- Corresponding Author:
- sujatha jayaraman
AMDM, Unilever R and D, Bangalore, No. 64, Whitefield, Bangalore-560 066, India
E-mail: sujatha.jayaraman@unilever.com
| Date of Submission | 05 October 2011 |
| Date of Revision | 30 August 2012 |
| Date of Acceptance | 31 August 2012 |
| Indian J Pharm Sci, 2012, 74 (4): 339-347 |
Abstract
The bark of Terminalia arjuna is known for its heart-health benefits in ayurvedic literature. This has been further supported by in vivo studies on animal and human volunteers. But there is no detailed study on identification of the active ingredients such as polyphenols. Polyphenols possesses antioxidant properties and are well-known health actives, it is important to characterise polyphenols in Terminalia arjuna. Aqueous extract of Terminalia arjuna bark was analysed for its composition and molecular weight distribution by dialysis. Compositional analysis revealed that it has 44% polyphenols and dialysis study showed that 70% of the polyphenols have molecular weight greater than 3.5 kDa. High performance liquid chromatography and liquid chromatography-mass spectrometry analysis of Terminalia arjuna, confirmed that it contains flavon-3-ols such as (+)-catechin, (+)-gallocatechin and (−)-epigallocatechin. Phenolic acids such as gallic acid, ellagic acid and its derivatives were also found in Terminalia arjuna extract. Ellagic acid derivatives were isolated and their spectral studies indicated that isolated compounds were 3-O-methyl-ellagic acid 4- O-β-D-xylopyranoside, ellagic acid and 3-O-methyl ellagic acid 3-O-rhamnoside. Hydrolysis and thiolysis studies of high molecular weight polyphenols indicated that they are proanthocyanidins. Given these results, it may be possible to attribute the heart-health effects of Terminalia arjuna to these polyphenols which may be responsible for the endothelial benefit functions like tea.
Keywords
Catechins, high performance liquid chromatography, liquid chromatography–mass spectrometry, proanthocyanidins, Terminalia arjuna, thiolysis
Terminalia arjuna (T. arjuna) is a large, evergreen, deciduous tree found in sub-Himalayan belt in India, e.g., Uttar Pradesh, Bihar and also in the Deccan region, mainly on the riversides. It belongs to Combretaceae family and attains a height of 20-30 m. It finds its place in ancient Indian medicinal literature like Charaka Samhita and Astang Hridayam. Ancient Indian physician Vagbhatta first advocated the use of this bark powder for the treatment of heart diseases. It is an essential ingredient in many ayurvedic preparations meant to improve cardiovascular health. It is known to provide antidyslipidaemic [1], hypocholesterolaemic [2] and antioxidant activity [1,3]. These were established by various in vivo studies on animal and human volunteers [2]. The plant has also been found to possess anticancer activity [4] and antibacterial activity [5].
The composition of the T. arjuna bark is not studied completely. A number of triterpenes were isolated from the bark of T. arjuna which include triterpene glycosides and aglycones. Some of the triterpenes isolated from the T. arjuna tree are arjunic acid [6,7], arjunolic acid [6,7] and arjungenin [6]. The triterpene glucosides isolated from the T. arjuna tree are arjunetin [6,8], arjunoglucoside I [8], arjunoglucoside II [8], arjunoglucoside III [9], arjunoside I [10] and arjunoside II [11]. The other compounds characterised from the T. arjuna tree are β-sitosterol [12] and terminic acid [12]. Three polyphenols, arjunin, arjunone and arjunolone have been isolated from T. arjuna [13,14].
Studies reported so far have mainly focused on the use of ethanol or aqueous-methanol extracts (AMEs) for studying its bioactivity. Also, terpene class of compounds present in T. arjuna are well studied for its bioactivity which account only for ~1% (w/w) of T. arjuna bark [15]. Our initial compositional investigation suggested that aqueous extract (AE) of T. arjuna bark is enriched in polyphenols. But there is no detailed study on characterisation of various types of polyphenols in AE of T. arjuna tree bark. As polyphenols are well-known antioxidants, it is important to quantify and characterise polyphenols in T. arjuna. In this study, we have carried out a detailed analysis of polyphenols in T. arjuna and addressed key questions like (a) total polyphenol content (b) molecular weight (MW) distribution of polyphenols and (c) identification of polyphenols in the extract.
Materials and Methods
HPLC-grade standards such as (+)-catechin, (−)-epicatechin, (+)-gallocatechin, (−)-epigallocatechin, (−)-epigallocatechin gallate, gallic acid and ellagic acid were purchased from Sigma-Aldrich Co., Bangalore, India. Analytical-grade solvents such as chloroform, methyl iso-butyl ketone (MiBK), ethyl acetate, n-butanol, methanol, conc. hydrochloric acid (36 N) and ethylenediaminetetraacetic acid sodium salt [EDTA] were purchased from Merck Limited India, Mumbai. Benzyl mercaptan (analytical grade), phenol (analytical grade), trifluoroacetic acid (TFA, analytical grade), Bradford reagent and polyvinyl polypyrrolidone (PVPP) were purchased from Sigma-Aldrich Co., Bangalore, India. For HPLC and LC-MS, HPLC-grade solvents such as methanol, acetonitrile, acetic acid and formic acid were purchased from Merck Limited India, Mumbai. All the deuteriated solvents used for NMR were procured from Cambridge Isotope Laboratories Inc, MA, USA.
