- *Corresponding Author:
- Ming Yuan
Department of Medicine Chongqing Medical and Pharmaceutical College Chongqing 401331, China
E-mail: qtx2015630@163.com
| Date of Received | 18 December 2021 |
| Date of Revision | 05 October 2022 |
| Date of Acceptance | 25 July 2023 |
| Indian J Pharm Sci 2023;85(4):1099-1109 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Capsaicin and dietary fiber are effective natural food ingredients to control the obesity and metabolic diseases. The aim of the present study was to investigate the improved anti-obesity effects by adding capsaicin to a high-fiber diet. Sprague–Dawley rats were fed with high fiber diet, and different doses of capsaicin. Plasma parameters, gut microbiota, bile acid and short-chain fatty acids were analyzed to detect the improved effects and possible mechanisms. The results showed that the addition of capsaicin further decreased the fasting blood glucose and insulin, and increased beta-muricholic acid, deoxycholic acid, chenodeoxycholic acid, and 3 beta-ursodeoxycholic acid when compared to only high fiber diet. Administration of 0.05 g/kg capsaicin showed the highest of tauro-alpha-muricholic acid sodium salt and tauro-beta-muricholic acid sodium salt and 0.1 g/kg capsaicin resulted in the highest of lithocholic acid, cholic acid and hyodeoxycholic acid. Capsaicin increased the abundance of microorganisms including Akkermansia, Allobaculum et al. and increased the short-chain fatty acids, especially acetic acid and butyric acid. Results from our study indicated that the addition of capsaicin have better effects to reduce the weight, insulin and fasting blood glucose, and the possibly mechanism can be due to the changes in bile acid composition, microbial abundance and shortchain fatty acids.
Keywords
Dietary fiber, capsaicin, bile acid, gut microbiota, short-chain fatty acids
There is an emerging health concern worldwide on overweight or obese due to their close relationship with several diseases, including cardiovascular disease, type 2 diabetes mellitus and various forms of cancer[1]. Although there are a number of antiobesity drugs available, diet-control interventions still remain to be the preferred therapy for the management of obesity. Numerous studies have confirmed that the supplementation with natural functional food may help reduce the risk of obesityrelated complications[2,3]. Dietary fiber is one of the functional ingredients which has beneficial effects on obesity-related metabolic diseases. Inulin is a soluble dietary fiber in fructan family extracted from chicory roots. It is considered to be a prebiotic that cannot be hydrolyzed by digestive enzymes in the human small intestine but can be fermented by probiotics[4]. Thus, it can modulate the composition and metabolic activity of the intestinal microbiota, which might potentially enhance the health of the host organism[5,6]. Some studies have demonstrated that early inulin intervention in High Dietary (HD) fed mouse can reduce the glucose metabolism disorders and gut dysbiosis in the offspring[7]. In addition, dietary fiber can be fermented by gut microbes into Short-Chain Fatty Acids (SCFAs), which have beneficial effects on the gut barrier and can mitigate obesity by regulating the endocrine activity[8]. Butyrate, in particular, is a key promoter of colon health and integrity, and can meet 60 %–70 % of the energy requirements necessary for colon cell proliferation and differentiation[9]. Therefore, it is becoming more and more popular to adjust the obesity and abnormal glucose and lipid metabolism by eating dietary fiber.
Capsaicin (CAP) is a key ingredient in natural chili peppers. It has recently attracted considerable attention due to its positive role on gut flora by eliminating the disease-causing enteric pathogens, and promoting the growth of beneficial bacteria[10,11]. There are several reports on the antimicrobial and anti-dysbiosis property of capsaicin by mediating the beneficial alteration of microbiota many studies also found that capsaicin can prevent the onset or development of diseases like obesity, diabetes, or inflammatory bowel diseases[7,12]. For example, Baboota et al.[13] reported that the oral administration of CAP can increase the weight loss of High Fat Diet (HFD) fed mice, and the changes in gut microbiota compositions was an important factor. Capsaicin can also increase the BA content and glucose metabolism[14].
Therefore, both CAP and dietary fiber are effective natural food ingredients to control the obesity and metabolic diseases, it is worthwhile to study whether there are enhanced effects on obesity by the combination of CAP and dietary fiber. But most of the existing studies are conducted with HFD and type 2 diabetes models; the role of capsaicin in high fiber diets is rarely studied. The present study was conducted to explore the improved anti-obesity effects by the addition of capsaicin to high fiber diet, and to explore the possible mechanism of CAP by investigating the intestinal microbiota homeostasis in Sprague–Dawley (SD) rats.
Materials and Methods
Materials:
CAP (≥95 %) was purchased from Henan bis-biotech co., Ltd. Sprague-Dawley (SD) rats were provided by Hunan SJA laboratory animal Co., Ltd. Inulin (≥90 %) was purchased from Henan Wanbang industrial co.,Ltd.
Animals and treatments:
Animal experiments were conducted with SD rats. The experimental protocols were approved by the Institutional Animal Care and Use Committee of Southwest University (IACUC-20201008-01). Rats (200±20 g) were housed in individual stainless steel cages at 25°±1°, with relatively humidity of 40 %–70 %, and artificially illuminated with fluorescent lamps in 12 h light/dark cycles. Before the experiment, the rats were provided free access to water and diet for 1 w, and then randomly divided into four groups; Negative Control (NC), HB, high fiber diet with medium dose (0.01 %) of capsaicin (HBM), and high fiber diet with low dose (0.005 %) of capsaicin (HBL). The ingredient composition of experimental group diets was shown in Table 1. The high fiber feed contained 10 % inulin supplement Bile Acidsed on the U.S. Standard AIN-93 feed formula[15].
| NC | HB | |
|---|---|---|
| Corn starch | 549.5 | 449.5 |
| Inulin | 0 | 100 |
| Soybean oil | 40 | 40 |
| a AIN-76 mineral mixture (% mixture) | 35 | 35 |
| Casein | 200 | 200 |
| b AIN-76 vitamin mixture (% mixture) | 10 | 10 |
| Sucrose | 100 | 100 |
| L-cystine | 3 | 3 |
| Cellulose | 50 | 50 |
| Choline chloride | 2.5 | 2.5 |
| Cholesterol | 10 | 10 |
Table 1: The Ingredient Compositions Of The Experimental Diets (G/Kg Diet).
