- *Corresponding Author:
- O. Astafyeva
Department of Biotechnology, Faculty of Biology, Astrakhan State University, pl. Shaumyana 1, 414000, Astrakhan, Russia
E-mail: astra39@list.ru
| Date of Submission | 07 September 2016 |
| Date of Revision | 03 August 2017 |
| Date of Acceptance | 15 March 2018 |
| Indian J Pharm Sci 2018;80(3): 434-441 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The composition of detectable small organic compounds in the ethanol extract of Achillea micrantha was defined by means of gas chromatography-mass spectrometric analysis. There were 71 low molecular weight organic compounds observed, two of which remained unidentified. The antibacterial activity of the extract was studied in respect to Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa using the agar diffusion test and serial dilutions to define minimum inhibitory concentration. In order to compare the antibacterial activity of the herb and blossom truss extract of A. micrantha, the extracts of A. millefolium and A. leptophylla was used. In relation to the microorganisms tested, a significant inhibitory effect was observed with the aqueous alcoholic extract of A. micrantha at the minimum inhibitory concentration of 0.05 µg/ml.
Keywords
Achillea micrantha, low molecular weight organic compounds, gas chromatography-mass spectrometry, antibacterial activity
The genus Achillea of compositae (Asteraceae) contains about 150 species. It vegetates mostly on the territory of Europe and Western Asia as well as in Australia, New Zealand and North America [1]. Different scientists managed to extract some chemical substances from the blossom truss and herb of yarrow, Achillea millefolium L., such as lactones, achillin, artillizin, grossmizin, michrantin, kaempferol 3-rhamnoside, campesterol [2,3], sesquiterpene lactones, sinthenin and micrantin [4,5], other various components of essential oils [6,7] and flavonoids [8].
The flavonoid content in the yarrow was considered to make antipyretic and haemostatic drugs [9]. In ethnomedicine, the herb and tips of flowering plants (anthodes) are used as a haemostatic agent in cases of internal haemorrhage, gastric disorders, external haemorrhage, inflammatory processes and metabolopathy [10-12]. There were reports of antioxidant activity of the extracts and essential oils of the plants sp. Achillea [13-15], as well as estrogenic [16], antiulcer [17,18], antitumoral [19], antisecretory (inhibiting intestinal motility) [20], immunomodulatory [21], fibrinogenic [22], antimicrobial [23-27], antifungal [28], and antiinflammatory [29,30] activities.
In traditional medicine, in contrast to A. micrantha Willd., the herb and blossom truss of A. millefolium, the chemical composition of which has been properly studied is mostly used. Nevertheless, literature reported that the antimicrobial effect of the extracts A. micrantha was much higher than that of A. millefolium [23]. The aim of this paper was to examine the chemical composition of the extracts of A. micrantha vegetative elements and blossom truss and their antibacterial activity in comparison with the extracts of other Achillea species.
Materials and Methods
Gas chromatography/mass spectrometry (GC/MS) analysis
The low-molecular weight organic compounds (LMWOC) of the extracts were defined by methods of GC/MS with the help of Shimadzu GC/MS (QP-5050А, Shimadzu, Japan) equipped with the software Class 5000 and chromato-mass spectrometer TRACE DSQ II (Thermo Electron Corporation) with quadrupole mass analyser. In the first case, the column of DBI, 30 m; 0.53 mm ID и 1.5 μm film (J&W Scientific) was used. As a mobile phase the carrier gas helium was applied. Ionization mode- EI, ionization voltage –70 ev. Temperature program: 5° (1 min)-150° (1 min) at 10°/ min-250° (5 min) at 5°/min-270° (2 min) at 3.5°/min. Detector temperature was set at 250° and the injector temperature was set at 280°.
In the second one, before the analysis started the preparation was 200-fold attenuated and transformed into hexane. The constitution of the compounds in the solution obtained was detected by means of the chromatomass spectrometer with quadrupole mass analyser. The column Thermo TR-5 ms SQC 15 м×0.25 mm phase ID 0.25 micron was applied. As the carrier gas helium was used. The mass spectra were tested in mode of scanning on mass full-scale range (30- 580 m/z) at a programmed temperature range (35°- 3 min, 2°/min to 60°-3 min, 2°/min to 80°-3 min, 4°/min to 120°-3 min, 5°/min to 150°-3 min, 15°/min to 240°- 10 min) with the subsequent step-by-step operation of chromatograms. The agents detected were identified with the help of mass spectral libraries (NIST-2005 and Wiley). For more precise identification the Kavats's retention index, obtained due to the use of the standards of alkanes C7-C30, was applied. The quantitative analysis was made through the internal standards, decafluorobenzophenone and benzophenone.