For all colorimetric measurements, Perkin Elmer Lambda 900 UV spectrophotometer was used. Shimadzu UFLC instrument with LC-M20AD pump, SPD-M20A UV detector and auto-sampler were used for the HPLC analysis of catechins and phenolic acids. The chromatogram was monitored at 278 nm. For isolation, Shimadzu preparative HPLC system equipped with two LC-8A pumps, SPD-20A UV/Vis detector and FRC-10A fraction collector was used to isolate ellagic acid derivatives. Isolation of these compounds was carried using a preparative reversed phase Phenomenex Luna C18 column (250×21.20 mm, 10 μm). Both proton and carbon NMR spectra were recorded using a Bruker AV400, a 400-MHz high-resolution multinuclear FT-NMR spectrometer. Chemical shifts for 1H-NMR and 13C-NMR spectra in DMSO-d6 solution were referenced internally to DMSO-d6 signals, using values of δ 2.49 for proton (middle peak) and δ 39.50 ppm for carbon (middle peak). Mass spectrum of isolated compounds was collected using electron spray ionisation (ESI)-ion trap (IT) MS from Bruker Esquire 3000. Products obtained after thiolysis were analysed by Bruker Esquire 3000 mass spectrometer coupled with Agilent 1100 HPLC. Mass spectra (MS) of the samples were recorded using electron spray ionisation source, equipped with ion trap mass analyser in the negative mode. Nitrogen was used as nebuliser gas with a flow rate of 8 l/min. Nebuliser pressure was maintained at 37 psi and nebuliser temperature at 365°. Mass scan range was from 50 to 3000 m/z. Each spectrum collected had maximum acquisition time of 200 mS with an average of eight spectra. Capillary exit voltage for the ion trap was kept at 90.8 V and of skim 1 was at 22.1 V.
Aqueous extraction
Dried powder of AE of T. arjuna bark was purchased from Natural Remedies Pvt. Ltd., Bangalore, India (Batch No. RD/1903 dated June 2008). Aqueous extraction procedure followed is described in brief: The sun-dried bark of T. arjuna was coarsely powdered and sieved through mesh No. 20 for uniform particle size. This powder was extracted in water (1:4) by refluxing for 2 h. The contents were filtered through a muslin cloth and the extraction process was repeated 2 more times. The filtrates were concentrated by distillation under vacuum and spray-dried. Certificate of analysis of the AE of T. arjuna from supplier included levels of total tannins as tannic acid, weight loss on drying, ash content, bulk density, levels of heavy metals and various microbial tests. Sample was stored in a sealed container and stored at 4° in cold room. Unless stated otherwise, AE of T. arjuna bark was used for all the analysis and isolation procedures reported in this paper.
Methanol extraction
Unprocessed T. arjuna bark was purchased from a local supplier (Channa Bassappa and Co., Bangalore, India) and specimens were authenticated by in-house Botanist of Unilever R and D, Bangalore. These specimens were further authenticated as per tests given in the Indian pharmacopeia 2010 edition. Specimen of the material has been deposited in the herbal lab at Unilever R and D, Bangalore. The dried bark of T. arjuna tree was powdered and sieved through mesh No. 80 for uniform size. This powder was extracted with 100 ml of 70% methanol–water at 50° for 15 min with continuous stirring. After extraction, the supernatant was decanted and the extraction was repeated 4 times. Supernatants from all four extractions were pooled together and centrifuged at 4000 rpm for 15 min to remove the insoluble particles. Supernatant obtained was dried using rotary evaporator to obtain powder of methanol extract of T. arjuna bark. Previous studies have proven intravenous administration of 70% alcohol extract of T. arjuna produced dose-dependent hypotension in anaesthetised dogs [16].
Compositional analysis
The total polyphenol content in the AE of T. arjuna was determined by Folin-Ciocalteu (FC) method as per ISO guidelines [17]. The total polyphenol was expressed as gallic acid equivalent. Phenol– sulphuric acid assay was used for the quantification of sugars. The total sugar was expressed as glucose equivalent [18]. Prior to analysis, the extracts were treated with PVPP [19] and shaken vigorously to remove polyphenols and proanthocyanidins which can interfere with the analysis. After treatment with PVPP, the mixture was centrifuged at 14,000 rpm for 15 min and colourless supernatant was collected. The supernatant was refluxed with 3 M trifluoroacetic acid (TFA) for 4 h. The mixture was evaporated to dryness in a rotary evaporator. The solids were quantitatively transferred and sugar content was quantified by phenol–sulphuric acid assay [18]. Bradford assay was used for estimation of proteins using bovine serum albumin as the standard [20]. Total ash content was determined by ashing accurately weighed extract at 500° for 5 h followed by gravimetric estimation of the residue. Total triterpenes in the AE of T. arjuna bark were quantified by its chloroform extraction followed by material testing with Liebermann–Buchard reagent [21]. Formation of reddish colour indicates the presence of triterpenes. The chloroform extract was rich in triterpenes which was confirmed by colour test for the triterpenes. The amount of triterpenes was estimated gravimetrically after evaporation of the chloroform fraction.