Sample collection:
The animal mortality and signs of toxicity were observed once a day throughout the trial duration. Body weight and food consumption were recorded once a week. After feeding for 4 w, SD rats were euthanized to collect blood, the cecal content, and small intestine content, and the contents were put into the sterile doffer tubes and immediately frozen in liquid nitrogen for 30 s and then stored at −80° for a further use. All the operations were performed under aseptic conditions.
Biochemical analysis:
Plasma Triglyceride (TG) and Total Cholesterol (TC) concentrations were determined using commercially available kits (BioSino, Beijing, China). Fasting blood glucose, High-Density Lipoprotein (HDL-C) and Low-Density Lipoprotein (LDL-C) were determined using automatic biochemical analyzer (URIT-8021A). Plasma insulin concentration was measured using a mouse insulin Enzyme Linked Immunosorbent Assay (ELISA) kit (Jiancheng, Nanjing, China)
Gut microbiota analysis by 16S Ribosomal Ribonucleic Acid (16S rRNA) gene sequencing:
Deoxyribonucleic Acid (DNA) preparation, Polymerase Chine Reaction (PCR) amplification, and pyrosequencing were performed as described by Li et al.[16]. Briefly, total DNA was extracted from 250 mg of cecal contents using E.Z.N.A.® Stool DNA kit (OMEGA, Bio-Tek, United States of America (USA)). A primer set (515F/926R) was used to amplify the V4 region of the 16S rRNA gene to analyze the gut microbiota. Each sample was amplified in triplicate with a reaction volume of 20 μl containing 1× Ex Taq™ PCR buffer, 10 pM each primer, 1.25 U Takara Ex Taq (TaKaRa Biotechnology Co., Ltd., Dalian, China), and 5 ng genomic DNA was amplified using the following program; 5 min at 95°, followed by 30 cycles of 94° for 30 s, then 56° for 30 s, and 72° for 30 s, and finally, 10 min at 72°. PCR products were purified using the QIAEX II Gel Extraction Kit (QIAGEN). Library preparation and pyrosequencing were performed at the Chinese National Human Genome Center in Shanghai with 454 GS FLX sequencing platform (Roche). The raw sequences were available on the National Center for Biotechnology Information (NCBI)/European Bioinformatics Institute (EBI) /DNA Data Bank of Japan (DDBJ) Sequence Read Archive (Accession No. DRA002627 and DRA0012641).
The average read length was 250 bp. By randomly sampling 30 681 sequences from each sample, the resulting sequences were rarefied separately, so that the sampling workload of the entire sample was equal. To predict the functional profiles of microbial communities, Operational Taxonomic Unit (OTU) analysis was conducted by clustering sequences at the 97 % similarity level (USEARCH, version 10.0), and 0.005 % of all sequences was used as the threshold to filter OTUs. For beta diversity, species diversity matrix was calculated by a variety of algorithms, such as binary Jaccard, Bray–Curtis, and unweighted UniFrac (only for bacteria). The Principal Coordinate Analysis (PCoA) results were plotted using R software.
Bile Acid analysis:
Bile Acid analysis by Ultra-High Performance Liquid Chromatography-Time of Flight/Mass Spectrometry (UHPLC-TOF/MS). 10 mg of small intestines contents was weighed and dissolved in 1000 μl methanol (-20), followed by vortexing for 60 s, and sonicating for 30min. The supernatants were collected after centrifugation at 12 000 rpm at 4° for 10 min, and then diluted by fifty-fold.
For Chromatographic analysis, ACQUITY UPLC® BEH C18 (2.1×100 mm, 1.7 μm, Waters Corp.) was used as the column. The injection volume was 5 μl, the mobile phase solvents were 0.01 % formic acid (A) and acetonitrile (B). Flow rate was 0.25 ml/min.
For Quadrupole-TOF (Q-TOF) mass spectrometry conditions, Electrospray Ionization (ESI) positive and negative ion modes were used for detection. The ion source temperature was 500° and the voltage was −4500 V. Nebulizing gas and auxiliary gas (nitrogen) were set at 50 psi, curtain gas was at 30 psi and collision gas at 6 psi. Lithocholic acid (LCA) and other 39 substance were used as the bile acid standards (Sigma, USA). The HPLC chromatogram of bile acid standards were presented in fig. 1 and the standard curves were provided in Table 2.
| Bile acid | Full name | Retention time (min) | linear equation | Correlation coefficien (R) |
|---|---|---|---|---|
| LCA | Lithocholic acid | 32.49 | Y=-1219.6+2452.7x | 0.996509278 |
| β-UDCA | 3β-Ursodeoxycholic acid | 20.61 | Y=-826.9+8195.7x | 0.997482682 |
| DCA | Deoxycholic acid | 27.88 | Y=3170.5+6339.8x | 0.998563291 |
| CDCA | Chenodeoxycholic acid | 27.3 | Y=-1829.4+3711.6x | 0.995607743 |
| HDCA | Hyodeoxycholic acid | 22.6 | Y=299.04+3227x | 0.997026219 |
| α-MCA | α-Muricholic acid | 15.84 | Y=-108.95+4376.1x | 0.995819998 |
| UCA | Ursocholic acid | 13.32 | Y=-447.66+1843.2x | 0.997831515 |
| β-MCA | β-Muricholic acid | 17.28 | Y=132.52+1879.9x | 0.996643952 |
| CA | Cholic acid | 21.65 | Y=1176.8+2948.6x | 0.999327695 |
| TLCA | Taurolithocholic acid sodium salt | 24.44 | Y=-496.67+12631x | 0.998144375 |
| TDCA | Taurodeoxycholic acid sodium salt | 18.92 | Y=285.19+9813x | 0.99745871 |
| TCDCA | Taurochenodeoxycholic acid | 17.56 | Y=-296.28+8556.7x | 0.998502153 |
| TCA | Taurocholic acid sodium salt | 12.96 | Y=-68.605+4988.1x | 0.999233726 |
| T-α-MCA | Tauro-α-muricholic acid sodium salt | 7.391 | Y=305.65+8644.9x | 0.995792518 |
| T-β-MCA | Tauro-α-muricholic acid sodium salt | 7.662 | Y=27.883+7597x | 0.99666197 |
Table 2: Calibration Curves Of Different (Bile Acids) In Standard Solution.