Plant material
The blossom trusses, leaves and footstalks of A. micrantha were picked in the spring-summer period (May-June) of 2010-11 at blossom-time on the territory of Volga Sands, Astrakhan region, Russia. Species identification was carried visually and microscopically by examining the morphology of blossoms in trusses, leaves and other characteristic features of the plants, and defined them with the determiner at the Botany Department, Astrakhan State University. While determining the antibacterial activity of A. micrantha extracts, the known medical plants of Achilleа genus, A. millefolium, as well as A. leptophylla Bieb., growing in Astrakhan region were used for comparison.
The dried plant raw material (blossom trusses, leaves and footstalks) was extracted with 40 % aqueous solution of ethanol at an indoor temperature during 7 d while being constantly stirred. Then the extract was filtered, with the spirit evaporated, pasteurized in the dry-air sterilizer at a temperature of 85°. The final product was used to examine the antibacterial activity of the extract obtained. In the course of our pasteurization of extracts, some of the organic molecules could be destroyed due to the high temperature, and they were ultimately not taken into account in the analysis. However, this was done in order to obtain an extract completely free of microorganisms for further experiments.
Antibacterial assay
The antibacterial activity of the extracts was tested using the agar diffusion test and counting the colony-forming units (CFUs) of test organisms, Staphylococcus aureus Rosenbach RNCIM В-1899, Escherichia coli Migula CK RNCIM В-1911, obtained from the Russian National Collection of Industrial Microorganisms, FSUE, State Research Institute of Genetics (Moscow, Russia); Pseudomonas aeruginosa Migula 1315, Staphylococcus еpidermidis Rosenbach 537, obtained from the regional infectious hospital. The minimum inhibitory concentration (MIC) was tested by means of count method of CFUs on solid medium. Gentamycin was used as the standard drug.
Results and Discussion
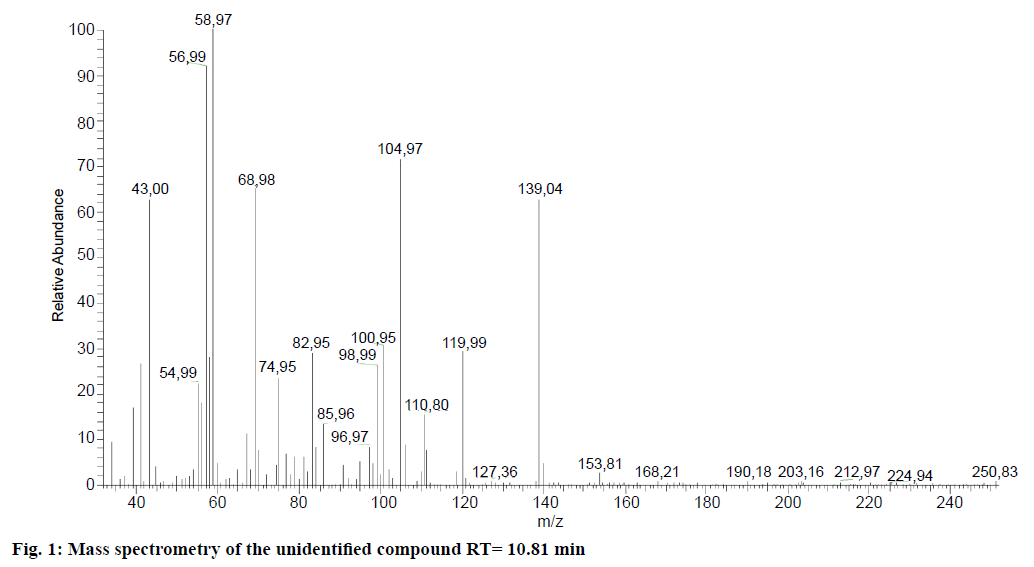
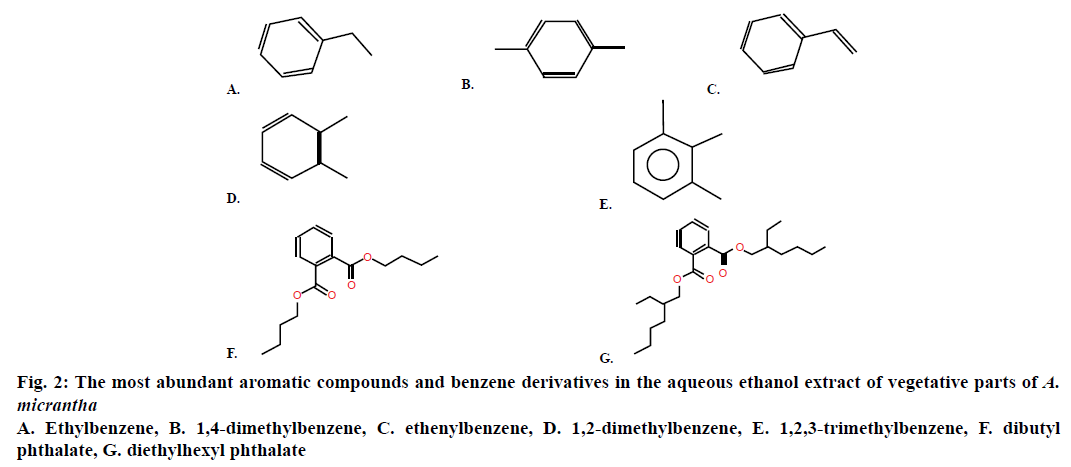
In the study of compositional analysis of aqueous alcoholic extracts of A. micrantha vegetative elements (leaves and footstalks) through GC/MS, there were a large number of LMWOC related to different groups of chemical compounds (Tables 1 and 2). Here with, aldehydes, alcohols and hydrocarbons were found prevailing (Table 2). Out of 55 defined compounds, one remained unidentified (Figure 1). The concentration of two compounds (butane-1,3-diol and hexanal) was the highest. Their proportion was about 63 % from the total LMWOC concentration in the solution. Hexanal participates in regulation of different plant reactions, among them the formation of plant defence mechanisms from external damage and plant-feeders [31-33]. This compound has antifungal and antimicrobial properties [34]. The substantial part of all LMWOCs were aromatic compounds and benzene derivatives (Table 1). The most abundant of them are given in Figure 2. All these compounds are bioactive.
