- *Corresponding Author:
- Aruna Jadhav
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, CBD Belapur, Navi Mumbai, Maharashtra 400614, India
E-mail: aruna.jadhav@bvcop.in
| Date of Received | 23 July 2021 |
| Date of Revision | 09 March 2022 |
| Date of Acceptance | 07 December 2022 |
| Indian J Pharm Sci 2022;84(6):1588-1592 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In the present study, an attempt has been made to develop a high-performance thin layer chromatography fingerprinting of gallic acid, piperine and curcumin from an Ayurvedic formulation, Dekofcyn tablet. Chromatographic separation was achieved on the plate pre-coated with silica gel 60F254 as the stationary phase. The solvent system consisted of toluene:ethyl acetate:formic acid:glacial acetic acid in the ratio (5:3.5:1:1 v/v/v/v) respectively. The retardation factor value was calculated at 0.35 (±0.02), 0.66 (±0.02) and 0.89 (±0.02) for gallic acid, piperine and curcumin respectively. Densitometric analysis was carried out in the isoabsorptive mode at 295 nm. The regression analysis was carried out in the range of 100-700 ng/spot for gallic acid and piperine, 400-1600 ng/spot for curcumin and the regression coefficient for all three markers was found to be 0.99 respectively. Validation of the method was performed according to international conference on hominization guidelines Q2 (R1). This method can also be used to evaluate other formulations containing these markers as one of the constituents, thus conforming to the need for ensuring the quality of an Ayurvedic formulation.
Keywords
High performance thin layer chromatography, fingerprinting, gallic acid, piperine, curcumin, quantification, validation
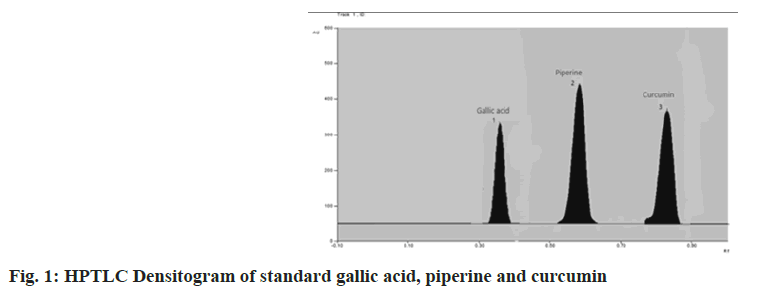
Standardization of herbal formulation is a crucial aspect for maintaining and assessing the standard and safety of the polyherbal formulation as these are combinations of quite one herb to attain the desired therapeutic effect. It decreases batch to batch variation, assures safety, efficacy, quality and acceptability of the polyherbal formulations[1,2]. Thin Layer Chromatography (TLC) and High Performance Thin Layer Chromatography (HPTLC) fingerprint profiles were used to determine the identity, purity and strength of the polyherbal formulation and also for fixing standards for this Ayurvedic formulation. HPTLC fingerprint is principally used to study low or moderate polarity compounds. This technique is extensively used in the pharmaceutical industry in process development, identification and detection of adulterants in herbal product[3,4]. In this work, standardization of marketed polyherbal formulation, i.e. Dekofcyn tablet was carried out. It consists of haldi (Curcuma longa), talispatra (Taxus buccata), shatavari (Asparagus racemose), piper (Piper longum), ashwagandha (Withania somnifera), amla (Emblica officinalis), kachura (Curcuma zedoria), vasaka (Adhatoda vasica), galo ghan (Tinospora cordifolia), dagdi pashanbhed (Bergenia ciliate), Suvarna mashik bhasma (A.F.I.-193)10 Puta, Abhrak bhasma (S.Y.S.-159)42 Puta, laghu malinivasanta Rasa (A.F.I.-271)[5]. It is used in the treatment of chronic cough, cold, sore throat and chronic oral allergies. Gallic acid is one of the constituents of amla. Therapeutically it is used as an antioxidant, anti-cancer, anti- inflammatory and anti-diabetics[6,7]. Scavenging of superoxide anions and inhibition of myeloperoxidase release activity, as well as possible interference with the assembly of active Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-oxidase, may account for the inhibition of the inflammatory process by gallic acid. Structure-activity relationship analysis showed that the ortho-dihydroxy group of gallic acid is important for the inhibitory effect[8]. Curcumin is an active constituent