Dialysis of aqueous extract of Terminalia arjuna
For dialysis, Snake Skin™ Dialysis tubing of degenerated cellulose of three different molecular weight cut-offs (MWCO) was procured from Thermo Scientific, India. MWCO ranges for these membranes were 3.5, 7 and 10 kDa. Dialysis bags were soaked in water for 30 min before use and were washed several times with water to condition the membranes. For dialysis experiments, 0.2-0.3 g of the AE of T. arjuna was solubilised in 20 ml of methanol–water mixture (1:1), sonicated and then filled in dialysis tubes. Time for complete dialysis was optimised by analysing solution outside dialysis tubing for total polyphenol content TPP content after every 24 h and observed that after 72 h, TPP levels were below the detection limit of FC assay. Hence, total dialysis time chosen was 72 h. Dialysis was carried in duplicates to evaluate repeatability. After dialysis, solutions retained inside dialysis tubings were dried using a freeze-drier. Solids were weighed accurately on a Mettler balance with a least count of 0.1 mg. Mass balance was determined from initial weight of sample and from the MW fractions. High MW fractions were further analysed for total polyphenol content by FC assay. Total polyphenols were expressed in gallic acid equivalents.
Solvent fractionation of aqueous extract of Terminalia arjuna
Two grams of the AE of T. arjuna was dissolved in 100 ml of water by constant stirring for 30 min. This aqueous solution of AE of T. arjuna was extracted sequentially with solvents of increasing polarity like chloroform, MiBK, ethyl acetate and n-butanol. Each extraction was carried out three times with approximately 200 ml of solvent. Each solvent fraction was collected separately, evaporated and weighed. The extracts will be further referred to as chloroform, MiBK, ethyl acetate, n-butanol and water extracts.
HPLC analysis of Terminalia arjuna bark extracts
Catechins in both AE of T. arjuna and AME were analysed by ISO method for catechin analysis [22]. Catechins in both the extracts were quantified using a HPLC method. For HPLC analysis, Phenomenx phenyl-hexyl, 5 μm, 4.6×250 mm HPLC column was used. The mobile phase A was 9% (volume fraction) acetonitrile, 2% (volume fraction) acetic acid with 20 μg/ml EDTA and mobile B was 80% (volume fraction) acetonitrile, 2% (volume fraction) acetic acid with 20 μg/ml EDTA. Separation was carried out at 1 ml/min flow rate with HPLC column maintained at 40° and detection at 278 nm. HPLC analysis was carried out using a gradient elution. Binary gradient conditions: 0% mobile phase B for 10 min, then over 15 min, a linear gradient to 32% mobile phase B and hold at this composition for 10 min. Then reset to 0% mobile phase B to equilibrate for 10 min before next injection.
Gallic acid, ellagic acid and ellagic acid derivatives were also analysed by the same method. Peaks of catechins and phenolic acids in the HPLC chromatogram were identified by comparing the retention time and UV spectra with standards. Standard compounds were used to generate calibration and using the calibration curves, catechins, gallic acid and ellagic acid were quantified. In case of ellagic acid derivatives, ellagic acid was used as a standard.
Isolation of ellagic acid derivatives
Twenty grams of AE of T. arjuna was taken and extracted with 300 ml of chloroform (3×100 ml). The residue was filtered and further extracted with 400 ml of ethyl acetate (4×100 ml). The ethyl acetate extract was evaporated to dryness. Dried powder was dissolved in methanol and centrifuged at 4000 rpm for 20 min to remove the insoluble residue. The supernatant was used for preparative HPLC injection. Mobile phase used was 0.3% formic acid (A) and acetonitrile (B), flow rate was 6 ml/min. A linear gradient elution method was used for the analysis starting with 20% of B, gradually increasing to 60% over a time of 40 min, following which concentration of B was reduced to 20% over 5 min. Then the column was allowed to equilibrate over a period of 5 min. Four major compounds eluting at retention times of 18.0, 20, 24.5 and 28.4 min were collected and dried. The identification of ellagic acid derivatives was performed by NMR spectral confirmation and mass spectral analysis.
Proanthocyanidin estimation
One milligram per millilitre of AE of T. arjuna was taken and dissolved in 1:1 methanol–water. 0.1 ml of this solution was taken and 0.6 ml of water was added followed by 6 ml of butanol–HCl (95:5). The mixture was refluxed for 50 min. Then it was cooled and absorbance was measured at 540 nm in UV spectrophotometer. Cyanidin chloride was used as a standard to generate calibration curve. From this calibration curve, total proanthocyanidin content was quantified and expressed as cyanidin chloride equivalent [23,24].