| Peak | Compound | Formula | RT | IK | C | % |
|---|---|---|---|---|---|---|
| 1 | hexan-2-one | C6H12O | 2.55 | 796 | 183.47 | 1.93 |
| 2 | butane-1,3-diol | C4H10O2 | 2.72 | 803 | 2000.78 | 21.08 |
| 3 | Hexanal | C6H12O | 2.97 | 812 | 3980.44 | 41.93 |
| 4 | Ethylbenzene | C8H10 | 4.11 | 853 | 68.31 | 0.72 |
| 5 | 1,4-dimethylbenzene | C8H10 | 4.34 | 861 | 279.39 | 2.94 |
| 6 | 3-methyloctane | C9H20 | 4.51 | 867 | 27.67 | 0.29 |
| 7 | Ethenylbenzene [styrene] | C8H8 | 5.03 | 886 | 86.04 | 0.91 |
| 8 | 1,2-dimethylbenzene [o-xylene] |
C8H10 | 5.08 | 888 | 89.39 | 0.94 |
| 9 | 5-methoxypentan-2-one | C6H12O2 | 5.9 | 911 | 65.49 | 0.69 |
| 10 | 1-(4-methylpentan-2-yloxy)propan-2-ol | C9H20O2 | 6.05 | 914 | 303.89 | 3.20 |
| 11 | 4,7,7-trimethylbicyclo[4.1.0]hept-3-ene [4-carene] | C10H16 | 6.58 | 925 | 31.59 | 0.33 |
| 12 | 1,2,3-trimethylbenzene | C9H12 | 9.38 | 986 | 144.93 | 1.53 |
| 13 | (2S)-2-tert-butyl-5-methylidene-1,3-dioxolan-4-one | C8H12O3 | 10.00 | 999 | 186.55 | 1.97 |
| 14 | 3,7,7-trimethylbicyclo[4.1.0]hept-3-ene [3-carene ] | C10H16 | 10.26 | 1004 | 37.00 | 0.39 |
| 15 | Unidentified compounds m/z 139 [M+], 59 (100) |
10.81 | 1013 | 70.48 | 0.74 | |
| 16 | 1-methyl-3-propan-2-ylbenzene [m-cymene] | C10H14 | 11.08 | 1017 | 70.39 | 0.74 |
| 17 | 1-methyl-4-prop-1-en-2-ylcyclohexene [d-limonene] | C10H16 | 11.3 | 1021 | 25.81 | 0.27 |
| 18 | 5-ethyl-2,2,3-trimethylheptane | C12H26 | 11.36 | 1022 | 50.50 | 0.53 |
| 19 | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene [γ-Terpinene] | C10H16 | 13.13 | 1052 | 25.88 | 0.27 |
| 20 | 2-methyldecane | C11H24 | 13.63 | 1060 | 51.34 | 0.54 |
| 21 | 3,7-dimethyldecane | C12H26 | 14.21 | 1070 | 37.98 | 0.40 |
| 22 | (6S,7aS)-6-Ethyl-hexahydro-pyrrolizin-3-one | C9H15NO | 14.43 | 1073 | 41.22 | 0.43 |
| 23 | 2,3,4-trimethyldecane | C13H28 | 15.08 | 1084 | 29.85 | 0.31 |
| 24 | 4-tert-butyl-1,3-thiazole | C7H11NS | 15.76 | 1096 | 22.06 | 0.23 |
| 25 | 3,3-diethyl-4,5-dimethylhex-4-en-2-one | C12H22O | 16.91 | 1111 | 42.16 | 0.44 |
| 26 | 1,1,3,3,5-pentamethylcyclohexane | C11H22 | 17.81 | 1123 | 25.90 | 0.27 |
| 27 | 1,5-diethyl-2,3-dimethylcyclohexane | C12H24 | 18.46 | 1131 | 54.05 | 0.57 |
| 28 | 1-methyl-2-pentylcyclohexane | C12H24 | 19.57 | 1145 | 40.84 | 0.43 |
| 29 | 2,3-dimethyl-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene | C12H22 | 22.17 | 1178 | 29.25 | 0.31 |
| 30 | 1,6-dimethyl-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene | C12H22 | 22.77 | 1186 | 15.36 | 0.16 |
| 31 | dodecane | C12H26 | 23.9 | 1200 | 106.91 | 1.13 |
| 32 | 2,6-dimethyl-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene | C12H22 | 24.17 | 1204 | 119.90 | 1.26 |
| 33 | 1,5-dimethyl-1,2,3,4,4a,5,6,7,8,8a-decahydronaphthalene | C12H22 | 24.58 | 1209 | 97.07 | 1.02 |
| 34 | 4,10-dimethylspiro[4.5]decane | C12H22 | 25.72 | 1224 | 24.26 | 0.26 |