of haldi, it possesses anti-inflammatory, anti-bacterial, anti-allergic and anti-cancer activities. A possible scenario by which curcumin exerts anti-inflammatory effect is by binding to its receptor and the ligand-receptor interaction activates signaling pathways leading to the up-regulation of Peroxisome Proliferator- Activated Receptor Gamma (PPAR-γ) and the subsequent suppression of inflammatory cytokine release[9,10]. Piperine is an active constituent of a piper; it possesses nervous system depressant, anti-inflammatory, antipyretic, analgesic, antioxidant and bioenhancer properties. Piperine enhances absorption from the gastrointestinal tract by various mechanisms and reduces the gut metabolism of drugs. Piperine enhances the bioavailability of curcumin and gallic acid by inhibition of cytochrome p450 enzymes. Due to these properties, the piper is one of the ingredients of many Ayurvedic formulations[11,12]. A literature survey showed that gallic acid, curcumin and piperine were found to possess anti-inflammatory, anti-allergic and anti-oxidant properties which contribute to the therapeutic effect expected in the formulation. Therefore, in the present work gallic acid, curcumin and piperine were selected as markers for HPTLC to standardize Dekofcyn tablets. Validation of the developed HPTLC method was carried out according to International Conference on Harmonization (ICH) guideline Q2 (R1) for linearity, accuracy, precision, repeatability, specificity, sensitivity and robustness[13]. Standard gallic acid, piperine and curcumin were procured from Yucca Enterprises Mumbai, Maharashtra, India. Analytical grades reagents toluene, ethyl acetate, acetic acid and formic acid were purchased from SD Fine Chem. Ltd., Mumbai, Maharashtra, India. The Marketed formulation of Dekofcyn tablet has been procured from the pharmacy at Navi Mumbai, Maharashtra, India. The HPTLC system consists of Camag Linomat V sample applicator equipped with 100 µl Hamilton syringe. Merck TLC aluminium plates (10×10 cm) pre-coated with silica gel 60 F254 was used as a stationary phase. Standard solutions of markers and samples were applied as a band by use of a Camag Linomat V sample applicator from the bottom edge of the chromatographic plate. Camag glass twin-trough chamber was used for the ascending development of the plate, previously saturated for 15 min at room temperature. The suitable wavelength for the HPTLC analysis was determined using Ultraviolet (UV) spectrophotometer by recording UV Spectrum in the range of 200-400 nm for individual drug solution of gallic acid, piperine and curcumin then overlaid the spectra. An overlain spectrum of these three markers shows the isoabsorptive wavelength at 295 nm. The analysis was carried out utilizing Camag TLC scanner-3 with Camag Win CATS (V 1.44 CAMAG) software having scanning speed of 20 mm/s and the slit dimension was kept at 5 mm×0.45 mm. After completion of scanning the Retardation factor (Rf) values, peak areas and spectra for all the markers were recorded. The stock solution of each marker was prepared by dissolving 25 mg of each marker in small quantity of methanol and volume was made up to 25 ml to obtain 1000 ppm stock solutions. Working solutions were prepared from standard stock solutions of gallic acid, piperine and curcumin by withdrawing aliquot of 1 ml from stock solutions of each marker compound and transferred in two separate volumetric flasks of 10 ml. The volume was made up with methanol to obtain solutions of 100 μg/ml. Solutions for calibration curve were prepared such that application of 15 μl volume gave a series spots covering over a range of 100- 700 ng/spot (100, 200, 300, 400, 500, 600, 700 ng/ spot) for gallic acid and piperine and 400-1600 ng/ spot (400, 600, 800, 1000, 1200, 1400, 1600 ng/ spot) for curcumin. These ranges were used for the construction of a calibration curve. 12 tablets were triturated and 5 g powder was accurately weighed. The powder was macerated using 25 ml of methanol for 1 h and filtered through Whatman filter paper no.41 and this procedure is repeated using fresh methanol. Both filtrates were combined and volume was made up to 50 ml with methanol, this solution was then further used for quantification. The linearity of an analytes is determined by plotting a graph of the area against the concentration of analytes and the test results were evaluated by calculating the linear regression coefficient (r2). The standard stock solution was diluted to obtain the range of 100–700 ng/spot solutions of gallic acid and piperine each, 400-1600 ng/spot for curcumin. Three sets of such solutions were evaluated. Every set was analyzed to obtain a calibration curve. The Standard Deviation (SD), coefficient of determination (r2), slope and intercept of the calibration curves were estimated to determine the method linearity. Recovery of gallic acid, piperine and curcumin from formulation was checked by spiking a known quantity of standards at three concentration levels (i.e. 80 %, 100 % and 120 % of the quantified amount) to the test samples in triplicate using HPTLC. This way recovery was calculated for nine determinations over a specified range and mean recovery was calculated. Determination of repeatability and intermediate precision was carried out by measuring intraday and inter-day variations in triplicate. Determination of gallic acid, piperine and curcumin simultaneously for three different concentration levels 100, 400, 700 ng/spot for gallic acid and piperine each, 400, 1000, 1600 ng/spot for curcumin was performed, respectively and the results were expressed as percent Relative Standard Deviation (% RSD). The system precision was carried out with 6 replicates of the same concentration of 400 ng/ spot for gallic acid and piperine each, 1000 ng/ spot for curcumin and % RSD was calculated. The quantitation limit could also be a parameter of quantitative assays for low levels of compounds in sample matrices and is used particularly for the determination of impurities or degradation of the material and is expressed as Limit of Detection (LOD)=3.3 σ/S and Limit of Quantification (LOQ)=10 σ/S where, σ=SD of response, S=Slope of the calibration curve which are obtained from the calibration curve of the individual maker compound. The amount for which signal to noise ratio are determined is 3 and 10 for LOD and LOQ respectively. Robustness of the method was studied in triplicate at 100 ng/spot and 700 ng/spot for gallic acid and piperine each, 400 ng/spot and 1600 ng/spot for curcumin, by deliberately making small changes such as chamber saturation time variations (15±5 min) and variation in mobile phase composition (5.5:3:0.5:1 v/v/v/v) and (4:2.5:0.5:0.5 v/v/v/v). The specificity of the method was performed by applying standard and test solutions and by confirming their Rf values and differentiating standards from other closely related compounds, showing that the method was specific. The peak purity was evaluated by comparing the spectrum of standards with the sample. The quantification of gallic acid, piperine and curcumin present in the Dekofcyn tablet was calculated using linear regression analysis. Quantification of markers was done by performing HPTLC analysis of test solutions i.e. 100 μg/ml concentrations extract according to the developed method. The area obtained for each marker from formulation extract was extrapolated on the respective calibration curve of that marker. Every analysis was performed in triplicate. In this study, the HPTLC method was developed for the simultaneous quantification of gallic acid, piperine and curcumin in marketed