Thiolysis
Thiolysis was achieved by using the method reported by Guyot et al. [23]. 100 μl of methanolic suspension (1 mg/ml) of the high MW (greater than 10 kDa) of AE of T. arjuna was taken in a HPLC vial. To this was added 100 μl of acidic methanol (3% v/v of conc. HCl) and 200 μl benzyl mercaptan (5% v/v in methanol). The vial was sealed and reaction was carried out at 40° using a metallic dry bath. After 30 min, the reaction mixture was cooled and analysed by LC-MS.
Liquid chromatography–mass spectrometry analysis
Products obtained after thiolysis were analysed by LC-MS. LC-MS analysis was carried using Agilent HPLC (1100 series) with Bruker esquire 3000 mass spectrometer equipped with ESI source and IT mass analyser. Instrumental conditions are given in detail in the earlier section. Separation of the thioethers formed was carried using reversed phase bonda pak C-18 column (3.90×3.9 mm) from Waters. Mobile phases used were 2% formic acid (A) and acetonitrile (B) with flow rate of 0.6 ml/min. Column oven temperature was maintained at 30°. Diode array detector at 280 nm was used for detection of peaks. The elution was performed with 7% B isocratic for 5 min; a linear gradient was then installed to reach 50% B at 35 min and 80% B at 40 min. Then 80% B at 40 min was reduced to 7% B at 45 min followed by 5 min of re-equilibration time. From the total ion chromatogram, peaks corresponding to thioethers were obtained. Molecular ion peaks and fragments obtained were compared with literature to arrive at building flavan-3-ol units of proanthocyanidins [23,25-28].
Results and Discussion
Compositional analysis of AE of T. arjuna extract included estimation of total polyphenol, sugars, protein, triterpenes and ash content. Compositional analysis of AE of T. arjuna showed that it contains less than 1% of triterpenes, 44% polyphenols, 12% sugars, 30% proteins and 5% ash. The amount of polyphenol in AE of T. arjuna is very high compared to other common sources of polyphenols such as tea and grapes [29]. Though AE of T. arjuna has such high polyphenol levels, only three polyphenols have been reported so far. Characterising polyphenols can provide insights to understand the health benefits of T. arjuna.
Polyphenol-enriched fractions were obtained by subfractionation of AE of T. arjuna with different solvents. Polarity-based fractionation of T. arjuna extract was carried out using solvents of increasing polarity. Chloroform was used to extract out nonpolar compounds such as triterpenes and lipids. Sequential extractions with MiBK and ethyl acetate were carried out to separate polyphenols such as catechins, flavonols and nonpolar polyphenols. Extraction with n-butanol separates the mid-polar polyphenols such as oxidised catechins and flavonol glycosides. Finally, the highly polar ingredients reside in the water fraction which could be tannins, polymerised polyphenols or polyphenols bound to proteins. Recovery data during fractionation showed that more than 70% of the solids were recovered into water. Water fraction was dark brown in colour indicating the presence of tannins or proanthocyanidins. 20% of solids recovered into n-butanol and the fraction was red coloured. Water and n-butanol fraction together accounted for more than 90% of total solids in AE of T. arjuna. Less than 1% of solids recovered into chloroform. Chloroform fraction of AE of T. arjuna was enriched with triterpenes as confirmed by testing with Liebermann-Buchard reagent [21].
Powders obtained after solvent fractionation were analysed for the total polyphenol content using FC assay. Recovery of polyphenols in AE of T. arjuna into various solvent fractions showed that water fraction had 65% polyphenols, n-butanol fraction had 27 and 3-4% was recovered into MiBK and ethyl acetate fractions. This indicates that majority of the polyphenols in AE of T. arjuna were highly polar. The nature of the polyphenols in each fraction was analysed with HPLC and LC/MS.
Polyphenols in plant extracts can be present in monomeric form or as oligomeric/polymeric forms due to oxidation. Hence, MW distribution of polyphenols in AE of T. arjuna was evaluated using dialysis. During dialysis of AE of T. arjuna, concentrations were kept below 10% w/v to ensure complete solubility of all components. Dialysis was carried in duplicate to evaluate repeatability and recovery. Recovery was found to be more than 93%. The amount of solids into different MW fractions showed that about 40% of the solids in AE extract had MW less than 3.5 kDa, whereas nearly 55% of total solids had MW greater than 10 kDa. This indicated that major fraction solids in AE of T. arjuna contained high MW compounds. This high MW fraction was deep brown in colour and is expected to contain polysaccharide, proteins and polymeric polyphenols such as proanthocyanidins.
Total polyphenol analysis of the fractions obtained from dialysis study was carried out. Data showed that 20% of polyphenols in AE of T. arjuna have MW less than 3.5 kDa whereas 70% of total polyphenols in AE of T. arjuna have MW greater than 10 kDa suggesting that the polyphenols in AE of T. arjuna are polymerised. In grapes and apples, levels of high polymeric polyphenols vary from 2 to 60 mg/100 g and in comparison, AE of T. arjuna has very high amounts of polymeric polyphenols [30].