| 35 | tetradecane | C14H30 | 37.59 | 1400 | 60.17 | 0.63 |
| 36 | dodecanoyl chloride | C12H23ClO | 39.35 | 1443 | 12.64 | 0.13 |
| 37 | 2,6-ditert-butyl-4-methylphenol | C15H24O | 41.8 | 1504 | 14.25 | 0.15 |
| 38 | hexadecane | C16H34 | 45.93 | 1600 | 19.07 | 0.20 |
| 39 | 4-pyrrolidin-1-ylbenzene-1,3-diol | C10H13NO2 | 47.06 | 1632 | 9.25 | 0.10 |
| 40 | undecylcyclopentane | C16H32 | 47.68 | 1650 | 7.16 | 0.08 |
| 41 | octadecane | C18H38 | 52.25 | 1800 | 14.70 | 0.15 |
| 42 | bis(2-methylpropyl) benzene-1,2-dicarboxylate [diisobutyl phthalate] |
C16H22O4 | 54.31 | 1871 | 24.67 | 0.26 |
| 43 | (z)-hexadec-11-enoic acid | C16H30O2 | 55.72 | 1945 | 13.23 | 0.14 |
| 44 | dibutyl benzene-1,2-dicarboxylate [dibutyl phthalate] |
C16H22O4 | 55.92 | 1960 | 71.54 | 0.75 |
| 45 | hexadecanoic acid | C16H32O2 | 56.02 | 1968 | 76.83 | 0.81 |
| 46 | ethyl hexadecanoate | C18H36O2 | 56.36 | 1993 | 14.62 | 0.15 |
| 47 | eicosane | C20H42 | 56.45 | 2000 | 24.02 | 0.25 |
| 48 | 5-[(1S,4aS,8aS)-5,5,8a-trimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]-3-methylpent-1-en-3-ol; [manool] |
C20H34O | 56.87 | 2044 | 15.26 | 0.16 |
| 49 | icos-1-ene | C20H40 | 57.04 | 2062 | 24.72 | 0.26 |
| 50 | methyl 10-octadecenoate | C19H36O2 | 57.69 | 2137 | 80.26 | 0.85 |
| 51 | docosane | C22H46 | 58.19 | 2200 | 47.85 | 0.50 |
| 52 | docos-1-ene | C22H44 | 58.63 | 2266 | 72.02 | 0.76 |
| 53 | bis(2-ethylhexyl) hexanedioate | C22H42O4 | 59.43 | 2392 | 41.60 | 0.44 |
| 54 | bis(2-ethylhexyl) benzene-1,2-dicarboxylate [diethylhexyl phthalate] |
C24H38O4 | 60.45 | 2538 | 72.06 | 0.76 |
| 55 | (6E,10E,14E,18E)-2,6,10,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaene [squalene] |
C30H50 | 63.65 | 2814 | 311.29 | 3.28 |
Table 1: Percent relative content of LMWOC in the aqueous ethanolic (40 % v/v) extract of vegetative parts of A. micrantha
| Group of substances | Relative content, % |
|---|---|
| Alcohols | 21.24 |
| Hydrocarbons | 14.45 |
| Esters | 3.21 |
| Ketones | 2.37 |
| Aldehydes | 41.93 |
| Diverse functional groups | 6.29 |
| Aromatic hydrocarbons | 7.78 |
| Nitrogen and sulphur containing compounds | 0.76 |
| Chlorine-containing compounds | 0.13 |
| Carboxylic acids | 0.95 |
| Phenols | 0.15 |
| Unidentified compounds | 0.74 |
Table 2: Percent Relative content of the main groups of substances in the Aqueous Ethanolic extract of Vegetative parts ofA. micrantha
It is interesting to have found some compounds, naphthalene derivatives (compounds 29, 30, 32, 33, Table 1), the share of which is 2.75 % of the total concentration of LMWOC. Among LMWOCs of vegetative parts extract of A. micrantha, three compounds related to phthalates were identified (diisobutyl phthalate, dibutyl phthalate, diethylhexyl phthalate); herewith their total share was quite large (1.77 %). The given group of substances is often considered as pollutants. However, the recent findings have shown that actinomycetes, fungus and plants (terraneous and aquatic ones) are able to synthesize phthalates, which participate in allelopathic interacting and perform protective functions [35-39].