formulation. For the development of the mobile phase, several trials were made using many solvents in different proportions by the linear ascending development method. The solvent system used was toluene:ethyl acetate:formic acid:glacial acetic acid in the ratio (5:3.5:1:1 v/v/v/v) showed good separation and resolution of the peaks without interference from other compounds present in extracts at Rf 0.35±0.02 for gallic acid, 0.66±0.02 for piperine and 0.89±0.02 for curcumin as shown in fig. 1. Linear responses for the markers gallic acid, piperine and curcumin were obtained at 100-700 ng/spot, 100- 700 ng/spot and 400-1600 ng/spot, respectively. Linearity was validated by interpreting the regression line and test by calculating the r2 as shown in Table 1. The % RSD of intraday precision and inter-day precision of gallic acid, piperine and curcumin was found to be less than 2 %, which was within the limits of acceptance as shown in Table 2. The system precision results showed that the repeatability of measurement of the area is precise with % RSD; 0.33 % for gallic acid, 1.17 % for piperine and 0.55 % for curcumin since their coefficient of variance is less than 2.0 % it is possible to conclude that the instrument has good precision (n=6). The recovery was found to be 100, 99.50 and 99.76 % for gallic acid, piperine and curcumin, respectively as shown in Table 3. The LOD and LOQ were found to be 13.297 ng/ spot, 40.296 ng/spot for gallic acid, 23.351 ng/ spot, 70.763 ng/spot for piperine and 35.321 ng/ spot, 107.036 ng/spot for curcumin, respectively as shown in Table 1. Two concentrations were spotted for each of the markers in triplicate. 100 ng/spot and 700 ng/spot of gallic acid and piperine each, 400 ng/spot and 1600 ng/spot for curcumin were the concentrations selected for analysis. Table 1 summarizes the results obtained in robustness. It shows that the % RSD value for all the results is less than 2 %. Hence, the method was found to be robust. Specificity was confirmed by comparing the Rf value of marker with that of the component obtained in the chromatogram of extract. Furthermore, a UV spectrum of each marker was overlaid on the UV spectra of components obtained from the extract of the Dekofcyn tablet. It was observed that other constituents in the extract did not interfere with peaks of Gallic acid, piperine and curcumin. Therefore, the method was found to be specific. The quantification of gallic acid, piperine and curcumin from Dekofcyn tablet extract was carried out in triplicate. The total amount of gallic acid, piperine and curcumin were found to be 0.27 % w/w, 0.16 % w/w, 0.65 % w/w, respectively. The summary of all validated parameters is shown in Table 1. Thus, above results revealed that the developed method enables rapid, precise, sensitive and highly accurate simultaneous estimation of gallic acid, piperine and curcumin. The developed method was validated consistent with ICH guidelines. This method can also be applied for the evaluation of these phytoconstituent from other Ayurvedic formulations. Thus, it also ensures the need for quality of an Ayurvedic formulation.
| Parameters | Gallic acid | Piperine | Curcumin |
|---|---|---|---|
| Rf | 0.35±0.02 | 0.66±0.02 | 0.89±0.02 |
| Linearity range (ng/spot) | 100-700 | 100-700 | 400-1600 |
| Correlation coefficient (r2) | 0.9989±0.0022 | 0.9982±0.0022 | 0.9989±0.0022 |
| Regression equation | Y=7.9961x-15.571 | Y=3.9909+1049.8 | Y=9.6751+78.831 |
| LOD (ng/spot) | 13.297 | 23.351 | 35.321 |
| LOQ (ng/spot) | 40.296 | 70.763 | 107.036 |
| Percent recovery (n=3) | 100 % | 99.50 % | 99.76 % |
| Precision (% RSD) | Precise | Precise | Precise |
| Robustness | Robust | Robust | Robust |
| Specificity | Specific | Specific | Specific |
Note: n=3 represents each, is an average of three observations