To identify the type of polyphenols present, AE of T. arjuna was analysed for major classes of polyphenols such as anthocyanidin, flavonols, (catechins/proanthocyanidin) and phenolic acids. To find the presence of flavonols and its glycosides, AE of T. arjuna was hydrolysed and analysed for its aglycones viz. kaempferol, myricetin, quercetin, using reported HPLC methods [23]. Aglycones were not detected indicating the absence of flavonols. AE of T. arjuna was also analysed for anthocyanidins using an HPLC method [28]. But peaks corresponding to anthocyanidins were not detected. Catechins, phenolic acids and proanthocyanidins in T. arjuna were characterised using standard methods.
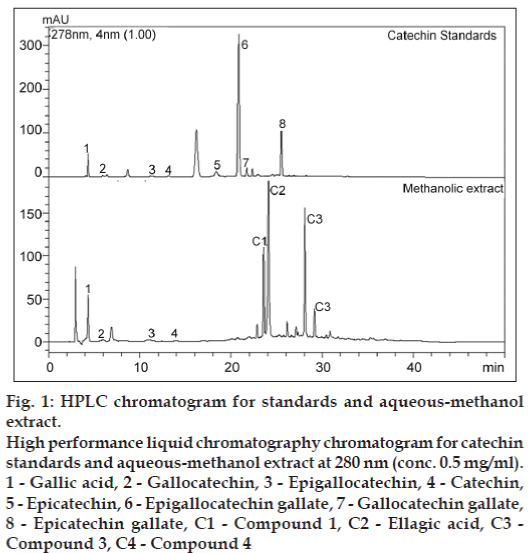
During catechin analysis of AE of T. arjuna, catechins were not detected. This could be due to degradation of catechins during harsh extraction conditions used for the manufacture of the AE of T. arjuna. To overcome such high-temperature degradation, mild extraction of T. arjuna bark was carried out using methanol+water mixture [70+30] at 50º. During catechin analysis of AME, catechin, gallocatechin and epigallocatechin were detected in AME as shown in fig. 1. Other catechins such as epicatechin, epicatechin gallate, epigallocatechin gallate and gallocatechin gallate were not detected. The quantification data for these compounds are provided in Table 1. Data showed that levels of (+)-catechin was twice the levels of gallocatechin. Levels of epigallocatechin were very low. AME had 1.5% w/w of catechins. Though levels of total polyphenols in AME are higher than polyphenols in tea or grapes, catechin levels in T. arjuna are significantly lower than that of green tea and grapes [28].
| Compounds | Concentration (mg/g) |
|---|---|
| Catechin | 8.85 ± 0.25 |
| Gallocatechin | 4.10 ± 0.06 |
| Epigallocatechin | 0.34 ± 0.01 |
| Gallic acid | 0.97 ± 0.11 |
| Ellagic acid and derivatives* | 98.1 ± 0.98 |
*Expressed as ellagic acid equivalent, AME=Aqueous-methanol extract
Table 1: Quantification data for catechins and phenolic acids in ame
Figure 1: HPLC chromatogram for standards and aqueous-methanol extract.
High performance liquid chromatography chromatogram for catechin standards and aqueous-methanol extract at 280 nm (conc. 0.5 mg/ml).
1 - Gallic acid, 2 - Gallocatechin, 3 - Epigallocatechin, 4 - Catechin,
5 - Epicatechin, 6 - Epigallocatechin gallate, 7 - Gallocatechin gallate,
8 - Epicatechin gallate, C1 - Compound 1, C2 - Ellagic acid, C3 - Compound 3, C4 - Compound 4
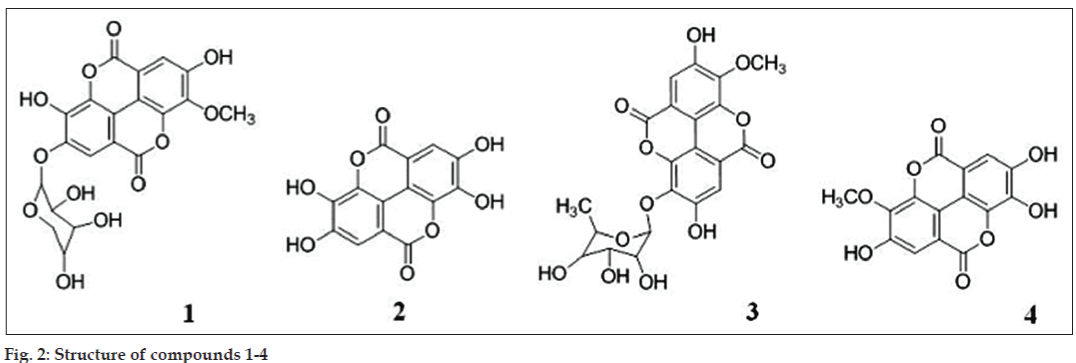
Compound 1 (fig. 2) was yellowish brown in colour. The ESI-MS analysis revealed a molecular ion at [M]− 446 consistent with MW of methyl-substituted ellagic acid and a pentose sugar. Fragment at m/z 300 corresponds to ellagic acid. 1H-NMR analysis showed presence of singlet at δ 7.3 ppm and 7.6 ppm corresponding to two aromatic protons in methyl-substituted ellagic acid moiety. Peak at δ 5.4 (d, 1.6 Hz) indicates anomeric proton of xylose and δ 4.3 (t, 1.6 Hz) corresponding to CHOH group of xylose adjacent to CH2. From spectral data and comparing it with literature, predicted structure for compound 1 is 3-O-methyl-ellagic acid 4-O-β-D-xylopyranoside [31].