SCFAs quantification by GC:
The SCFAs in the cecal contents were determined according to the method described by Zhang et al. Cecal contents (0.5 mg); 0.5 mg cecal contents were weighed and dissolved in 2 ml mixtures containing crotonic acid (5 mmol/l) and sodium hydroxide (10 mmol/l). The supernatants were collected after centrifugation at 10 000 rpm at 4° for 15 min[17].
Quantification analysis of fecal SCFAs was undertaken as previously described using an Agilent 7890 A. The chromatographic conditions were set as; injection volume 1 μl, inlet temperature 220°, column flow 0.95 ml/min, column temperature 90°, equilibrium time 0.5 min, temperature increased to 150° by 5°/min, retention time 9 min, detector temperature 230°, hydrogen flow 40 ml/min, air flow 400 ml/min, and tail blowing flow 40 ml/min.
The retention time of each chromatographic peak was used for qualitative analysis, and the peak area was used for quantification. GC chromatogram of SCFAs standard was shown in fig. 2 and the standard curves were provided in Table 3.
| SCFAs | Linear equation | Correlation coefficient (R2) |
|---|---|---|
| Acetic acid | y=62.583x-795.68 | 0.9914 |
| Propionic acid | y=121.56x-1144.4 | 0.9963 |
| i-butyric acid | y=171.47x-942.01 | 0.9988 |
| n-butyric acid | y=171.6x-1250.1 | 0.998 |
| i-valeric acid | y=193.82x-893.63 | 0.9996 |
| n-valeric acid | y=204.8x-843.75 | 0.9997 |
Table 3: Calibration Curves Of Different Scfas In Standard Solution.
Metabolite analysis by LC-MS/MS:
A 100 mg sample of cecal contents was weighed and mixed thoroughly into 800 μl of acetonitrile/methanol (1:1, w/v). The mixture was incubated for 60 min at -20°, then centrifuged at 16 000 rpm and 4° for 20 min. The upper phase was collected and vacuum dried, then dissolved in 100 μl acetonitrile:water (1:1, w/v) for analysis.
Pretreated cecal content samples were analyzed using an Agilent 1290 Infinity LC system (Agilent, USA) coupled with a Pegasus HT TOF mass spectrometer (LECO, USA). An ACQUITY UPLC BEH Amide 1.7 μm, 2.1 mm×100 mm column was used to separate the derivatives. The injection volume was set at 5 μl, and column temperature was set to 25°. The flow rate was 0.3 ml/min. Chromatographic mobile phase A was water+25mM ammonium acetate+25 mM ammonia water, while phase B was acetonitrile.
Mass spectra were acquired under the following settings; ion source gas 1 (Gas1) was 60, ion source gas 2 (Gas2) was 60, curtain gas (CUR) was 30, source temperature was 600°, Ion Sapary Voltage Floating (ISVF): ±5500 V, TOF MS scan m/z range was 60-1200 Da, product ion scan m/z range was 25-1200 Da, TOF/MS scan accumulation time was 0.15 s/spectra, product ion scan accumulation time was 0.03 s/spectra, secondary mass spectrometry has information dependent acquisition, Declustering Potential (DP) was ±60 V, collision energy was 30 Ev. Isotopes within 4 Da were excluded, and six candidate ions were monitored per cycle.
LC-TOF/MS raw data were first analyzed by MSDIAL software with the Xtensible Computational Mass Spectrometry (XCMS) dataBile Acidse, including raw peak extraction, data Bile Acidseline filtering and calibration, peak alignment, deconvolution analysis, peak identification and peak area integration. Subsequently, normalized data were input to the SIMCA 14.1 software package (Umetrics AB, Umea, Sweden). After mean centralizing and unit variance scaling, multivariate analyses, such as Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), were performed to visualize metabolic differences between the four groups. Finally, differentially expressed metabolites were identified and characterized by searching the online Kyoto Encyclopedia of Genes and Genomes (KEGG, http://www.genome.jp/kegg/).
Statistical analysis:
All data were expressed as mean and standard deviation (n=8). Data were subjected to one-way analysis of variance using Origin 8.5 and Statistical Package for the Social Sciences (SPSS) version 20.0. The differences among groups were examined by Duncan’s multiple-range test. p<0.05 was considered to be statistically significant.
Results and Discussion
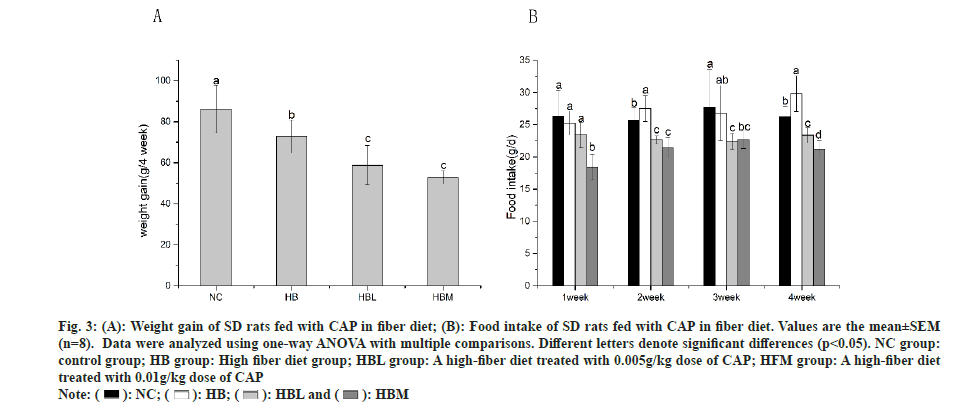
After feeding for 4 w, the weight gain of SD rats was shown in fig. 3A. Compared to the NC group, the weight gain in HB, HBL and HBM groups were significant decreased, especially in HBM and HBL groups. It is well known that dietary fiber has positive effects on body weight loss, which is accord with our research. The data of food intake in fig. 3B showed that the rats in HBL and HBM groups recorded much lower food intake than that in NC and HB groups after 2 w of feeding.
Fig. 3: (A): Weight gain of SD rats fed with CAP in fiber diet; (B): Food intake of SD rats fed with CAP in fiber diet. Values are the mean±SEM (n=8). Data were analyzed using one-way ANOVA with multiple comparisons. Different letters denote significant differences (p<0.05). NC group: control group; HB group: High fiber diet group; HBL group: A high-fiber diet treated with 0.005g/kg dose of CAP; HFM group: A high-fiber diet treated with 0.01g/kg dose of CAP.
The effects of CAP on blood biochemical parameters of rats fed with different diets were shown in Table 3. There were no significant differences among the treatment groups in plasma TG, TC, Low-Density Lipoprotein Cholesterol (LDL-C), and High Density Lipoprotein Cholesterol (HDL-C). However, for the insulin content, it was significantly decreased in rats fed with CAP (HBL and HBM groups), when compared with HB. HBM group showed the lowest fasting blood, but only a slight decrease in fasting blood was detected in HBL group with comparison to HB group.
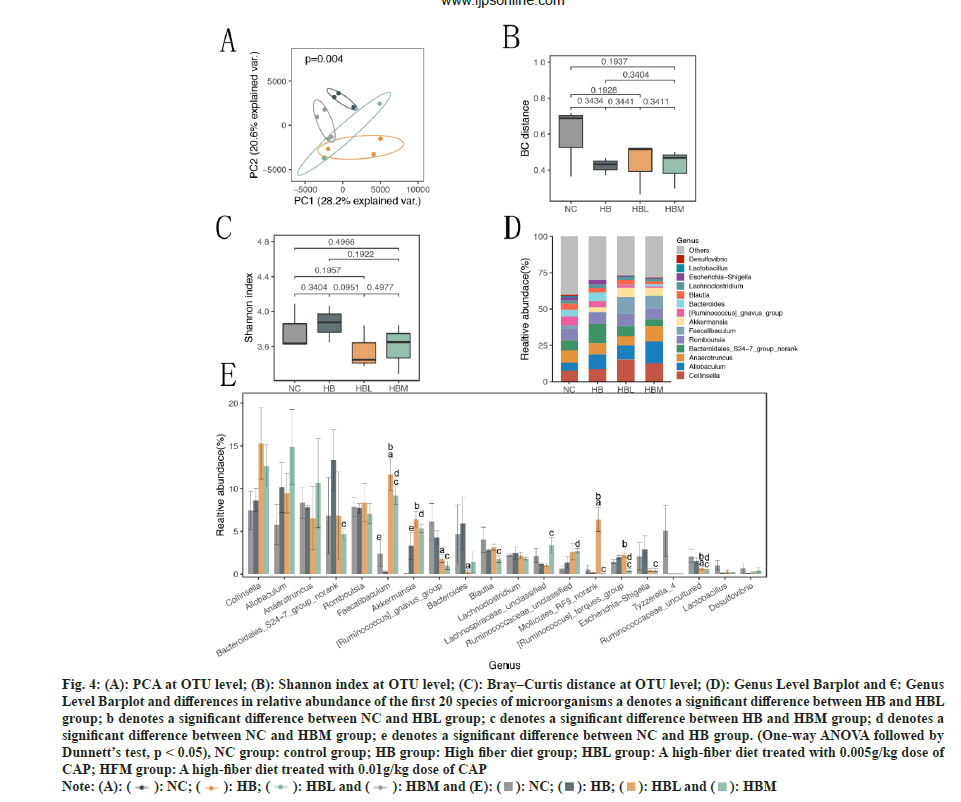
In order to understand the effects of CAP on the intestinal microflora profile of SD rats fed with dietary fiber, fecal samples were harvested at the 4th w of the experiment, the V3 and V4 16S rRNA variable gene regions in the fecal samples were sequenced using the Illumina MiSeq platform. We characterized 633 OTUs from 36549 high-quality sequences. The PCA was performed Bile Acidsed on OUTs and presented in fig. 4A, which showed the similarities and differences among different samples. The NC group was notably separated from HB group, a clear separation was observed among the HB and HBL, HBM, suggesting that the gut microbiota was substantially changed by CAP treatment. Fig. 4B exhibited the Bray–Curtis (BC) distances on the beta (β)-diversity of gut microbiota in rats with different diets. It can be seen that there were only slight differences in β-diversity of gut microbiota among the HB, HBL and HBM groups. But Shannon index in fig. 4C showed significant differences between HBL and HB groups. Results of both alpha and PCA analyses indicated that the intestinal flora of SD rats treated by CAP were significantly changed. The histogram of community structure at the genus level of intestinal flora was shown in fig. 4D, and the top 20 most dominant genera were shown in fig. 4E. Compared with HB group, CAP-fed groups decreased the Ruminococcaceae uncultured and Ruminococcus gnavus group but increased the Faecalibaculum. Similar concentration in Akkermansia was found in HB, HBL and HBM groups, but it was higher than NC group. And a significant lower concentration in bacteroidales_S24−7 was observed in HBM group than HB group.