Table 1: Results of Method Validation Studies
| Compound | Concentration (ng/spot) | Intra-day | Inter-day | ||||
|---|---|---|---|---|---|---|---|
| Mean area (AU) | SD | % RSD | Mean area (AU) | SD | % RSD | ||
| Gallic acid | 100 | 829 | 2.44949 | 0.29 | 833 | 2.94362 | 0.35 |
| 400 | 3164 | 16.5730 | 0.52 | 3162 | 29.4089 | 0.92 | |
| 700 | 5658 | 25.4602 | 0.44 | 5649 | 28.9137 | 0.51 | |
| Piperine | 100 | 754.16 | 12.3902 | 1.64 | 750.59 | 2.7581 | 0.23 |
| 400 | 3161.62 | 26.1594 | 0.82 | 312.92 | 22.4600 | 0.71 | |
| 700 | 5561.47 | 24.5420 | 0.44 | 5550.58 | 12.0851 | 0.29 | |
| Curcumin | 400 | 3971.35 | 18.8692 | 0.47 | 3967.19 | 73.4281 | 1.85 |
| 1000 | 9754.25 | 25.0343 | 0.25 | 9156.71 | 318.616 | 1.32 | |
| 1600 | 15417.6 | 108.421 | 0.70 | 15456.3 | 658.601 | 1.42 | |
Note: Each result is an average of three observations. Concentration levels used for precision parameter was 100, 400, 700 ng/spot for gallic acid and piperine and 400, 1000, 1600 ng/spot for curcumin
Table 2: Intraday and Interday Precision Results for Gallic Acid, Piperine and Curcumin
| Drug | % Level | Amount of marker added (ng) | Total amount (ng) | Amount of drug recovered (ng) | % Recovery | Average recovery (%) |
|---|---|---|---|---|---|---|
| Gallic acid | 80 | 649 | 1416 | 1418 | 100.14 | 100 |
| 100 | 812 | 1624 | 1625 | 100 | ||
| 120 | 974 | 1786 | 1783 | 100 | ||
| Piperine | 80 | 391.38 | 881.06 | 872.12 | 98.9 | 99.5 |
| 100 | 489.68 | 976.36 | 979.64 | 100.02 | ||
| 120 | 587.61 | 1077.29 | 1075.35 | 99.81 | ||
| Curcumin | 80 | 1812.41 | 1812.41 | 4077.93 | 99.45 | 99.76 |
| 100 | 2265.52 | 2265.52 | 4531.04 | 100.2 | ||
| 120 | 2718.62 | 2718.62 | 4984.14 | 99.81 |
Note: Each result is an average of three observations performed at 80, 100 and 120 % levels
Table 3: Recovery Study of Gallic Acid, Piperine and Curcumin
Conflict of interests:
The authors declared no conflict of interests.
References
- Sharma AK, Gaurav SS, Balkrishna A. A rapid and simple scheme for the standardization of polyherbal drugs. Int J Green Pharm 2009;3(2):134-40.
- Ahmad I, Aqil F, Owais M. Turning medicinal plants into drugs. Mod Phytomed 2006;384:67-72.
- Pattanaya P, Jena RK, Panda SK. HPTLC fingerprinting in the standardization of Sulaharan yoga: An ayurvedic tablet formulation. Int J Pharm Sci Rev Res 2010;3(2):33-6.
- Soni K, Naved T. HPTLC-Its applications in herbal drug industry. Pharm Rev 2010;4:112-7.
- Dekofcyn tablets-benefits, dosage, ingredients, side effects. Ayurmedinfo; 2018.
- Entessar HA, Al-Mosawe AS, Al-Saadi I. The Extraction and purification of gallic acid from the pomegranate rind. Al-Mustanisinyah J Sci 2012;23:184-92.
- Sawant L, Pandita N, Prabhakar B. Determination of gallic acid in Phyllanthus emblica Linn. dried fruit powder by HPTLC. J Pharm Bioallied Sci 2010;2(2):105-8.
[Crossref] [Google Scholar] [PubMed]
- Kroes BV, Van den Berg AJ, Van Ufford HQ, Van Dijk H, Labadie RP. Anti-inflammatory activity of gallic acid. Planta Med 1992;58(6):499-504.
[Crossref] [Google Scholar] [PubMed]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: A component of tumeric (Curcuma longa). J Alt Complement Med 2003;9(1):161-8.
[Crossref] [Google Scholar] [PubMed]
- Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-γ activation. PPAR Res 2008;2007:89369.
[Crossref] [Google Scholar] [PubMed]
- Shaikh SU, Jain VA. Development and validation of a RP-HPLC method for the simultaneous determination of curcumin, piperine and camphor in an ayurvedic formulation. Int J Pharm Pharm Sci 2018;10:115-21.
- Singh A, Deep A. Piperine: A bioenhancer. Int J Pharm Res Technol 2011;1(1):1-5.
- ICH, Q2 (R1), International Conference on Harmonization of Technical Requirement for Registration of Pharmaceuticals for Human use. Validation of Analytical Procedure: Text and Methodology; 2005.