Compound 2 was identified as ellagic acid (fig. 2) based on the retention time of authentic standard. This was further confirmed by ESI-MS analysis which showed a prominent molecular ion peak at 300 corresponding to ellagic acid. 1H-NMR study revealed only one aromatic proton at δ 7.3 (s).
Mass spectrum of compound 3 (fig. 2) had a molecular ion peak at m/z [M]− 461 (molecular ion peak) and fragments at m/z 300, 351 and 343. Fragment at m/z at 300 indicates ellagic acid, 315 correspond to methyl derivative of ellagic and 343 is indicative of methyl ellagic acid plus carbonyl group which can be contributed by the sugar moiety. 1H-NMR analysis showed presence of two aromatic protons at δ 7.5 (s) and 7.6 (s), methoxy signal at δ 4.0 (s) contributed by –OCH3 group attached to ellagic acid, anomeric proton of rhamnoside group at δ 5.48 (d, 1.6 Hz), 3.71 δ (d, J = 4 Hz) and 3.70 δ (s) correspond to methine proton and 1.13δ (d, J = 6 Hz) presence of one 6-deoxycarbon. There was a broad hump at 6.5-6.6 ppm because of OH groups. 13C-NMR spectra revealed peaks at δ17.1, 60.8, 69.6, 69.9, 70.2, 71.9, 99.4, 99.6, 110.9, 113.2, 114.6, 139.9, 147.3, 152.4, 158.9. These data were concordant with published 13C-NMR signals for 3-O-methyl ellagic acid 3-O-rhamnoside from alternate sources [32,33].
Compound 4 (fig. 2) is sugar derivative of 3-O-methyl ellagic acid but the exact structural elucidation could not be done. ESI-MS data showed molecular ion peak at [M]− at m/z 486 and fragments at m/z 314 corresponding to 3-O-methyl ellagic acid and 300 corresponds to ellagic acid. Proton NMR peaks at δ 7.41, 7.53 and 3.9 indicated the backbone of the compound is ellagic acid with substitution at 3 or 4 position.
The AE of T. arjuna was tested for proanthocyanidins. Proanthocyanidins on treatment with acid get depolymerised and are converted to highly coloured anthocyanidins absorbing at 530 to 550 nm [23]. AE of T. arjuna after acid hydrolysis turned red with high absorbance at 540 nm. This confirmed the presence of proanthocyanidins. The proanthocyanidins were quantified by spectrophotometry and expressed as cyanidin chloride (standard anthocyanidins) equivalent. It was observed that 7.5±0.3% of the AE of T. arjuna was composed of proanthocyanidins.
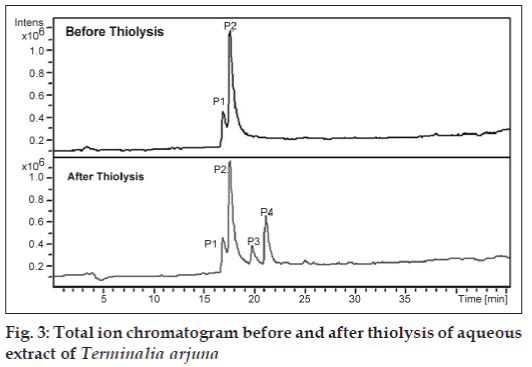
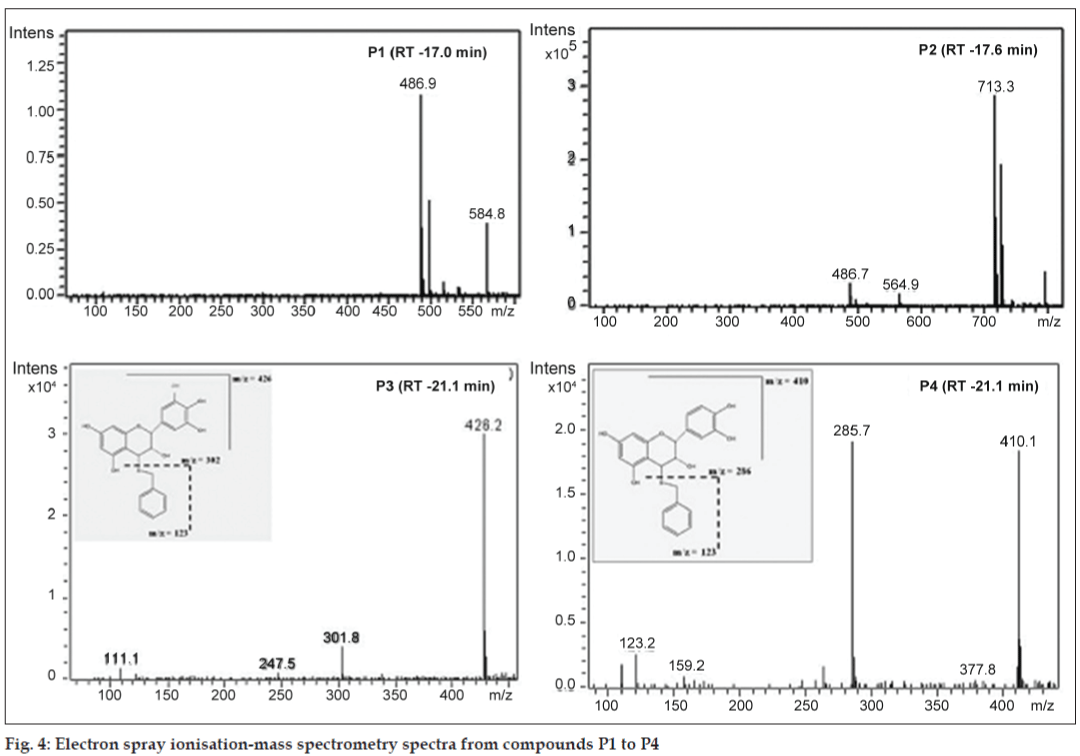
The building units in proanthocyanidins were determined by thiolysis [23,27]. The total ion chromatogram (TIC) of the AE after thiolysis showed two new peaks as shown in fig. 3. The new peak at 19.8 min has molecular ion peak at m/z 426 and fragment at m/z 302. The m/z 302 indicates gallocatechin moiety. Based on fragmentation pattern and the reported LC-MS methods (fig. 4), the new peak at 19.8 min would be identified as gallocatechin thioether or epigallocatechin thioether. The peak at 21.3 min has fragments at m/z 411, m/z 123 and m/z 286 (fig. 4). Fragment at m/z 286 indicates catechin moiety and m/z 123 indicates mercaptan moiety. Hence, from MS data, the new compound formed after thiolysis could be catechin or epicatechin thioether which is expected to have molecular ion peak at 411. From the MS fragmentation and comparing it with literature, we propose that the proanthocyanidins in T. arjuna are polymers of (+/−)-catechin and (+/−)-gallocatechin [23,25-28].