In ethanol extract of A. micrantha truss, 19 compounds were identified (Table 3). Out of the 19 compounds, identified in the truss extract of milfoil Parviflorus, 17 related to terpenes and their derivatives. This fact can be indicative of substantial antibacterial activity of this extract, as well as of vegetative parts extract where the proportion of terpenoids is also big, as the high antibacterial activity is intrinsic for terpenes and their derivatives [40-42].
| No. | Compound | Formula | IK | Relative content, % |
|---|---|---|---|---|
| 1 | 4,6,6-trimethylbicyclo[3.1.1]hept-3-ene [α-pinene] |
С10Н16 | 939 | 2.46 |
| 2 | 3,3-dimethyl-2-methylidenebicyclo[2.2.1]heptane [Camphene] | С10Н16 | 945 | 0.5 |
| 3 | 4-methylidene-1-propan-2-ylbicyclo[3.1.0]hexane [Sabinene] | С10Н16 | 974 | 0.1 |
| 4 | 6,6-dimethyl-4-methylidenebicyclo[3.1.1]heptane [β-pinene] | С10Н16 | 982 | 2.1 |
| 5 | 1-methyl-4-prop-1-en-2-ylcyclohexene [Limonene] |
С10Н16 | 1029 | 1.3 |
| 6 | 2,2,4-trimethyl-3-oxabicyclo[2.2.2]octane [1,8-cineol] |
С10Н18О | 1043 | 8.47 |
| 7 | 1-methyl-4-propan-2-ylcyclohexa-1,4-diene [γ-terpinene] |
С10Н16 | 1079 | 4.9 |
| 8 | 3,7-dimethylocta-1,6-dien-3-ol [Linalool] |
С10Н18О | 1097 | 0.56 |
| 9 | Unidentified | 1132 | 2.93 | |
| 10 | 4,7,7-trimethylbicyclo[2.2.1]heptan-3-one [Camphor] |
С10Н16О | 1156 | 10.62 |
| 11 | 4,7,7-trimethylbicyclo[2.2.1]heptan-3-ol [Borneol] |
С10Н18О | 1169 | 1.37 |
| 12 | 2-(4-methylcyclohex-3-en-1-yl)propan-2-ol [Terpineol] |
С10Н18О | 1174 | 1.53 |
| 13 | (6S)-3-methyl-6-propan-2-ylcyclohex-2-en-1-one [Piperitone] |
С10Н16О | 1224 | 34.15 |
| 14 | (4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl) acetate [Bornyl acetate] |
С12Н20О2 | 1237 | 1.32 |
| 15 | 2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one [Carvone] |
С10Н14О | 1242 | 24.93 |
| 16 | dibutyl benzene-1,2-dicarboxylate [Dibutyl phthalate] |
C16Н22O4 | 1960 | 1.37 |
| 17 | Octadecanoic acid | C18Н36O2 | 2100 | 0.45 |
| 18 | (3S,8S,9S,10R,13R,14S,17R)-17-[(2R,5R)-5-ethyl-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol [β-sitosterol] |
С29Н50О | 3200 | 0.7 |
| 19 | (3S,8S,9S,10R,13R,14S,17R)-17-[(E,2R,5S)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol [Stigmasterol] |
С29Н48О | 3230 | 0.24 |
Table 3: Percent Relative content of LMWOC in the Aqueous Ethanolic (40 % v/v) extract of Inflorescences of A. micrantha
The given chemical compounds, the majority of which refer to the group of biologically active terpenes and their derivatives were extracted with 40 % ethanol from the yarrow blossom trusses, were also the ones most commonly found in essential oils. The offered preparation method of extracting these compounds with the help of ethanol made it possible to obtain a complex of organic substances, which were the constituents of some essential oils with antimicrobial properties.