Fig. 4: (A): PCA at OTU level; (B): Shannon index at OTU level; (C): Bray–Curtis distance at OTU level; (D): Genus Level Barplot and €: Genus Level Barplot and differences in relative abundance of the first 20 species of microorganisms a denotes a significant difference between HB and HBL group; b denotes a significant difference between NC and HBL group; c denotes a significant difference between HB and HBM group; d denotes a significant difference between NC and HBM group; e denotes a significant difference between NC and HB group. (One-way ANOVA followed by Dunnett’s test, p < 0.05), NC group: control group; HB group: High fiber diet group; HBL group: A high-fiber diet treated with 0.005g/kg dose of CAP; HFM group: A high-fiber diet treated with 0.01g/kg dose of CAP.
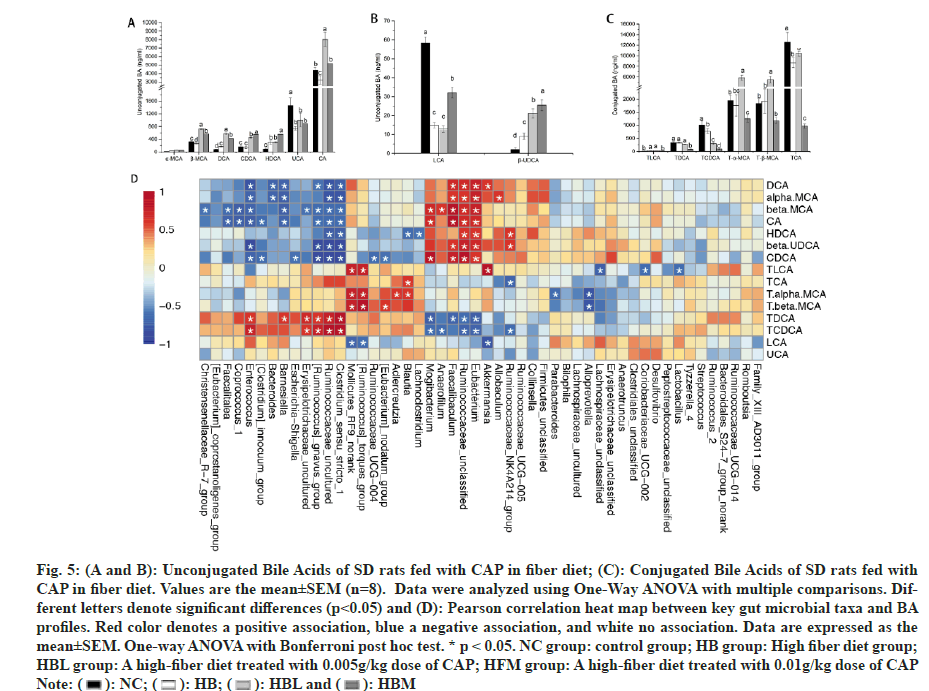
Bile acids are synthesized in the liver from cholesterol and released into the intestine to help the digestion of dietary lipids. To determine whether the weight loss in the capsaicin-treated groups was associated with the changes in bile acids, the composition and content of 15 BA species in small intestine were assayed, and the results were shown in fig. 5. Compared with HB and NC, the unconjugated bile acids including Beta-Muricholic acid (β-MCA), DCA, CDCA, and β-UDCA were significantly increased in CAP fed rats (HBL and HBM groups). And higher LCA, CA and HDCA were observed in HBM than that in HB (fig. 5A and fig. 5B). Besides, compared with NC, the Ursocholic Acid (UCA) and LCA were significant decreased in HBL, HBM, and HB (fig. 5A and fig. 5B). CAP-fed rats (HBL and HBM) also showed lower conjugated Bile Acids and Taurochenodeoxycholic Acid (TCDCA) than only fiber-fed rats (HB) (fig. 5C). Higher Tα-MCA and Tβ-MCA were found in HBL (fig. 5C). And HBM showed lower Taurocholic acid Sodium Salt (TCA) than HB (fig. 5C).
Fig. 5: (A and B): Unconjugated Bile Acids of SD rats fed with CAP in fiber diet; (C): Conjugated Bile Acids of SD rats fed with CAP in fiber diet. Values are the mean±SEM (n=8). Data were analyzed using One-Way ANOVA with multiple comparisons. Different letters denote significant differences (p<0.05) and (D): Pearson correlation heat map between key gut microbial taxa and BA profiles. Red color denotes a positive association, blue a negative association, and white no association. Data are expressed as the mean±SEM. One-way ANOVA with Bonferroni post hoc test. * p < 0.05. NC group: control group; HB group: High fiber diet group; HBL group: A high-fiber diet treated with 0.005g/kg dose of CAP; HFM group: A high-fiber diet treated with 0.01g/kg dose of CAP.
A Pearson correlation analysis was conducted to link the gut microbiota genera and bile acid profiles, and the heatmap of Pearson correlation analysis was presented in (fig. 5D). The results showed that Allobaculum was positively correlated with Alpha-Muricholic acid (α-MCA). Akkermansia was negatively correlated with LCA and positively associated with Taurolithocholic acid Sodium Salt (TLCA) and DCA. Lactobacillus was negatively correlated with TLCA. Bacteroides was negatively correlated DCA and α-MCA.
SCFAs are the final products of dietary fibers and resistant starch through microbial fermentation, and they play an important role in the prevention and treatment of obesity[18]. Acetic, propionic and butyric acids can serve as an energy source to human intestine epithelium[19]. The fecal SCFA excretion in SD rats fed with different diets were shown in Table 4. The highest acetic acid, butyric acid, i-butyric acid and total SCFAs were found in HBM. No significant differences were found in propionic acid, i-valeric acid, and n-valeric acid in CAP groups.
| NC | HB | HBL | HBM | |
|---|---|---|---|---|
| Plasma TC (mmol/l) | 3.07±0.353a | 3.19±0.198a | 3.00±0.014a | 3.23±0.269a |
| Plasma TG (mmol/l) | 0.66±0.007a | 0.56±0.0283a | 0.68±0.120a | 0.58±0.148a |
| LDL-Cholesterol (mmol/l) | 1.29±0.297a | 1.39±0.049a | 1.19±0.184a | 1.34±0.035a |
| HDL-Cholesterol (mmol/l) | 1.28±0.113a | 1.66±0.459a | 1.31±0.396a | 1.40±0.120a |
| Insulin (mU/l) | 50.26±5.742a | 49.87±0.007a | 35.47±1.273b | 36.82±4.200b |
| Fasting blood glouse (mmol/l) | 4.82±0.064a | 4.41±0.318ab | 3.97±0.014bc | 3.71±0.120c |
Table 4: Effects Of Cap On Plasma Parameters In Sd Rats (N=8).
The aim of this work was to study the impact of the CAP on gut microbes and metabolites in SD rats fed with dietary fiber. The results showed that capsaicin can significantly reduce the food intake and body weight gain of the rats fed with fiber diet. Bile acids, intestinal flora and metabolites were determined in order to investigate whether the weight loss was caused by reduced feed intake or by some other changes.
Many studies have proven that dietary fiber can regulate the metabolism of human body20. In our study, the addition of capsaicin to dietary fiber can further reduce the insulin levels, especially with the addition of 0.1 mg/kg capsaicin. At the same time, the combination of capsaicin and fiber was more effective on reducing fasting blood glucose. These results indicated that the addition of capsaicin in dietary fiber has a more regulatory effect on glucose metabolism.
Bile acids, derived from cholesterol in the liver, are an important compound in the gut lumen in response to ingestion of dietary fat. In the lower gastrointestinal tract, primary bile acids are converted to secondary bile acids through microbial metabolism, and secondary bile acids are positively associated with chronic disease, e.g., liver disease and colorectal cancer[21,22]. Both primary and secondary bile acids are strong signaling molecules in fat metabolism. In the liver, intestine, and other tissues, bile acids are also strong signaling molecules activating Farnesoid-X Receptor (FXR) and G-Protein-Coupled Bile Acid Receptor (GPBAR1/TGR5), and bile acids can help to the regulate several systemic endocrine functions, including lipid and glucose metabolism, immune responses, and energy metabolism[23,24].
The most potent ligands for FXR are CDCA, DCA, LCA, CA, Glycocholic Acid (GCA) and β-MCA[25], among which CDCA has the greatest potency, followed by DCA, LCA, and finally CA[23]. Conversely, TGR5 is mainly activated by the secondary bile acids, LCA and TLCA[23], and it is predominantly expressed in the enteric nervous system and influences distal gut motility[26,27]. Furthermore, bile acids are harmful when at higher concentrations, particularly those with higher hydrophobicity[28]. Therefore, changes in the composition of the bile acid pool may impact the health-related outcomes. In our research, the addition of capsaicin significantly increased CDCA, DCA, CA, and β-MCA contents (fig. 5A), indicating that CAP may activate the FXR signaling pathway via BA metabolism. Moreover, the activation of FXR is involved in lipid, glucose, and drug metabolism[29], inflammation[30], as well as epithelial cell proliferation[31]. This may be one reason for the changes of insulin and fasting blood glucose caused by capsaicin. LCA was increased significantly in medium dose of CAP, yet TLCA was decreased. Further researches were required to study the influences of CAP on distal gut motility.
The changes of gut bile acid levels are usually associated with bacterial growth and inflammation[32]. In the proximal small intestine, bile acids can protect gut mucosa against pathogens due to their amphipathic property and solubilizing ability. In the distal small intestine, this protection can be mediated by increased synthesis and secretion of antimicrobial factors from intestinal epithelium by gene induction through interaction with FXR[33]. Therefore, the composition of bile acids directly affects the changes of intestinal microorganisms.
Akkermansia was negatively correlated with FXR agonists LCA and positively associated with TLCA and DCA. Tauro-Beta-Muricholic Acid (Tβ-MCA) is a powerful (gut microbiota-sensitive) FXR antagonist in mice[34]. Some studies have shown that Lactobacillus is negatively correlated with FXR antagonists (MCA)[35] and positively associated with FXR agonists, such as TCA. Our results showed a similar trend, but only slight differences were observed (fig. 5D), probably due to the small sample size. In addition, Lactobacillus was negatively correlated with TLCA. And Bacteroides was negatively correlated DCA and α-MCA.
The gut microbiota is a collection of archaea, bacteria, and eukarya that have evolved over thousands of years to form a symbiotic relationship with human hosts[36]. These microbial populations influence metabolic, immune and defense systems in the intestine and consequently, human health[19,37]. In our research, at the species level, CAP reduced the OTU assigned to the Bacteroidales S24-7, Ruminococcaceae uncultured, and Ruminococcus gnavus group, but enhanced Faecalibaculum and Akkermansia. Akkermansia is a mucin-degrader bacterium and has many beneficial effects such as anti-inflammatory properties[38], antiobesity[ 39,40], mitigate inflammation, regulation of glucose metabolism[41], and has the ability to produce SCFAs[42]. The increased Akkermansia suggested that CAP can reduce intestinal inflammation, improve glucose metabolism, and enhance weight loss in rats. Bacteroidales_S24-7 is a kind of carbohydratefermenting bacteria that produces SCFAs[43]. It can degrade complex polysaccharides into acetate, propionate, and succinate[44]. However, we did not find a decrease in acetic acid and propionic acid in our results, one of the possible reasons may be the action of other microorganisms that produced SCFAs. Compared with HB, acetic acid , butyric acid and total SCFAs was significantly increased in HBM (Table 5), which may be caused by the increase in some microbes, such as Akkermansia, Allobaculum et al. Allobaculum is a beneficial intestinal bacterium that produces lactic, butyric acids and small amounts of ethanol from glucose[45,46], resulting in the decrease of glucose digestion. Medium dose of CAP increased the Allobaculum, leading to the differences in SCFAs content.
| NC | HB | HBL | HBM | |
|---|---|---|---|---|
| Acetic acid (mmol/l) | 13.90±0.11c | 15.06±0.733b | 15.98±0.554ab | 16.03±0.370a |
| Propionic acid (mmol/l) | 10.55±0.09a | 10.62±0.18a | 10.70±0.30a | 10.95±0.17a |
| butyric acid (mmol/l) | 5.86±0.242b | 5.92±0.033b | 5.94±0.032b | 6.37±0.074a |
| i-butyric acid (mmol/l) | 7.88±0.135b | 8.04±0.115b | 7.99±0.101b | 8.42±0.143a |
| i-valeric acid (mmol/l) | 4.96±0.022a | 5.10±0.159a | 5.00±0.048a | 5.18±0.195a |
| n-valeric acid (mmol/l) | 4.46±0.027a | 4.53±0.151a | 4.45±0.004a | 4.59±0.174a |
| Total SCFAs (mmol/l) | 44.48±5.338b | 49.27±1.491ab | 50.06±9.77ab | 51.54±1.255a |
Note: Different letters in the same row indicate statistical difference (p<0.05)
Table 5: Fecal Scfa Excretion In Sd Rats Fed With Different Diets (N=8).
It is well known that SCFAs suppress inflammation, regulate the gut bacterial ecology[47], and glucose and lipid metabolism. Inulin, the main component of HD fiber feed in our study, was found to be able to generate the short chain free fatty acids[48], and with the addition of capsaicin, the concentration of SCFAs significantly increased, especially in the 0.1 g/kg group. Therefore, the loss of body weight may be the result of the combined action of reduced feed intake and changes in intestinal flora.
Dietary fiber and CAP are promising ingredients for preventing obesity and related metabolic disorders[8,14]. Our research used these two elements together and found that the addition of capsaicin in dietary fiber enhanced the regulation of glucose metabolism and body weight. The possible mechanism can be related to the regulation on the composition of bile acids and gut microbiome. The changes in the abundance of some microbes, such as Akkermansia, Allobaculum, increased the content of short-chain fatty acids, and then modulated the weight gain and glucose metabolism; however, elucidation of the detailed mechanism requires further experiments.
Acknowledgement:
This research was supported by National Natural Science Foundation of Chongqing (cstc2019jcymsxmx0439) and Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1726396).
Author’s contributions:
Ting Gong and Haizhu Wang contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Andersen DK. Diabetes and cancer: Placing the association in perspective. Curr Opin Endocrinol Diabetes Obes 2013;20(2):81-6.
[Crossref] [Google Scholar] [PubMed]
- Du H, Feskens E. Dietary determinants of obesity. Acta Cardiol 2010;65(4):377-86.
- Silva TA, Quigley SP, Kidd LJ, Anderson ST, McLennan SR, Poppi DP. Effect of a high crude protein content diet during energy restriction and re-alimentation on animal performance, skeletal growth and metabolism of bone tissue in two genotypes of cattle. Plos One 2021;16(2):e0247718.
- Alles MS, Hautvast JG, Nagengast FM, Hartemink R, van Laere KM, Jansen JB. Fate of fructo-oligosaccharides in the human intestine. Br J Nutr 1996;76(2):211-21.
[Crossref] [Google Scholar] [PubMed]
- Roberfroid MB, van Loo JA, Gibson GR. The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 1998;128(1):11-9.
[Crossref] [Google Scholar] [PubMed]
- Flickinger EA, Loo JV, Fahey GC. Nutritional responses to the presence of inulin and oligofructose in the diets of domesticated animals: A review. Crit Rev Food Sci Nutr 2003;43(1):19-60.
[Crossref] [Google Scholar] [PubMed]
- Wang JX, Zhang XJ, Li Q, Wang K, Wang Y, Jiao JQ, et al. microRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circulation Res 2015;117(4):352-63.
- Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Reply to comment on: Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients 2020;12(8):2326.
[Crossref] [Google Scholar] [PubMed]
- Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 2008;100(2):297-305.
[Crossref] [Google Scholar] [PubMed]
- Rondanelli M, Opizzi A, Perna S, Faliva M, Solerte SB, Fioravanti M, et al. Acute effect on satiety, resting energy expenditure, respiratory quotient, glucagon-like peptide-1, free fatty acids, and glycerol following consumption of a combination of bioactive food ingredients in overweight subjects. J Am Coll Nutr 2013;32(1):41-9.
[Crossref] [Google Scholar] [PubMed]
- Zsiboras C, Matics R, Hegyi P, Balasko M, Petervari E, Szabo I, et al. Capsaicin and capsiate could be appropriate agents for treatment of obesity: A meta-analysis of human studies. Crit Rev Food Sci Nutr 2018;58(9):1419-27.
[Crossref] [Google Scholar] [PubMed]
- Wang J, Li P, Liu S, Zhang B, Hu Y, Ma H, et al. Green tea leaf powder prevents dyslipidemia in high-fat diet-fed mice by modulating gut microbiota. Food Nutr Res 2020;64.
[Crossref] [Google Scholar] [PubMed]
- Baboota RK, Murtaza N, Jagtap S, Singh DP, Karmase A, Kaur J, et al. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J Nutr Biochem 2014;25(9):893-902.
[Crossref] [Google Scholar] [PubMed]
- Meléndez?Martínez AJ. An overview of carotenoids, apocarotenoids, and vitamin A in agro?food, nutrition, health, and disease. Mol Nutr Food Res 2019;63(15):1801045.
[Crossref] [Google Scholar] [PubMed]
- Afrose S, Hossain MS, Maki T, Tsujii H. Karaya root saponin exerts a hypocholesterolemic response in rats fed a high-cholesterol diet. Nutr Res 2009;29(5):350-4.
- Li T, Long M, Gatesoupe FJ, Zhang Q, Li A, Gong X. Comparative analysis of the intestinal bacterial communities in different species of carp by pyrosequencing. Microb Ecol 2015;69(1):25-36.
[Crossref] [Google Scholar] [PubMed]
- Kalhan SC. Microbial fermentation of starch: Its impact on the range of acceptable carbohydrate intake. J Pediatr Gastroenterol Nutr 2018;66(4):S42-5.
[Crossref] [Google Scholar] [PubMed]
- Wang B, Yao M, Lv L, Ling Z, Li L. The human microbiota in health and disease. Engineering 2017;3(1):71-82.
- Song X, Zhong L, Lyu N, Liu F, Li B, Hao Y, et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genomics Proteomics Bioinformatics 2019;17(1):64-75.
[Crossref] [Google Scholar] [PubMed]
- Chiang JY. Targeting bile acids and lipotoxicity for NASH treatment. Hepatol Commun 2017;1(10):1002-4.
- Navarro SL, Levy L, Curtis KR, Elkon I, Kahsai OJ, Ammar HS, et al. Effect of a flaxseed lignan intervention on circulating bile acids in a placebo-controlled randomized, crossover trial. Nutrients 2020;12(6):1837.
[Crossref] [Google Scholar] [PubMed]
- Kim H, Fang S. Crosstalk between FXR and TGR5 controls glucagon-like peptide 1 secretion to maintain glycemic homeostasis. Lab Anim Res 2018;34(4):140-6.
[Crossref] [Google Scholar] [PubMed]
- Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 2015;5(2):135-44.
- Liu L, Liu Z, Li H, Cao Z, Li W, Song Z, et al. Naturally occurring TPE-CA maintains gut microbiota and bile acids homeostasis via FXR signaling modulation of the liver–Gut axis. Front Pharmacol 2020;11:12.
[Crossref] [Google Scholar] [PubMed]
- Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, et al. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology 2013;144(1):145-54.
[Crossref] [Google Scholar] [PubMed]
- Poole DP, Godfrey C, Cattaruzza F, Cottrell GS, Kirkland JG, Pelayo JC, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil 2010;22(7):814-e228.
[Crossref] [Google Scholar] [PubMed]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol 2009;15(14):1677.
[Crossref] [Google Scholar] [PubMed]
- Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol 2013;368(1-2):17-29.
[Crossref] [Google Scholar] [PubMed]
- Jena PK, Sheng L, Nguyen M, Di Lucente J, Hu Y, Li Y, et al. Dysregulated bile acid receptor-mediated signaling and IL-17A induction are implicated in diet-associated hepatic health and cognitive function. Biomarker Res 2020;8(1):59.
[Crossref] [Google Scholar] [PubMed]
- Di Ciaula A, Garruti G, Baccetto RL, Molina-Molina E, Bonfrate L, Portincasa P, et al. Bile acid physiology. Ann Hepatol 2018;16(1):S4-14.
[Crossref] [Google Scholar] [PubMed]
- Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 2014;30(3):332.
[Crossref] [Google Scholar] [PubMed]
- Hofmann AF, Eckmann L. How bile acids confer gut mucosal protection against bacteria. Proceed Natl Acad Sci 2006;103(12):4333-4.
[Crossref] [Google Scholar] [PubMed]
- Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013;17(2):225-35.
[Crossref] [Google Scholar] [PubMed]
- DiMarzio M, Rusconi B, Yennawar NH, Eppinger M, Patterson AD, Dudley EG. Identification of a mouse Lactobacillus johnsonii strain with deconjugase activity against the FXR antagonist T-β-MCA. PLoS One 2017;12(9):e0183564.
[Crossref] [Google Scholar] [PubMed]
- Moeller AH, Hahn BH, Pusey AE, Lonsdorf EV, Muller MN, Georgiev AV, et al. Cospeciation of gut microbiota with hominids. Am J Phys Anthropol 2017;162:289-90.
- Kundu P, Blacher E, Elinav E, Pettersson S. Our gut microbiome: The evolving inner self. Cell 2017;171(7):1481-93.
[Crossref] [Google Scholar] [PubMed]
- Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016;7(1):12015.
[Crossref] [Google Scholar] [PubMed]
- Van Dorsten FA, Peters S, Gross G, Gomez-Roldan V, Klinkenberg M, De Vos RC, et al. Gut microbial metabolism of polyphenols from black tea and red wine/grape juice is source-specific and colon-region dependent. J Agricul Food Chem 2012;60(45):11331-42.
[Crossref] [Google Scholar] [PubMed]
- Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 2015;64(6):872-83.
[Crossref] [Google Scholar] [PubMed]
- de La Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care 2017;40(1):54-62.
[Crossref] [Google Scholar] [PubMed]
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004;54(5):1469-76.
[Crossref] [Google Scholar] [PubMed]
- Monk JM, Lepp D, Wu W, Pauls KP, Robinson LE, Power KA. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J Nutr Biochem 2017;49:89-100.
[Crossref] [Google Scholar] [PubMed]
- Xu C, Liu J, Gao J, Wu X, Cui C, Wei H, et al. Combined soluble fiber-mediated intestinal microbiota improve insulin sensitivity of obese mice. Nutrients 2020;12(2):351.
[Crossref] [Google Scholar] [PubMed]
- Greetham HL, Gibson GR, Giffard C, Hippe H, Merkhoffer B, Steiner U, et al. Allobaculum stercoricanis gen. nov., sp. nov., isolated from canine feces. Anaerobe 2004;10(5):301-7.
- Lagkouvardos I, Lesker TR, Hitch TC, Gálvez EJ, Smit N, Neuhaus K, et al. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 2019;7(1):1-5.
[Crossref] [Google Scholar] [PubMed]
- Elena H, Young W, Rosendale D, Reichert-Grimm V, Riedel CU, Conrad R, et al. RNA-based stable isotope probing suggests Allobaculum spp. as particularly active glucose assimilators in a complex murine microbiota cultured in vitro. Biomed Res Int 2017;2017:1829685.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Yu K, Chen H, Su Y, Zhu W. Caecal infusion of the short?chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb Biotechnol 2018;11(5):859-68.
[Crossref] [Google Scholar] [PubMed]
- Reddy YS, Srivalliputturu SB, Bharatraj DK. The effect of lead (Pb) exposure and iron (Fe) deficiency on intestinal lactobacilli, E. coli and yeast: A study in experimental rats. J Occup Health 2018;60(6):475-84.
[Crossref] [Google Scholar] [PubMed]