A close look at the total ion chromatogram after thiolysis revealed the compound eluting at 17.6 min do not reduce in intensity after thiolysis. It has a strong fragment at m/z 713 as shown in fig. 3. 713 is a characteristic fragment for trimeric procyanidin (fig. 4) [34]. A-type proanthocyanidins do not undergo thiolytic degradation because of double linkages between polymer units [31]. Based on these data, we can propose that the compound is an A-type trimeric procyanidin [34]. The compound eluting at 6.9 min can be arjunic acid or arjunolic acid both of which have MW of 488 g/mol [11]. Further experiments need to be done to confirm the same.
We have, for the first time, reported a detailed analysis of polyphenols in AE of T. arjuna bark. Extract had 44% polyphenols by weight and majority of polyphenols are polymeric in nature. Catechin, gallocatechins, a significant amount of ellagic acid and its derivatives are detected in the methanol extract. Given these results, it may also be possible to attribute the heart-health effects for T. arjuna to these poly phenols which may be responsible for the endothelial benefit functions, like tea. It may be these polyphenols that have potential nitric oxide generation activity which does benefit positively the endothelial functions.
Acknowledgements
The authors thank Dr. D. B. A. Narayana for his valuable input while carrying out this study.
References
- Chander R, Singh K, Khanna AK, Kaul SM, Puri A, Saxena R. Antidyslipidemic and antioxidant activities of different fractions of Terminaliaarjunastem bark. J Clin Biochem 2004;19:141-8.
- Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminaliaarjuna tree-bark powder: A randomised placebo-controlled trial. J Assoc Physicians India 2001;49:231-5.
- Raghavan B, Kumari SK. Effect of Terminaliaarjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J Physiol Pharmacol 2006;50:133-42.
- Pettit GR, Hoard MS, Doubek DL, Schmidt JM, Pettit RK, Tackett LP, et al. Antineoplastic agents 338. The cancer cell growth inhibitory.Constituents of Terminaliaarjuna (Combretaceae). J Ethnopharmacol1996;53:57-63.
- Singh DV, Gupta MM, Santha Kumar TR, Saikia D, Khanuja SP. Antibacterial principles from the bark of Terminaliaarjuna. CurrSci 2008;94:27-9.
- Singh DV, Gupta MM, Tripathi AK, Prajapati V, Kumar S. Arjunetin from Terminaliaarjuna as an insect feeding-deterrent and growth inhibitor. Phytother Res 2004;18:131-4.
- Verma SC, Jain CL, Padhi MM, Devalla RB. Microwave extraction and rapid isolation of arjunic acid from Terminaliaarjuna (Roxb. ex DC.) stem bark and quantification of arjunic acid and arjunolic acid using HPLC-PDA technique. J Sep Sci 2012;35:1627-33.