The complex of the detected compounds in the extracts A. micrantha had a notable inhibitory effect on the test strains studied. As evidenced from the Table 4, the most inhibitory effect on S. aureus was found in the A. micrantha blossom truss extract similar to the effect produced by gentamycin. The extracts of other parts of A. micrantha featured stronger antibacterial effect than the ones of A. millefolium. In this study the extracts of A. micrantha have demonstrated more antibacterial activity towards the strain S. aureus.
| Samples | Diameter of zone of inhibition M ± m. mm | |||
|---|---|---|---|---|
| S. aureus | P. aeruginosa | E. coli | S. еpidermidis | |
| Gentamycin | 42.0 ± 1.4 | 26.0 ± 0.8 | 16.0 ± 0.5 | 33.2 ± 0.3 |
| A. millefolium Bl. truss | 15.2 ± 1.8 | 12.4 ± 1.2 | 10.0 ± 0.4 | 22.8 ± 0.8 |
| A. millefolium leaf | 14.1 ± 1.5 | 11.8 ± 0.9 | 7.0 ± 1.7 | - |
| A. millefolium herb | 13.4 ± 2.3 | 13.0 ± 1.3 | - | - |
| A. micranta Bl. truss | 39.0 ± 0.4 | 21.0 ± 0.8 | 14.0 ± 1.5 | 25.2 ± 1.5 |
| A. micranta leaf | 17.3 ± 1.2 | 16.6 ± 1.4 | 10.0 ± 2.4 | - |
| A. micranta herb | 20.5 ± 1.2 | 19.5 ± 1.0 | 9.7 ± 1.3 | - |
Table 4: The Comparative Antimicrobial Activity of the Plant Extracts by Agar Diffusion Test
Besides, the study of effect of different concentrations (MIC) of the extracts A. micrantha and A. millefolium on the strain S. aureus (CFU counting method) showed more intense inhibiting effect of extract dilution A. micrantha (Table 5). The most active concentrations in MIC proved to be 5.0, 2.5, 0.25 μg/ml. It should be noted, that the antibacterial activity of the concentration of A. micrantha 0.025 μg/ml was 1.8 as high as that of the extract A. millefolium (Table 5). The study of different extract concentrations A. millefolium, A. micrantha and A. leptophylla by means of agar diffusion test also revealed more intense inhibiting effect of the extract A. micrantha in respect of S. aureus (MIC value= 5.0, 0.05 μg/ml; Table 6). As it was detected, the extracts of A. micrantha in all the concentrations studied 5.0, 0.5 and 0.05 μg/ ml showed more antibacterial action than those of A. millefoluim and A. leptophylla. The inhibitory activity of the extract is similar (compared to) to that of gentamycin, and the antibacterial action of the extract with 0.05 concentrations is 2.5 times higher than that of gentamycin (Table 6).
| Concentration of extracts active agents | CFUs. M ± mm | |
|---|---|---|
| A. millefolium | A. micrantha | |
| Control without extract | 158.4 ± 1.3 | 213.2 ± 2.5 |
| 0.25 µg/ml | 50 ± 0.6 | 26.8 ± 0.9 |
| 0.5 µg/ml | 15.8 ± 0.7 | 17 ± 0.9 |
| 2.5 µg/ml | 3.6 ± 0.2 | 8.6 ± 0.5 |
| 5.0 µg/ml | 1.7 ± 0.2 | 4.7 ± 1.03 |
Table 5: Minimum Inhibitory Concentration of the Plant Extracts in Terms of Staphylococcus aureus
| Samples | (M ± mm).mm | ||
|---|---|---|---|
| 5.0 µg/ml | 0.5 µg/ml | 0.05 µg/ml | |
| A. micrantha | 39.0 ± 0.4 | 20.0 ± 0.5 | 25.2 ± 0.8 |
| A. millefolium | 16.5 ± 0.2 | 10.1 ± 0.1 | 0 |
| A. leptophylla | 20.5 ± 1.2 | 0 | 0 |
| Gentamycin | 42.0 ± 1.4 | 22.5 ± 0.1 | 10.0 ± 0.1 |
Table 6: The Comparative Antimicrobial Activity (MIC) of Plant Extracts in Respect of Staphylococcus aureus
As per the results of the study, the detected antimicrobial action of the extract A. micrantha, similar due to its activity to chemical specific antibiotic, is determined by the content of terpenic and phenolic compounds as its major constituents, and other detected in the course of study compounds.
