- *Corresponding Author:
- Hongyan Zhao
Department of Gastroenterology, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang 150000, China
E-mail: drzhaohongyan123@163.com
| This article was originally published in a special issue, “Emerging Therapeutic Interventions of Biopharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(3) Spl Issue “45-53” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Cancer of the stomach is one of the most frequent forms of cancer in adults. The treatment for gastric cancer with cisplatin brought a serious side effect of drug resistance. Cinobufagin could reverse the side effect still remains unknown. The object of the current research was to examine the role of cinobufagin in reversing the resistance of cisplatin in treatment of gastric cancer. Drug screening was used to isolate the cisplatin-resistant SGC7901-DDP cell line. The pathway variation in SGC7901-DDP was assayed by Western blot. Then, the cell line SGC7901-DDP was subjected to hypoxia treatment. The cell viability and apoptosis proportion was examined in hypoxia treatment. In addition, the underlying mechanism was explored by probing the protein kinase B/mammalian target of rapamycin signal pathway. Furthermore, the antagonistic action of cinobufagin on the cell line SGC7901-DDP was examined by three different treatments on SDC7901-DDP cell line. After treatments, the cell viability, the apoptosis proportion and protein kinase B/mammalian target of rapamycin signal pathway was assayed respectively by cell counting kit-8, flow cytometer and Western blot. The results indicated that the cells in the hypoxia+cinobufagin group were less likely to survive than cells in the other three groups. In addition, the western operation illustrated that the protein kinase B/mammalian target of rapamycin pathway and hypoxia-inducible factor-alpha factor was inhibited in cinobufagin+hypoxia group compared to that in hypoxia group. The results indicated that cinobufagin could decrease the cell viability, increase the apoptosis proportion and reverse the cisplatin resistance in SGC7901-DDP cell line by protein kinase B/mammalian target of rapamycin signal pathway. Lastly, the in vitro assay was completed and the result demonstrated that cinobufagin could inhibit the lump growth and reverse the cisplatin resistance. The cinobufagin could reverse the cisplatin resistance for gastric cancer therapy through protein kinase B/mammalian target of rapamycin signal pathway.
Keywords
Gastric cancer, cinobufagin, cisplatin, protein kinase B/mammalian target of rapamycin signal pathway, hypoxia
One of the most common and dangerous cancers in the world is Gastric Cancer (GC), adds a lot to the global cancer load[1, 2]. The absence of sufficient sensitive and specific biomarkers that would allow for early diagnosis of this malignancy contributes to the tragically high mortality rate of 75 % among the approximately 990 000 people diagnosed with GC each year[3]. The current standard of care for stomach cancer results in a low median overall survival rate. Therefore, the key to improving the treatment impact of stomach cancer is ongoing research and development of novel targeted medications and techniques. Research into novel medications that target faulty signaling pathways in GC has been a major focus in the medical community over the last several decades[4].
Bufonis venenum (Chansu) a Latin scientific name is Bufo melanostictus or Bufo bufo venom is a kind of traditional Chinese medicine that involves extracting the white fluid from the auricle and the skin glands of the Bufo gargarizans Cantor or Bufo melanostictus Schneider and drying it[5]. Many studies have shown that the Chansu injection can fight different types of cancer in humans, including lung cancer, stomach cancer, liver cancer, pancreatic cancer and gallbladder cancer. This is true whether it is used by itself or with other chemotherapeutic drugs[6]. Cinobufagin is one kind of cardiotonic steroid isolated from Chansu and it inhibits the growth of breast cancer, osteosarcoma and colorectal cancer by persuading apoptosis. Furthermore, it can turn colon cancer cells that are resistant to multiple drugs around[7].
Cisplatin is a very good anticancer drug that is used to treat many types of cancer, such as ovarian, endometrial, lung, bladder, head and neck, testicular, breast and colon cancer. The recognition of the drug’s efficacy in treating cancer is linked to its ability to induce full or partial remission or to stabilize the progression of the illness. Nevertheless, its primary drawback is in the adverse consequences linked to its toxicity, including nephrotoxicity, neurotoxicity, ototoxicity and several others[8]. Cisplatin therapy’s main downside is that it does not kill cancer cells. Resistance of cisplatin varies on forms of cancer. Both innate and learned resistances play a role in protecting organisms from harmful substances. When a medicine is initially effective but loses that status over time due to acquired resistance, we say that the resistance was there from the outset of therapy. It is possible for cells to become resistant to cisplatin via modifying their drug uptake, influx and efflux drug detoxification by cellular thiols, drug target and Deoxyribonucleic Acid (DNA) repair mechanisms[9].
The drug resistance of platinum-based systemic chemotherapy becomes an essential problem that bounds the perfect therapeutic effects in cancer treatments[10]. Numerous things, such as changed drug sites and better DNA repair pathways, can lead to drug tolerance and so on. Hypoxia, a common phenomenon occurred in most malignant tumors is involved in drug resistance and tumorigenesis[2]. Hypoxia in the tumor is a feature of almost all types of cancer and is strongly linked to how deadly the tumor is[11]. Drug resistance is one of several hypoxia-induced phenotypic alterations thought to be regulated by Hypoxia-Inducible Factor-1 (HIF-1)[12].
However, the precise protective mechanisms Aminolevulinic Acid (ALA) employs downstream of Protein Kinase B (AKT) are yet unknown. It has been established that Akt activation and downstream pathways drive the bulk of growth factor-induced responses in circulating endothelial cells. Downstream of Akt is a called mammalian Target of Rapamycin (mTOR), which is a master regulator of protein synthesis and cell development. Akt may activate mTOR indirectly[13]. There are several parts of the signaling pathway that are controlled by mTOR that affect the appearance and biology of tumors and tumor-like lesions in bone and soft tissue. These include proteins Akt, p70S6-Kinase (S6K), and Eukaryotic initiation factor 4 Ebinding Protein 1 (4E-BP1). Together, these proteins are called mTOR cassette proteins[14]. In order to investigate the mechanism under the drug resistance on the GC, the cis-platinum resistant cell line SGC7901 was selected and used to explore the role of hypoxia in the platinum resistant. The problem of conflict by Bufonis venenum was also examined in gastric cell line SGC7901-DDP. Research like these might pave the way for a new standard for dealing with cis-platinum resistant GC.
Materials and Methods
Reagents:
Corning Incorporated, Shanghai China, supplied Dulbecco's Modified Eagle Medium (DMEM)/F12 medium and the Fetal Bovine Serum (FBS) got from the Tianhang Biotechnology Co., Ltd., (Hangzhou, China). Penicillin/streptomycin and trypsin solution were supplied by beyotime Biotechnology (Haimen, China). The SGC7901 and cis-platinum resistant cell line SGC7901 (SGC7901-DDP) were purchased from Fenghuishengwu Inc. The following antibodies were used; rabbit HIF-α, rabbit AKT, rabbit p-AKT, rabbit mTOR, rabbit p-mTOR, rabbit Glycogen Synthase Kinase-3 Beta (p-GSK3Β), rabbit Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) and rabbit Proline-Rich Akt Substrate of 40 kDa (PRAS40). These antibodies were all obtained from Cell Signal Technology (CST) Shanghai Yuanye Bio-Technology Co., Ltd., supplied the bufagin and cisplatinum.
Cell culture:
The human GC cell lines SGC7901 and SGC7901-DDP were maintained at 37°. 5 % Carbon dioxide (CO2) in complete medium that consisted of DMEM/F12, 10 % FBS, 5 % L-glutamine and 100 U/ml penicillin-streptomycin (Life Technologies, New York, United States of America (USA)). The medium was changed every 3 d. A 1:3 ratio was used for cell sub culturing when the cells reached 80 %-90 % convergence. In order to avoid mycoplasma contamination from affecting the experiment, the mycoplasma contamination test was conducted and the report indicated that the cell was free of mycoplasma. In addition, the Short Tandem Repeat (STR) analysis was also performed for SGC7901 cell line. A sample SGC7901, submitted to Fenghui Biotechnology, shanghai, for STR (cell line) testing. The sample, a cell line with a quantity of one, underwent DNA extraction using Axygen’s genome extraction kit followed by amplification with the 21-STR amplification protocol. Analysis was performed on the ABI 3730XL genetic analyzer for STR loci and the sex gene Amelogenin. Table 1 shows the basic condition for the genotype sample test.
| Company number | Multiple alleles | Matching cell lines | Cell bank | EV value | Match description |
|---|---|---|---|---|---|
| 20210927-01 | Have | SGC-7901 | EXPASY | 1 | Exact Match |
Table 1: Basic condition for genotype test.
Test results for sample 20210927-01 revealed a perfect match for its DNA typing in the cell line database. Searching with EXPASY, the corresponding cell line was identified as SGC-7901, known as CVCL_0520 best. Notably, this cell line exhibits multiple alleles. Multi-allelic refers to the phenomenon of genes with three or more alleles. The results of cell typing in this test were good.
Cell viability assay:
100 μl cell postponement was seeded in each well of 96-well plate and the culture plate was placed in an incubator for 36 h under the condition of 37° and 5 % CO2. Then 10 μl Cell Counting Kit-8 (CCK-8) solutions were added to each well. After 4 h incubation for the culture plate, 450 nm wavelength was selected and used to assay the cell feasibility. Three replications of the experiment were conducted.
Cell apoptosis assay:
When the cell staged in the logarithmic stage, they were digested with trypsin without Ethylenediaminetetraacetic Acid (EDTA), centrifuged at 300 g for 5 min and washed twice with precooled Phosphate Buffer Solution (PBS) three times. After PBS was discarded, 100 μl 1×binding buffer was added to the cell suspension. In addition, 5 μl Annexin V-Fluorescein Isothiocyanate (FITC) and 10 μl Propidium Iodide (PI) staining solutions were together added in the cell suspension and mix gently. After that, the mixtures were left to react at room temperature for 15 min, shielded from light. Afterwards, 400 μl of 1×binding buffer was added in every tube, mixed well and then set on ice. The next step was to use flow cytometry on these samples.
Cell treatment:
In the cisplatinum resistance experiment, the cell line SGC7901-DDP and SGC7901 were cultured in 1640 medium. They were separately treated with different dose of cisplatinum at 0 µM, 1 µM, 10 µM, 50 µM, 100 µM and 1000 µM. After 36 h incubation, the cell viability was assayed. Then the cell viability Inhibition Rate (IC) was calculated.
IC=1×100 %-(experimental group absorbance value/control group absorbance value)×100 %
The drug resistance index was obtained with Half-Maximal Inhibitory Concentration (IC50) and the formula were as follows; drug Resistance Index (RI)=IC50 (SGC7901/DDP)/IC50 (SGC7901). In the pathway activation assay between SGC7901-DDP and SGC7901 cell lines, the cell SGC7901-DDP was treated with 3.3 µM cisplatinum additionally. Then the protein was extracted and subjected to signal pathway western analysis. During the hypoxia treatment, the cell line SGC7901-DDP cell was cultured and split into four groups including the hypoxia group, control group, control+cinobufagin group and hypoxia+cinobufagin group. The dose for cinobufagin was at 100 nM. All cells in these treatments were subjected to cell viability assay, cell apoptosis assay and signal pathway analysis. In the combined treatment experiments, test subjects were randomly assigned to one of three group’s hypoxia, hypoxia+cisplatinum and hypoxia+cisplatinum+cinobufagin. The concentration for cisplatinum and cinobufagin were at 3.3 µM and 100 nM. All these treatments were subjected to cell viability assay, cell apoptosis analysis and signal pathway analysis by Western blot.
Western blot:
The cold Radio-Immunoprecipitation Assay (RIPA) lysis solution were directly placed in the cell culture dish for cell lysis or used for tissue homogenate buffer. Then the concentration of protein was measured by Bicinchoninic Acid Assay (BCA) method. The immunoblotting step as follows; the samples were performed with Sodium Dodecyl Sulphate (SDS)-page electrophoresis and the protein was transferred to nitrocellulose membrane (Whatman™) by blot apparatus rendering to company’s guidelines (Bio-Rad). A primary antibody was then added to the membranes and left to incubate overnight at 37°. After that, Horseradish Peroxidase (HRP)-conjugated goat anti-rabbit antibody (1:500) was added to the membrane and left to sit for a while. Lastly, the membranes were placed in Enhanced Chemiluminescence (ECL) immunoblotting detection reagents (Boster Incorporated) based on manufacturer's specifications. To control for equal protein loading, membranes were also probed for GAPDH and gray values were normalized to GAPDH.
Xenograft tumor study in nude mice:
The male, 4 w old nude mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. The average weight of each mouse is about 15 gm. The total number of nude mice was sixteen and they were randomly divided into two groups. The animals were kept in accordance with the standards set out by the American Association for the Accreditation of Laboratory Animal Care (AAALC). All operations of animal experiments were conducted according to the protocols authorized by the Harbin Medical University. These experiments were approved by the Animal Ethics Committee of Harbin Medical University (No: 2022-DWSYLLCZ-76). Injecting SGC7901-DDP cells subcutaneously at the nape of the neck was done using 1 ml syringe. The mice were observed after 1 w and the cinobufagin and cisplatinum was injected subcutaneously around the tumor. The dose was 0.08 mg cisplatinum for each mouse/time. In addition, the cinobufagin were 0.04 µg for each mouse at each time, except the cisplatinum with the same dose in the group. The volume of tumor was determined by the measurement of the major axis and minor axis. After 3 w feeding, three mice died in each group and these nude mice were killed by cervical dislocation. Therefore, the result displayed five tumor lump. Following tumor recovery, a little section of each tumor’s tissue was flash-frozen. An extra fraction of every tumor was hydrolyzed by the RIPA adjuvant. Then the corresponding protein was measured by the Western blot.
Results and Discussion
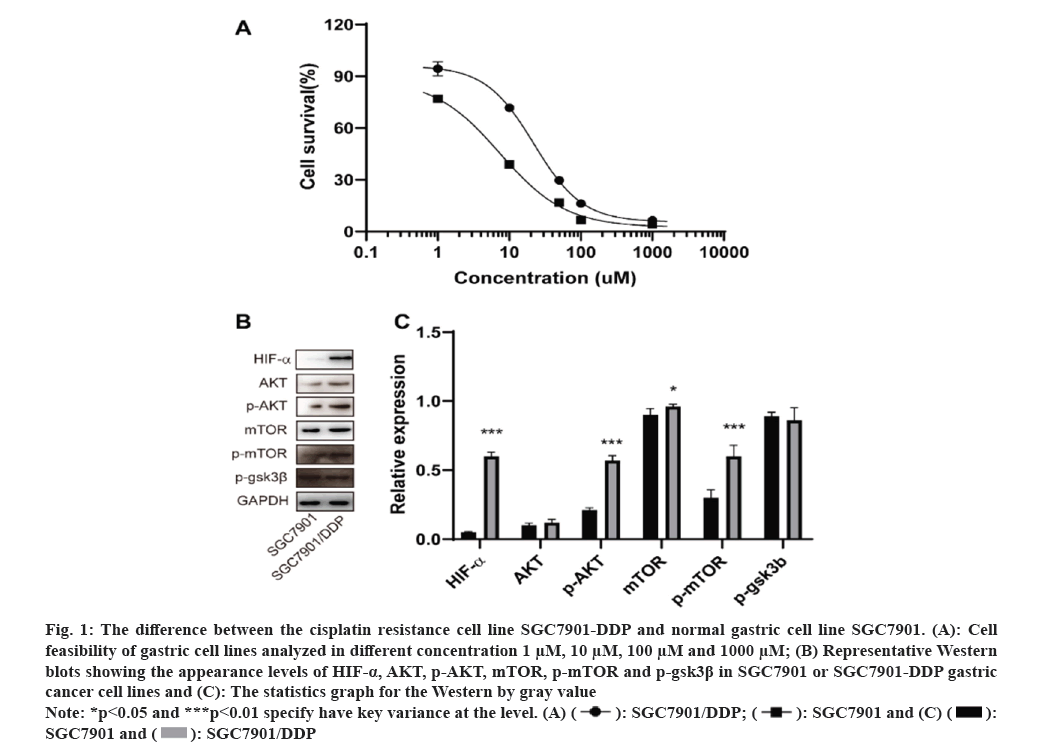
In order to verify the drug resistance of stomach cancer cell line SGC7901-DDP, the cell viability by CCK-8 kit was detected. The results shown that the drug RI of SGC7901-DDP cells to DDP was 1.55, >1.5. It displayed that SGC7901-DDP cells of GC were successfully constructed, as shown in fig. 1A and Table 2.
Fig. 1: The difference between the cisplatin resistance cell line SGC7901-DDP and normal gastric cell line SGC7901. (A): Cell
feasibility of gastric cell lines analyzed in different concentration 1 μM, 10 μM, 100 μM and 1000 μM; (B) Representative Western
blots showing the appearance levels of HIF-α, AKT, p-AKT, mTOR, p-mTOR and p-gsk3β in SGC7901 or SGC7901-DDP gastric
cancer cell lines and (C): The statistics graph for the Western by gray value.
Note: *p<0.05 and ***p<0.01 specify have key variance at the level. 
 .
.
| IC50 of DDP (µM) | |
|---|---|
| SGC7901-DDP | 1.345 |
| SGC7901 | 0.8671 |
| RI | 1.55 |
Table 2: MDR validation for SGC7901/DDP cell.
In order to approve which pathway was involved in the SGC7901-DDP cell resistant, the Western pathway factors such as HIF-α, AKT, mTOR and GSK3Β were performed. The results indicated that, compared to SGC7901 cell line, the protein for HIF-α, AKT, p-AKT, mTOR and p-mTOR were up regulated shown in fig. 1B. In addition, no significant variation was found in the protein level for p-gsk3β between SGC7901-DDP and SGC7901 cell lines in fig. 1C. Therefore, we thought that the pathway AKT-mTOR was involved in the SGC7901 cisplatinum resistant. In addition, the upregulation of HIF-α indicated that the cell proliferation of SGC7901-DDP was independent of the oxygen consumption.
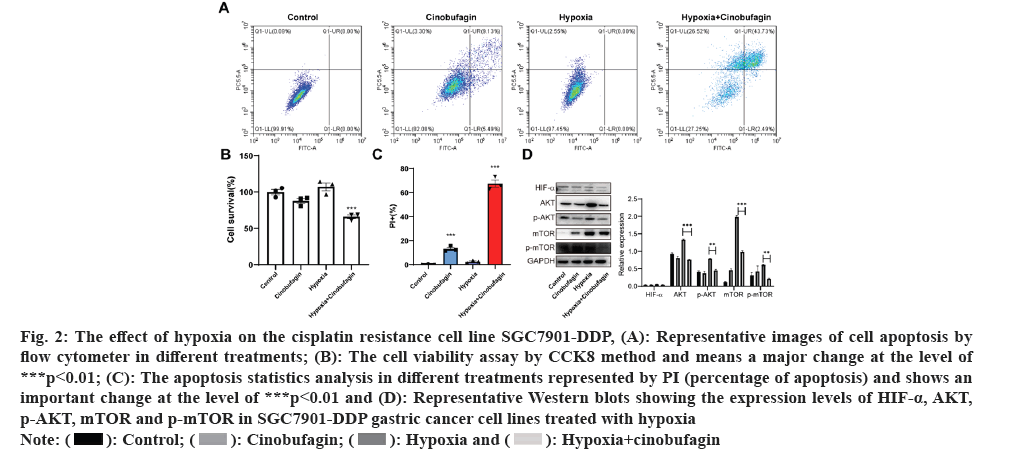
In order to authorize the effect of hypoxia+cinobufagin on the cell growth, the experiments were divided into four groups. After culture, the cell viability analysis was performed. The results indicated that the hypoxia could enhance the cell viability and cinobufagin could inhibit the cell growth. Fig. 2A shows that compared to the other three groups, hypoxia+cinobufagin had significantly reduced cell viability. Furthermore, the cell apoptosis was also assayed. According to fig. 2B and fig. 2C, the apoptosis rate was clearly greater in the hypoxia+cinobufagin group compared to the other three groups. In addition, the pathway was examined by Western blot. The results shows that AKT-mTOR pathway were all decreased significantly related to that in other three groups. Meanwhile, the factor HIF-α was also probed and it was obviously reduced compared to that in hypoxia+cinobufagin group shown in fig. 2D. The result means cinobufagin could reverse the effect of hypoxia contributing to the cell growth.
Fig. 2: The effect of hypoxia on the cisplatin resistance cell line SGC7901-DDP, (A): Representative images of cell apoptosis by
flow cytometer in different treatments; (B): The cell viability assay by CCK8 method and means a major change at the level of
***p<0.01; (C): The apoptosis statistics analysis in different treatments represented by PI (percentage of apoptosis) and shows an
important change at the level of ***p<0.01 and (D): Representative Western blots showing the expression levels of HIF-α, AKT,
p-AKT, mTOR and p-mTOR in SGC7901-DDP gastric cancer cell lines treated with hypoxia.
 .
.
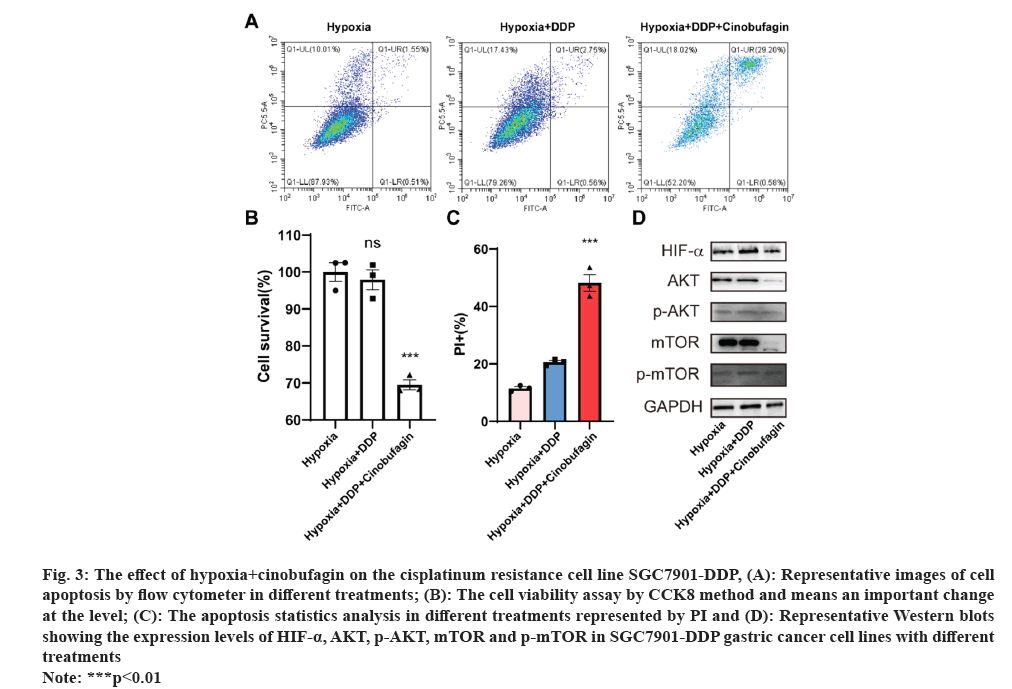
In order to confirm the cinobufagin reverse the cisplatin resistance in cell line SGC7901-DDP. The combination drugs were used for the cell culture. There were three groups used in the research including hypoxia, hypoxia+cisplatin and hypoxia+cinobufagin. The results on the cell viability indicated that the hypoxia+DDP+cinobufagin group had a marked decrease in the cell viability compared to that in other three groups shown in fig. 3A.
Fig. 3: The effect of hypoxia+cinobufagin on the cisplatinum resistance cell line SGC7901-DDP, (A): Representative images of cell
apoptosis by flow cytometer in different treatments; (B): The cell viability assay by CCK8 method and means an important change
at the level; (C): The apoptosis statistics analysis in different treatments represented by PI and (D): Representative Western blots
showing the expression levels of HIF-α, AKT, p-AKT, mTOR and p-mTOR in SGC7901-DDP gastric cancer cell lines with different
treatments.
Note: ***p<0.01.
Fig. 3B and fig. 3C show the results of the apoptosis experiment, which showed that the hypoxia+DDP+cinobufagin group had a much greater apoptosis rate than the other two groups. Finally, Western blotting was used to examine the route as well. The results shows that the AKT, p-AKT, mTOR and p-mTOR were all down regulated related to that in other two groups expressed in fig. 3D. Moreover, that the level of HIF-alpha was decreased by the cinobufagin means the cinobufagin reverse the cisplatin resistance effect.
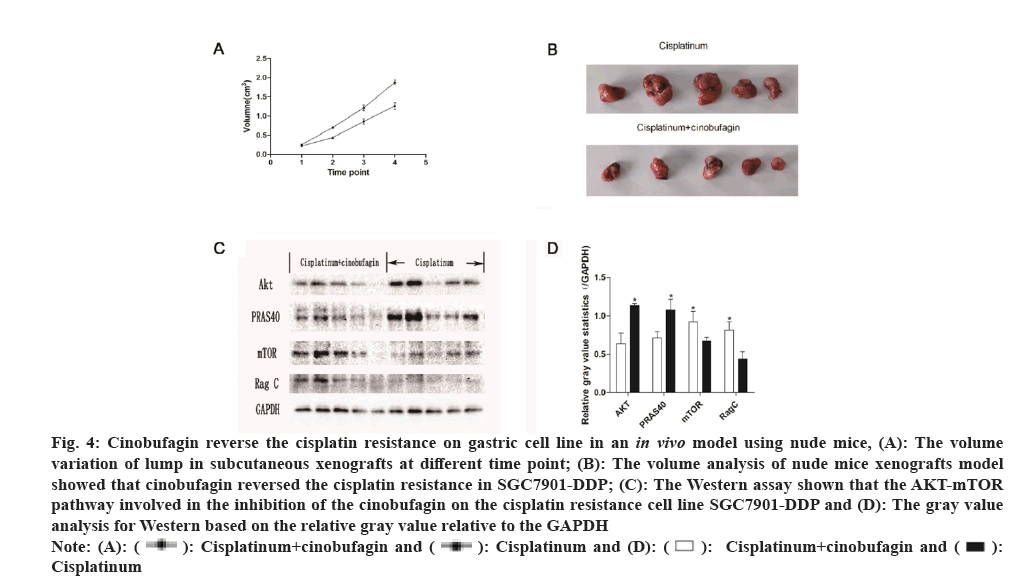
To assess whether cinobufagin influences the growth of gastric carcinoma in vivo, xenograft models were created by injecting the tumor cell lines MKN45 subcutaneously and then the medicine of cinobufagin+cisplatinum or cisplatinum were injected into the tumor lump. The tumor growth curve analysis shows that the volume in the cinobufagin+cisplatinum group was considerably smaller than that in cisplatinum group shown in fig. 4A-fig. 4C.
Fig. 4: Cinobufagin reverse the cisplatin resistance on gastric cell line in an in vivo model using nude mice, (A): The volume
variation of lump in subcutaneous xenografts at different time point; (B): The volume analysis of nude mice xenografts model
showed that cinobufagin reversed the cisplatin resistance in SGC7901-DDP; (C): The Western assay shown that the AKT-mTOR
pathway involved in the inhibition of the cinobufagin on the cisplatin resistance cell line SGC7901-DDP and (D): The gray value
analysis for Western based on the relative gray value relative to the GAPDH.
 Cisplatinum.
Cisplatinum.
The tumor lump was recovered from the nude mouse model and part tissue was used for protein extraction. Then the Akt-mTOR signal pathway was probed. In total, the four genes were examined, including Akt, PRAS40, mTOR and Rag C. The western results indicated that, compared with the cisplatinum group, the cisplatinum+cinobufagin group have more mTOR and Rag C expression and less Akt and PRAS40 expression. The phenomenon showed that mTOR and Ragc were involved in the proliferation for the SGC7901-DDP cell line (fig. 4D). On the contrary, the Akt and PRAS40 have the antagonistic effect on the cell growth.
Chemotherapy has not been successful in treating cancer in many cases because patients have acquired resistance to the drugs used in the treatment. Cancer treatment resistance has several causes and manifestations, such as tumor heterogeneity, tumor load and growth dynamics, physical obstacles, the immune system, and the microenvironment[15]. Among them, intratumoral hypoxia microenvironment were found to exist in many solid tumors, which triggers a series of metabolic shifts involving many factors such as HIFs. Hypoxia is a common cause of cancer patients developing resistance to therapy drugs[12]. Cisplatin is one of the most powerful broad-spectrum anticancer medications used in the treatment of a variety of malignancies. The remarkable characteristics of cisplatin appear to be the reason behind its efficacy. It can enter cells through various pathways, where it forms various DNA-platinum adducts. At the same time, it triggers a cellular defense mechanism by activating or repressing numerous genes, leading to significant changes in epigenetics and genetics[16]. As a result, we need to further research to overcome the resistance of cisplatin and therefore the cinobufagin was introduced to our study and hoped to reverse the resistance of cisplatin.
Drug resistance was a complicated process, which correlated with many factors such as hypoxia, metabolism and many signal pathways. In our study, the signal pathway AKT-mTOR was examined between the SGC7901 and SGC7901-DDP. The outcomes displayed that the molecular AKT, mTOR and their phosphorylated state were all up regulated in SGC7901-DDP cell line related to that in SGC7901 cell line. Based on this, we speculated that AKT-mTOR pathway was involved in the cisplatin resistance in gastric cell line SGC7901. In addition, the hypoxia -inducible factor alpha was also detected and increased in the SGC7901-DDP cell line. The results mean the drug resistance for SGC7901-DDP was connected with the hypoxia.
In order to confirm whether the cisplatin resistance was correlated with the hypoxia, the cell line SGC7901-DDP was treated with hypoxia. In the results, we found that the hypoxia could increase the cell feasibility. However, in the hypoxia+cinobufagin group, the cell feasibility was obviously decreased related to that in other three groups. The phenomenon means the hypoxia was contribute to the drug resistance in cell line SGC7901-DDP. It was reported that AKT signaling pathway plays a critical role in cell proliferation, maintenance and death. Typically, Phosphatidylinositol-3 Kinase (PI3K) activates AKT at particular phosphorylation sites on Thr-308 or Ser-473. Once activated, AKT may phosphorylate its substrate to carry out its function[17]. The kinase for serine and threonine PI3K/AKT/mTOR is a network of proteins that work together to control cell growth. AKT is an important part of this network. Therefore, the pathway was probed in our study of the hypoxia treatment. The in vitro investigation demonstrated that hypoxia+cinobufagin could inhibit the AKT-mTOR pathway and HIF alpha expression related to that in hypoxia group. In addition, the in vivo model assay indicated that the gene AKT was compact in the cinobufagin+cisplatinum group relative to that in the cisplatinum group. The findings showed that cinobufagin could reduce AKT expression, which in turn inhibited stomach cancer growth. The findings corroborated the hypothesis that phytochemical-induced Akt inhibition contributes to a decrease in cancer cell viability and tumor growth.
The primary function of PRAS40, which is a member of mTOR complex 1 and an AKT substrate, was as a 14-3-3 binding protein. Protein degradation, immunological modulation, senescence, cell survival, proliferation, metastasis, apoptosis and PRAS40 are all crucial processes in many species[18]. Multiple diseases, including cancer and insulin resistance, have been associated with PI3K-AKT-mTOR pathway dysregulation[19]. According to reports, PRAS40 is also implicated in cancer cell death. In our study, the cinobufagin+cisplatinum group showed that the gene pRRAS40 was reduced compared to that in cisplatinum group. The results demonstrated that cinobufagin could inhibit the GC proliferation by inhibit the gene PPAS40 expression. The results were in accord with the PRAS40 is usually was found to be increased in the tumor such as breast and lung cancers. In our study, the gene was inhibited by the cinobufagin and reduced the tumor volume[20].
Serine/threonine kinase MTOR promotes cell proliferation and growth as the catalytic component of mitogen-activated AKT MTORC1 and MTORC2[21]. Previous studies have shown that the PI3k/Akt/mTOR pathways are highly overexpressed in primary colorectal cancer[22]. Nevertheless, the current data suggests that several cellular ageing processes rely on mTOR-dependent signaling for their upkeep or execution[23]. Therefore, the mTOR could dual role in the proliferation of cancer. Our study showed that, in the cinobufagin+cisplatinum group, mTOR expression was improved. However, the SGC7901-DDP, the expression of mTOR was decreased. The result means that the mTOR possibly has a different role in different cell line. Combined with the tumor growth curve, we speculated that cinobufagin inhibit the tumor growth by improving the mTOR expression in cell line SGC7901-DDP.
The recruitment of mTORC1 to lysosomes by the Rag GTPases has the potential to control cell proliferation and growth in reaction to amino acid supply. In order for Rag heterodimers to bind to mTORC1, their nucleotide status is essential. One of them is the Rag C-mTORC1 signaling cascade that begins with the protein attaching to the mTORC1 subunit[24]. One of the rapamycin complexes, mTORC1 links the availability of nutrients, energy, and growth factors to the rate of cell proliferation. Active Rags by direct binding to the mTORC1 component Raptor recruit the lysosomal membrane. Our study showed that gene RagC expression was improved in cinobufagin+cisplatinum group related to that in the cisplatinum group[25]. Since the tumor volume in cinobufagin+cisplatinum group was bigger than that in the cisplatinum group. Therefore, we speculated that the gene Rag C have the cancer inhibitory role in the cancer progression by the cinobufagin.
In conclusion, the cisplatin resistance was related with the hypoxia and the cinobufagin could reverse the resistance. The cell viability assay for SGC7901-DDP indicated that its cell viability was higher than that of cell line SGC7901. The pathway analysis in SGC7901-DDP revealed that the AKT-mTOR pathway and HIF-alpha factor were up regulated in SGC7901-DDP cell line related to that in SGC7901 cell line. In addition, our tests showed that the cells in the hypoxia+cinobufagin group were less likely to survive than cells in the other three groups. Furthermore, the western operation illustrated that the AKT-mTOR pathway and HIF-alpha factor were inhibited by cinobufagin. Furthermore, we found that AKT-mTOR pathway was remarkably increased by hypoxia. In order to further confirm our speculation, the reversing assay by cinobufagin was carried out. The results indicated that cinobufagin could decrease the cell viability, increase the apoptosis proportion and reverse the cisplatin resistance in SGC7901-DDP cell line by AKT-mTOR pathway. Lastly, the in vitro assay was completed and the result demonstrated that cinobufagin could inhibit the lump growth and reverse the cisplatin resistance.
Ethical approval:
All operations of animal experiments were conducted according to the protocols authorized by the Harbin Medical University. These experiments were approved by the Animal Ethics Committee of Harbin Medical University (No: 2022-DWSYLLCZ-76).
Funding:
The project was supported by Heilongjiang Natural Science Foundation of China (General Project) under the project “Bufalin reverse the cisplatin resistance through the AKT signaling pathway in GC cells” (No: H2018026).
Conflict of interests:
The authors declared no conflict of interests.
References
- Puliga E, Corso S, Pietrantonio F, Giordano S. Microsatellite instability in Gastric Cancer: Between lights and shadows. Cancer Treat Rev 2021;95:102175.
[Crossref] [Google Scholar] [PubMed]
- Wang D, Zhao C, Xu F, Zhang A, Jin M, Zhang K, et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics 2021;11(6):2860.
[Crossref] [Google Scholar] [PubMed]
- Kozak J, Forma A, Czeczelewski M, Kozyra P, Sitarz E, Radzikowska-Buchner E, et al. Inhibition or reversal of the epithelial-mesenchymal transition in gastric cancer: Pharmacological approaches. Int J Mol Sci 2020;22(1):277.
[Crossref] [Google Scholar] [PubMed]
- Jiang L, Gong X, Liao W, Lv N, Yan R. Molecular targeted treatment and drug delivery system for gastric cancer. J Cancer Res Clin Oncol 2021;147:973-86.
[Crossref] [Google Scholar] [PubMed]
- Apryani E, Ali U, Wang ZY, Wu HY, Mao XF, Ahmad KA, et al. The spinal microglial IL-10/β-endorphin pathway accounts for cinobufagin-induced mechanical antiallodynia in bone cancer pain following activation of α7-nicotinic acetylcholine receptors. J Neuroinflammation 2020;17(1):75.
[Crossref] [Google Scholar] [PubMed]
- Deng X, Sheng J, Liu H, Wang N, Dai C, Wang Z, et al. Cinobufagin promotes cell cycle arrest and apoptosis to block human esophageal squamous cell carcinoma cells growth via the p73 signalling pathway. Biol Pharm Bull 2019;42(9):1500-9.
[Crossref] [Google Scholar] [PubMed]
- Li X, Chen C, Dai Y, Huang C, Han Q, Jing L, et al. Cinobufagin suppresses colorectal cancer angiogenesis by disrupting the endothelial mammalian target of rapamycin/hypoxia-inducible factor 1α axis. Cancer Sci 2019;110(5):1724-34.
[Crossref] [Google Scholar] [PubMed]
- Quintanilha JC, Saavedra KF, Visacri MB, Moriel P, Salazar LA. Role of epigenetic mechanisms in cisplatin-induced toxicity. Crit Rev Oncol Hematol 2019;137:131-42.
[Crossref] [Google Scholar] [PubMed]
- Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem 2019;88:102925.
[Crossref] [Google Scholar] [PubMed]
- Mao X, Nanzhang, Xiao J, Wu H, Ding K. Hypoxia-induced autophagy enhances cisplatin resistance in human bladder cancer cells by targeting hypoxia-inducible factor-1α. J Immunol Res 2021;2021:8887437.
[Crossref] [Google Scholar] [PubMed]
- Kim MC, Hwang SH, Yang Y, Kim NY, Kim Y. Reduction in mitochondrial oxidative stress mediates hypoxia-induced resistance to cisplatin in human transitional cell carcinoma cells. Neoplasia 2021;23(7):653-62.
[Crossref] [Google Scholar] [PubMed]
- Bao MH, Wong CC. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells 2021;10(7):1715.
[Crossref] [Google Scholar] [PubMed]
- Xie R, Li X, Ling Y, Shen C, Wu X, Xu W, et al. Alpha-lipoic acid pre-and post-treatments provide protection against in vitro ischemia-reperfusion injury in cerebral endothelial cells via Akt/mTOR signaling. Brain Res 2012;1482:81-90.
[Crossref] [Google Scholar] [PubMed]
- Dobashi Y, Suzuki S, Sato E, Hamada Y, Yanagawa T, Ooi A. EGFR-dependent and independent activation of Akt/mTOR cascade in bone and soft tissue tumors. Mod Pathol 2009;22(10):1328-40.
[Crossref] [Google Scholar] [PubMed]
- Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature 2019;575(7782):299-309.
[Crossref] [Google Scholar] [PubMed]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev 2012;64(3):706-21.
[Crossref] [Google Scholar] [PubMed]
- Dukaew N, Chairatvit K, Pitchakarn P, Imsumran A, Karinchai J, Tuntiwechapikul W, et al. Inactivation of AKT/NF-κB signaling by eurycomalactone decreases human NSCLC cell viability and improves the chemosensitivity to cisplatin. Oncol Rep 2020;44(4):1441-54.
[Crossref] [Google Scholar] [PubMed]
- Qi Z, Zhang T, Song L, Fu H, Luo H, Wu J, et al. PRAS40 hyperexpression promotes hepatocarcinogenesis. EBioMedicine 2020;51:102604.
[Crossref] [Google Scholar] [PubMed]
- Wang H, