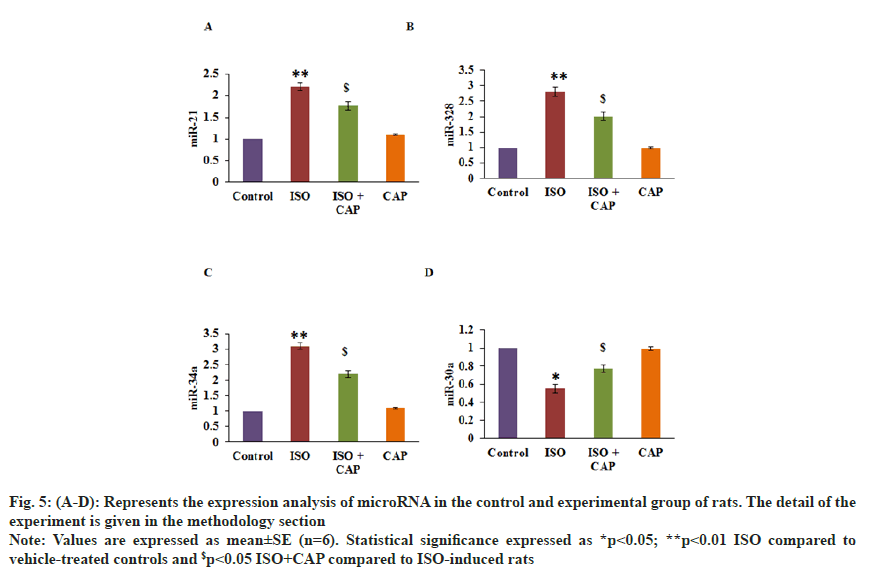
The role of CAP in the cardioprotective mechanism was assessed through the expression of miRs by PCR. Fig. 5 shows the expression level of mRNA, and the results established an augmented level of miR-21 (2.2-fold), miR-34a (3.1-fold), miR-328 (2.8-fold), with reduced miR-30a in ISO induced rats. Whereas levels of these miRs were found reinstated (p<0.01) in CAP pre-treatment proposes that the interaction of microRNAs is one of the major signaling regulators in the activation of heart arrest mechanism (fig. 5).
Fig. 5: (A-D): Represents the expression analysis of microRNA in the control and experimental group of rats. The detail of the experiment is given in the methodology section.
Note: Values are expressed as mean±SE (n=6). Statistical significance expressed as *p<0.05; **p<0.01 ISO compared to vehicle-treated controls and $p<0.05 ISO+CAP compared to ISO-induced rats.
The current investigation was aimed at exploring the potential curative effects of CAP in using the cardioprotective activities in ISO-induced MI and the subsequent cardiac hypertrophy and fibrosis in rats. MI induced by administering ISO is a known method to study cardiac failure in animals[16] and we have examined various cardiac, biochemical and histopathological parameters in establishing the protective effect of CAP. Administration of ISO at low doses regularly for 4 w has induced cardiac hypertrophy[17] and the observed myocardial changes were taken at the elevated levels of ISO in circulation[18]. The administration of ISO has induced extensive necrosis in the endocardium[19] and myocardial lesions[20] that indicates the cardiomyocytes loss are the initial triggers of heart failure[21]. The Left Ventricular (LV) function and the myocardial contractility are affected by hypertrophy[22]. The altered electrical activity of the heart does reflect the heart abnormalities and can be effectively measured using Electrocardiogram (ECG) parameters, and it was found that the modulated ECG parameters in the present ISO induced rats confirm the onset of cardiac failure[23]. Interestingly, with the treatment of CAP there was a significant restoration in the ECG parameters of the heart functions which was evident from the histological analysis suggesting the protective effects of CAP.
ISO overdose has induced LV dilation and hypertrophy[22] with the decreasing ventricular power to efficiently empty the ventricle and thus increasing the diastolic pressure. The ventricle dilates due to the stretching of the myocytes, thus thinning the myocardium. The integrity of the myocardial membrane of normal control rats was examined in the histological staining results and found that it is intact without any infiltration of the immune cells or necrosis and the same when compared in the ISO-treated rats have shown a marked increase in the infiltration of macrophages and lymphocytes into the myocardium and edema with necrosis was also observed[24]. While, CAP treatment has reduced the edema and the infiltration of inflammatory cells[25] indicating a cardio protective effect of CAP in ISO-treated rats.
Classic biomarkers that are expressed during heart failure were evaluated. The ideal biomarker that is having its presence in the blood during myocardial necrosis is cardiac troponin[26]. It indicates the apoptosis of myocytes, myocyte cell turnover and necrosis[27,28]. C-Myc has recently been used as a biomarker for cardiac myopathy as well as a marker for myocardial injury[29,30]. The serum concentration of c-Myc has been correlated with the cardiac structure of the patients and it is an indication of the transition from cardiac hypertrophy to myocardial fibrosis. CK although has been use as a reliable marker for cardiac myopathy for confirmation of the diagnosis, monitor the progress of the disease and to gauge the infarct size[31]. In order to establish the veracity of acute chest pain[32,33] in the ISO treated animals, we have evaluated h-FABP as a biomarker for the injured myocardium and is high in these animals. These results confirm that the increase in the cardiac stress markers evidenced in ISO induced rats. While rats administered with CAP elicited the abrogation in these markers projecting the cardioprotective role of CAP.
ECM is the main component of myocardial tissue and since collagens are found to be the major components of the ECM, there has been an increased deposition of collagen and collagen turnover is noted to be an essential marker in the diagnosis of myocardial fibrosis. The regulation of ECM and its turnover is regulated by MMPs, and they can degrade much protein of ECM[34,35]. An increase in MMP2 and MMP9 are found to be correlated to increase in the expression of periostin and connective tissue growth factor[36] and osteopontin increased its expression in the myocardial injured heart participating in the wound healing. Another important marker that is known to be highly expressed in the myocardial fibrosis is TGF-β which in its active form binds to the TGF-β receptor in the canonical pathway, recruits and phosphorylates the downstream signaling molecules of SMAD2 or SMAD3[37] and are then released to form an intracellular complex with mediator SMAD4.
The intracellular complexes of SMAD bind to the promoter regions of genes[38] that encode alpha-Smooth Muscle Actin (alpha-SMA), PAI‐1[39], collagens and fibronectin[37] that are significantly upregulated in the ISO induced cardiac myotropic animals that are in the end stage of the disease with these pro-fibrogenic genes playing a significant role in the differentiation of cardiac fibroblasts to myofibroblasts and ECM deposition in the myocardial tissue[37]. CAP treatment has in-turn inhibited the TGF-β[15] and thereby the downstream effector genes that are pro-fibrogenic in the ISO-treated animals get reduced to normal levels and prevents cardiac fibrosis in the final group.
In the development of heart failure, LV remodeling plays an important role and also LV hypertrophy. Hence many of the transcriptional factors are activated in the cardiomyocytes and they are dependent on the calcium homeostasis[40]. Among them NFAT1, GATA4 is highly expressed[41] in the ISO treated cardiac failure animals and their transcriptional pathways are dependent on Calcium (Ca2+) homeostasis in the cardiac remodeling in a heart attack[42]. The upregulation of nucleostemin in the injured myocardium that is in sync with the cardiac remodeling is dependent on the high expression of the pro-proliferative transcription factor, c-Myc that is increased in the injured hearts of ISO treated animals[43]. The reduced contractile properties in heart failure is due to the reduction in the expression of SERCA2a that mediates the uptake of Ca2+ from the sarcoplasm into the sarcoplasmic reticulum in cardiomyocytes[44]. Hence, there was a reduction of SERCA2a in ISO-treated animals. Under the condition of heart pressure, the salt water balance and blood pressure are maintained by an increase in the expression of ANP[45] and a decreased level of expression of ATF3[46] that deteriorates into cardiac hypertrophy. The trends in the cardiac infarction markers have been seen as a reversal with the CAP treatment indicating a protective effect after MI.
miRs known to be highly expressed in cardiac fibrosis have been evaluated. miR-21 is highly expressed in all the ISO-treated animals as they are playing a critical role in the cardiac fibrosis after MI through the TGF-β/ SMAD signaling[47]. We have observed that miR-34a expression was highly expressed in the cardiac fibroblasts and it would activate the TGF-β1 fibrogenic activity and by targeting SMAD4, miR-34a is known to be directly involved in the progression of cardiac fibrosis[48]. miR-328 that is upregulated in the infarcted myocardium is known to activate TGF-β1 and increase the collagen production in fibroblasts. It is observed to be elevated its expression in the diseased heart[49] of ISO-treated animals. The expression of miR-30a in the cardiac fibrosis animals decreases with the increase in the periostin whereas the CAP treated animals have reversed its trend suggesting protective effects offered by miR-30a in the prevention of cardiac fibrosis[50]. Similarly, miR-15 is upregulated in the cardiac hypertrophy and fibrosis[51] with other molecules like miR-133a and miR-29b also contributing to the cardiac fibrosis by modulating the collagen, type I and alpha 1 expression. These miRs decreased their expression on CAP treatment with an upsurge in the expression of miR-26a, indicating a therapeutic intervention with this molecule in cardiac fibrosis[52].
We have provided extensive evidence for establishing the role played by TGF-β in heart attack and cardiac remodeling, and the protective effect of our candidate molecule CAP in inhibiting the TGF-β and prevention of adverse cardiac remodeling. These studies performed on young animals may not give an elaborate picture of the consequences of TGF-β inhibition and hence may be repeated with studies involving the senescent mice with diabetes to understand the TGF-β inhibition and the effectiveness of using CAP in MI and cardiac remodeling.
Author contributions:
Shan Wang and Jing Li contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Holdright DR, Taggart P, Sutton P, Swanton H. Myocardial reperfusion injury: Experimental evidence and clinical relevance. Eur Heart J 1996;17(11):1760-1.
[Crossref] [Google Scholar] [PubMed]
- Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol 2014;11(5):276-89.
[Crossref] [Google Scholar] [PubMed]
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest 2013;123(1):92-100.
[Crossref] [Google Scholar] [PubMed]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357(11):1121-35.
[Crossref] [Google Scholar] [PubMed]
- Lahnwong S, Palee S, Apaijai N, Sriwichaiin S, Kerdphoo S, Jaiwongkam T, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol 2020;19(1):1-3.
- Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71(4):549-74.
[Crossref] [Google Scholar] [PubMed]
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol 2000;35(3):569-82.
[Crossref] [Google Scholar] [PubMed]
- Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther 2009;123(2):255-78.
[Crossref] [Google Scholar] [PubMed]
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 2013;10(1):15-26.
[Crossref] [Google Scholar] [PubMed]
- Lajiness JD, Conway SJ. Origin, development and differentiation of cardiac fibroblasts. J Mol Cell Cardiol 2014;70:2-8.
[Crossref] [Google Scholar] [PubMed]
- Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, et al. Transforming growth factor-β function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation 2002;106(1):130-5.
[Crossref] [Google Scholar] [PubMed]
- Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin and collagen. Am J Physiol 1992;262(6):H1861-6.
[Crossref] [Google Scholar] [PubMed]
- Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 2003;113(6):685-700.
[Crossref] [Google Scholar] [PubMed]
- Heldin CH, Miyazono K, Ten Dijke P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 1997;390(6659):465-71.
[Crossref] [Google Scholar] [PubMed]
- Lee TY, Chang HH, Chen JH, Hsueh ML, Kuo JJ. Herb medicine Yin-Chen-Hao-Tang ameliorates hepatic fibrosis in bile duct ligation rats. J Ethnopharmacol 2007;109(2):318-24.
- Zhou R, Xu Q, Zheng P, Yan L, Zheng J, Dai G. Cardioprotective effect of fluvastatin on isoproterenol-induced myocardial infarction in rat. Eur J Pharmacol 2008;586(1-3):244-50.
[Crossref] [Google Scholar] [PubMed]
- Kralova E, Mokran T, Murin J, Stankovicova T. Electrocardiography in two models of isoproterenol-induced left ventricular remodeling. Physiol Res 2008;57(2):S83-9.
[Crossref] [Google Scholar] [PubMed]
- Mészáros J, Khananshvili D, Hart G. Mechanisms underlying delayed afterdepolarizations in hypertrophied left ventricular myocytes of rats. Am J Physiol Heart Circ Physiol 2001;281(2):H903-14.
[Crossref] [Google Scholar] [PubMed]
- Goldspink DF, Burniston JG, Ellison GM, Clark WA, Tan LB. Catecholamine-induced apoptosis and necrosis in cardiac and skeletal myocytes of the rat in vivo: The same or separate death pathways? Exp Physiol 2004;89(4):407-16.
[Crossref] [Google Scholar] [PubMed]
- Rona G. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Arch Pathol 1959;67(4):443-55.
- Jalil JE, Janicki JS, Pick R, Abrahams C, Weber KT. Fibrosis-induced reduction of endomyocardium in the rat after isoproterenol treatment. Circ Res 1989;65(2):258-64.
[Crossref] [Google Scholar] [PubMed]
- Grimm D, Elsner D, Schunkert H, Pfeifer M, Griese D, Bruckschlegel G, et al. Development of heart failure following isoproterenol administration in the rat: Role of the renin–angiotensin system. Cardiovasc Res 1998;37(1):91-100.
[Crossref] [Google Scholar] [PubMed]
- Kannan MM, Quine SD. Ellagic acid ameliorates isoproterenol induced oxidative stress: Evidence from electrocardiological, biochemical and histological study. Eur J Pharmacol 2011;659(1):45-52.
[Crossref] [Google Scholar] [PubMed]
- Panda S, Kar A, Ramamurthy V. Cardioprotective effect of vincristine on isoproterenol-induced myocardial necrosis in rats. Eur J Pharmacol 2014;723:451-8.
[Crossref] [Google Scholar] [PubMed]
- Cai Y, Zheng Q, Sun R, Wu J, Li X, Liu R. Recent progress in the study of Artemisiae Scopariae Herba (Yin Chen), a promising medicinal herb for liver diseases. Biomed Pharmacother 2020;130:110513.
[Crossref] [Google Scholar] [PubMed]
- Chapman AR, Fujisawa T, Lee KK, Andrews JP, Anand A, Sandeman D, et al. Novel high-sensitivity cardiac troponin I assay in patients with suspected acute coronary syndrome. Heart 2019;105(8):616-22.
[Crossref] [Google Scholar] [PubMed]
- Eggers KM, Lindahl B. Application of cardiac troponin in cardiovascular diseases other than acute coronary syndrome. Clin Chem 2017;63(1):223-35.
[Crossref] [Google Scholar] [PubMed]
- Garg P, Morris P, Fazlanie AL, Vijayan S, Dancso B, Dastidar AG, et al. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Int Emerg Med 2017;12(2):147-55.
[Crossref] [Google Scholar] [PubMed]
- Kaier TE, Twerenbold R, Puelacher C, Marjot J, Imambaccus N, Boeddinghaus J, et al. Direct comparison of cardiac myosin-binding protein C with cardiac troponins for the early diagnosis of acute myocardial infarction. Circulation 2017;136(16):1495-508.
[Crossref] [Google Scholar] [PubMed]
- Anand A, Chin C, Shah AS, Kwiecinski J, Vesey A, Cowell J, et al. Cardiac myosin-binding protein C is a novel marker of myocardial injury and fibrosis in aortic stenosis. Heart 2018;104(13):1101-8.
[Crossref] [Google Scholar] [PubMed]
- Al-Hadi HA, Fox KA. Cardiac markers in the early diagnosis and management of patients with acute coronary syndrome. Sultan Qaboos Univ Med J 2009;9(3):231-46.
[Google Scholar] [PubMed]
- Das UN. Heart-type fatty acid-binding protein (H-FABP) and coronary heart disease. Indian Heart J 2016;68(1):16-8.
[Crossref] [Google Scholar] [PubMed]
- Bertinchant JP, Polge A. Diagnostic and prognostic value of heart-type fatty acid-binding protein (H-FABP), an early biochemical marker of myocardial injury. Arch Mal Coeur Vaiss 2005;98(12):1225-31.
[Google Scholar] [PubMed]
- Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: Severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol 2011;151(1):18-33.
[Crossref] [Google Scholar] [PubMed]
- Fan D, Takawale A, Basu R, Patel V, Lee J, Kandalam V, et al. Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis and diastolic dysfunction. Cardiovasc Res 2014;103(2):268-80.
[Crossref] [Google Scholar] [PubMed]
- Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, et al. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol 1998;30(11):2411-22.
[Crossref] [Google Scholar] [PubMed]
- Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J Clin Invest 2017;127(10):3770-83.
[Crossref] [Google Scholar] [PubMed]
- Euler-Taimor G, Heger J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc Res 2006;69(1):15-25.
[Crossref] [Google Scholar] [PubMed]
- Flevaris P, Khan SS, Eren M, Schuldt AJ, Shah SJ, Lee DC, et al. Plasminogen activator inhibitor type I controls cardiomyocyte transforming growth factor-β and cardiac fibrosis. Circulation 2017;136(7):664-79.
[Crossref] [Google Scholar] [PubMed]
- Barry SP, Townsend PA. What causes a broken heart-molecular insights into heart failure. Int Rev Cell Mol Biol 2010;284:113-79.
[Crossref] [Google Scholar] [PubMed]
- Liang Q, de Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD. The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo. J Biol Chem 2001;276(32):30245-53.
[Crossref] [Google Scholar] [PubMed]
- Cortes R, Rivera M, Roselló-Lletí E, Martínez-Dolz L, Almenar L, Azorín I, et al. Differences in MEF2 and NFAT transcriptional pathways according to human heart failure aetiology. PLoS One 2012;7(2):e30915.
[Crossref] [Google Scholar] [PubMed]
- Hariharan N, Quijada P, Mohsin S, Joyo A, Samse K, Monsanto M, et al. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J Am Coll Cardiol 2015;65(2):133-47.
[Crossref] [Google Scholar] [PubMed]
- Park WJ, Oh JG. SERCA2a: A prime target for modulation of cardiac contractility during heart failure. BMB Rep 2013;46(5):237-43.
[Crossref] [Google Scholar] [PubMed]
- Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene 2015;569(1):1-6.
[Crossref] [Google Scholar] [PubMed]
- Zhou H, Shen DF, Bian ZY, Zong J, Deng W, Zhang Y, et al. Activating transcription factor 3 deficiency promotes cardiac hypertrophy, dysfunction and fibrosis induced by pressure overload. PLoS One 2011;6(10):e26744.
[Crossref] [Google Scholar] [PubMed]
- Yuan J, Chen H, Ge D, Xu Y, Xu H, Yang Y, et. Mir-21 promotes cardiac fibrosis after myocardial infarction via targeting Smad7. Cell Physiol Biochem 2017;42(6):2207-19.
[Crossref] [Google Scholar] [PubMed]
- Huang Y, Qi Y, Du JQ, Zhang DF. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets 2014;18(12):1355-65.
[Crossref] [Google Scholar] [PubMed]
- Du W, Liang H, Gao X, Li X, Zhang Y, Pan Z, et al. MicroRNA-328, a potential anti-fibrotic target in cardiac interstitial fibrosis. Cell Physiol Biochem 2016;39(3):827-36.
[Crossref] [Google Scholar] [PubMed]
- Yuan CT, Li XX, Cheng QJ, Wang YH, Wang JH, Liu CL. MiR-30a regulates the atrial fibrillation-induced myocardial fibrosis by targeting snail 1. Int J Clin Exp Pathol 2015;8(12):15527-36.
[Google Scholar] [PubMed]
- Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, et al. The microRNA-15 family inhibits the TGFβ-pathway in the heart. Cardiovasc Res 2014;104(1):61-71.
[Crossref] [Google Scholar] [PubMed]
- Angelini A, Li Z, Mericskay M, Decaux JF. Regulation of connective tissue growth factor and cardiac fibrosis by an SRF/MicroRNA-133a axis. PloS One 2015;10(10):e0139858.
[Crossref] [Google Scholar] [PubMed]