Conflict of interests
None declared.
Financial support and sponsorship
Nil.
References
- Khani A, Asghari J. Insecticide activity of essential oils of Mentha longifolia, Pulicaria gnaphalodes and Achillea wilhelmsii against two stored product pests, the flour beetle, Tribolium castaneum and the cowpea weevil, Callosobruchus maculates. J Insect Sci 2012;12:73.
- Hatam NAR, Yousif NJ, Porzel A, Seifert K. Sesquiterpene lactones from Achillea micrantha. Phytochemistry 1992;21(6):2160-62.
- Souza TM, Rangel VLBI, Pietro Rr RCL, Santos LE, Moreira RRD. Phytochemical screening of Achillea millefolium harvested at Araraquara-Sp. Rev Bras Plantas Med 2006;8:151-54.
- Rustaiyan A, Sharif Z, Tajarodi A, Sadjadi AS. Sesquiterpene lactones from Achillea micrantha. Phytochemistry 1987;26(10):2856-57.
- Mahmoud AA, Al-Shihry SS, Hegazy MEF. A new epimeric sesquiterpene lactone from Achillea liguistica. Rec Nat Prod 2012;6(1):21-7.
- Toncer O, Basbag S, Karaman S, Diraz E, Basbag M. Chemical composition of essential oils of some Achillea species growing wild in Turkey. Int J Agric Biol 2010;12:527-30.
- Asimova SS, Glushenkova AI, Vinogradova VI. Lipids, lipophilic components and essential oils from plant sources. Berlin: Springer; 2012.
- Valant-Vetschera KM. On the identity of five species of Achillea sect. Millefolium subsect Filipendulinae (Compositae, Anthemideae). Willdenowia 1999;29:141-46.
- Benetis R, Radusiene J, Janulis V. Variability of phenolic compounds in flowers of Achillea millefolium wild populations in Lithuania. Medicina (Kaunas) 2008;44(10):775-81.
- Priya KS, Gnanamani A, Radhakrishnan N, Babu M. Healing potential of Datura alba on burn wounds in albino rats. J Ethnopharmacol 2002;83:193-99.
- Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol 2005;100:100-07.
- Saeidina S, Gohari AR, Mokhber-Derfuli N, Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru 2011;19(3):173-86.
- Konyalioglu S, Karamenderes CF. The protective effects of Achillea L. species native in Turkey against H2O2-induced oxidative damage in human erythrocytes and leucocytes. J Ethnopharmacol 2005;102:221-27.
- Turkoglu I, Turkoglu S, Celik S, Kahyaoglu N. Antioxidant and antimicrobial activities of Turkish endemic Achillea species. African J Microbiol Res 2010;4(19):2034-42.
- Vitalini S, Beretta G, Iriti M, Orsenigo S, Basilico N, Dall’Acqua S, et al. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim Pol 2011;58(2):203-9.
- Innocentia G, Vegetob E, Dall-Acqua S, Cianab P, Giorgettia M, Agradib E, et al. In vitro estrogenic activity of Achillea millefolium L. Phytomedicine 2007;14:147-52.
- Niazmand S, Khoshnood E. The effects of Achillea wilhelmsii extract on rat’s gastric motility at basal and vagal stimulated conditions. Iran J Basic Med Sci 2010;14(2):151-57.
- Potrich FB, Allemand A, da Silva LM, dos Santos AC, Baggio CH, Freitas CS, et al. Antiulcerogenic activity of hydroalcoholic extract of Achillea millefolium L. involvement of the antioxidant system. J Ethnopharmacol 2010;130:85-92.
- Lopes FCM, Benzatti FP, Junir CMJ, Moreira RRD, Carlos IZ. Effect of the essential oil of Achillea millefolium L. in the production of hydrogen peroxide and tumor necrosis factor-α in murine macrophages. Rev Bras Cienc Farm 2005;31(3):401-05.
- Babaei M, Abarghoei ME, Akhavan MM, Ansari R, Vafaei AA, Taherian AA, et al. Antimotility effect of hydroalcolic extract of yarrow (Achillea millefolium) on the guinea-pig ileum. Pak J Biol Sci 2010;10(20):3673-77.
- Sharififar F, Pournourmohammadi S, Arabnejad M. Immunomodulatiry activity of aqueous extract of Achillea wilhelmsii C. Kosh. in mice. Indian J Exp Biol 2009;47:668-71.
- Hemmati AA, Arzi A, Adinehvand A, Mostofi NE, Mozaffari AR, Jalali A. Yarrow (Achillea millefolium L.) extract impairs the fibrogenic effect of bleomycin in rat lung. J Med Plants Res 2011;5(10):1843-49.
- Sukhenko LT. Biologically active substances of some plants and the mechanisms of their antimicrobial activity. Nat Sci 2010;3:166-76.
- Magiatis P, Skaltsouis A-L, Haroutounian SA. Chemical composition and in vitro antimicrobial activity of the essential oils of three Greek Achillea species. Z Naturforsch C 2002;57:287-90.
- Kharma A, Hassawi D. The Antimicrobial activity and genetic relationship of Achillea species. Biotechnology 2006;5(4):501-07.
- Baser KHC, Demirci B, Demirci F, Kocak S, Akinci C, Malyer H, et al. Composition and antimicrobial activity of the essential oil of Achillea multifidi. Planta Med 2008;68:941-3.
- Karaalp C, Yurtman AN, Yavasoglu NUK. Evaluation of antimicrobial properties of Achillea L. flower head extracts. Pharm Biol 2009;47(1):86-91.
- Kordali S, Gakir A, Arkin TA, Mete E, Arkin A, Aydin T, et al. Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola Hub-Mor and Achillea biebersteinii Afan. (Asteraceae). Ind Crops Prod 2000;29:562-70.
- Salvagnini LE, Migliato KF, Isaac VLB, Correa MA, Salgado NRN, Pietro RCLR. Evaluation of efficacy of preservatives associated with Achillea millefolium L. extract against Bacillus subtilis. Braz J Microbiol 2006;37:75-7.
- Lakshimi T, Geetha RV, Roy A, Kumar SA. Yarrow (Achillea millefolium Linn.) a herbal medicanl plant with broad therapeutic use – a review. Int J Pharm Sci Rev Res 2011;9(2):136-41.
- Fall R, Karl T, Hansel A, Jordan A, Lindinger W. Volatile organic compounds emitted after leaf wounding: On-line analysis by proton-transfer-reaction mass spectrometry. J Geophys Res 1999;104(13):15963-74.
- Arimura G, Matsui K, Takabayashi J. Chemical and molecular ecology of herbivore-induced plant volatiles: proximate factors and their ultimate functions. Plant Cell Physiol 2009;50(5):911-23.
- Jüttner F, Messina P, Patalano C, Zupo V. Odour compounds of the diatom Cocconeis scutellum: effects on benthic herbivores living on Posidonia oceanica. Mar Ecol Prog Ser 2010;400:63-73.
- Lanciotti R, Belletti N, Patrignani F, Gianotti A, Gardini F, Guerzoni ME. Application of hexanal, (E)-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J Agric Food Chem 2003;51:2958-63.
- Xuan TD, Chung M, Khanh TD, Tawata S. Identification of Phytotoxic Substances from Early Growth of Barnyard Grass (Echinochloa crusgalli) Root Exudates. J Chem Ecol 2006;32:895-906.
- Roy RN, Laskar S, Sen SK. Dibutyl phthalate, the bioactive compound produced by Streptomyces albidoflavus 321.2. Microbiol Res 2006;161(2):121-26.
- Qiming X, Haidong C, Huixian Z, Daqiang Y. Chemical composition of essential oils of two submerged macrophytes, Ceratophyllum demersum L. and Vallisneria spiralis L. Flavour Fragr J 2006;21:524-26.
- Qiming X, Haidong C, Huixian Z, Daqiang Y. Allelopathic activity of volatile substance from submerged macrophytes on Microcystin aeruginosa. Acta Ecologica Sinica 2006;26(11):3549-54.
- Kurashov EA, Krylova JV, Mitrukova GG, Chernova AM. Low-molecular-weight metabolites of aquatic macrophytes growing on the territory of Russia and their role in hydroecosystems. Contemp Probl Ecol 2014;7(4):433-48.
- Solís C, Becerra J, Flores C, Robledo J, Silva M. Antibacterial and antifungal terpenes from Pilgerodendron uviferum (D. Don) Florin. J Chil Chem Soc 2004;49(2):157-61.
- Togashi N, Inoue Y, Hamashima H, Takano A. Effects of two terpene alcohols on the antibacterial activity and the mode of action of farnesol against Staphylococcus aureus. Molecules 2008;13:3069-76.
- Gupta N, Saxena G, Kalra SS. Antimicrobial activity pattern of certain terpenoids. Int J Pharm Biol Sci 2011;2(1):87-91.