- Row LR, Murti PS, Rao GS, Sastry CS, Rao KV. Chemical examination of Terminaliaarjuna: Part XIII – Isolation and structure determination of Arjunetin from Terminaliaarjuna. Indian J Chem 1970;8:772-5.
- Honda T, Murae T, Tsuyuki T, Takahashi T, Sawai M. Arjungenin, arjunglucoside I, and arjunglucoside II. A new triterpenesglucoside from Terminaliaarjuna. Bull. Chem. Soc. Jpn 1976;49:3213-8.
- Tsuyuki T, Yuriko H, Honda T, Takahashi T, Matsushita K. A new triterpenesglucoside from Terminaliaarjuna. Arjunglucoside III. Bull. Chem. Soc. Jpn 1979;52:3127-8.
- Anjaneyulu AS, Prasad AV. Chemical examination of roots of Terminaliaarjuna(Roxb) Wright and Arnot: Part I – Characterisationof two new triterpenoid glycosides. Indian J Chem 1982;21B: 530-3.
- Anjaneyulu AS, Prasad AV. Structure of terminic acid, a dihydroxytriternene carboxylic acid from Terminaliaarjuna. Phytochemistry 1983;22:993-8.
- Kandil FE, Nassar MI. A tannin anti-cancer promotor from Terminaliaarjuna. Phytochemistry 1998;47:1567-8.
- Honda T, Murae T, Tsuyuki T, Takahashi T. A structure of arjunin. A new sapogenin from Terminaliaarjuna.Chem Pharm Bull 1976;24:178-80.
- Singh DV, Verma RK, Gupta MM, Kumar S. Quantitative determination of oleane derivatives in Terminaliaarjuna by high performance thin layer chromatography. Phytochem Anal 2002;13:207-10.
- Nammi S, Gudavalli R, Babu BS, Lodagala DS, Boini KM. Possible mechanisms of hypotension produced 70% alcoholic extract of Terminaliaarjuna(L.) in anaesthetized dogs. BMC Complement AlternMed 2003;3:5-8.
- ISO, 2005a. ISO14502-1: 2005 Determination of substances characteristic of green and black tea. Part 1. Content of total polyphenols in tea—colorimetric method using Folin–Ciocalteu reagent.
- Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature 1951;168:167-168.
- Mitchell AE, Hong YJ, May JC, Wright CA, Bamforth CW. A comparison of Polyvinylpolypyrrolidone (PVPP), Silica Xerogel and a Polyvinylpyrrolidone (PVP)-silica co-product for their ability to remove polyphenols from beer. J Inst Brew 2005;111:20-5.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Tripathi VK, Pandey VB, Udupa KN, Rücker G. Arjunolitin, a triterpene glycoside from Terminaliaarjuna. Phytochemistry 1992;31:349-51.
- ISO, 2005b. ISO14502-2: 2005 Determination of substances characteristic of green and black tea. Part 2. Content of catechins in green tea—method using high performance liquid chromatography.
- Guyot S, Marnet N, Laraba D, Sanoner P, Drilleau JF. Reversed-phase HPLC following Thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a french cider apple variety (Malusdomestica Var. Kermerrien). J Agric Food Chem 1998;46:1698-705.
- Nitao JK, Birr BA, Nair MG, Herms DA, Mattson WJ. Rapid quantification of proanthocyanidins (condensed tannins) with a continuous flow analyzer. J Agric Food Chem 2001;49:2207-14.
- Wang H, Helliwell K. Determination of flavonols in green and black tea leaves and green tea infusions by high performance chromatography. Food Res Int 2001;34:223-7.
- Meagher, LP, Lane G, Sivakumaran S, Tavendale MH, Fraser K. Characterization of condensed tannins from Lotus species by thiolytic degradation and electrospray mass spectrometry. Animal Feed SciTechnol 2004;117:151-63.
- Fu C, Loo AE, Chia FP, Huang D. Oligomericproanthocyanidins from mangosteen pericarps. J Agric Food Chem 2007;55:7689-94.
- Callemien D, Guyot S, Collin S. Use of thiolysis hyphenated to RP-HPLC-ESI(-)-MS/MS for the analysis of flavonoids in fresh lager beers. Food Chem 2008;110:1012-8.
- Aqil F, Ahmad I, Mehmood Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk J Biol 2006;30:177-83.
- dePascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem 2000;48:5331-7.
- Zafrilla P, Ferreres F, Tomás-Barberán FA. Effect of processing and storage on the antioxidant ellagic acid derivatives and flavonoids of redraspberry (Rubusidaeus) jams. J Agric Food Chem 2001;49:3651-5.
- Kim JP, Lee IK, Yun BS, Chung SH, Shim GS, Koshino H, et al.Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus. Phytochemistry 2001;57:587-91.
- Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminaliabellerica, Terminaliachebula and Terminaliahorrida): Characterization, quantitation and determination of antioxidantcapacities. Phytochemistry 2010;71:1132-48.
- Nyman NA, Kumpulainen JT. Determination of anthocyanidins in berries and red wine by high-performance liquid chromatography. J Agric Food Chem 2001;49:4183-7.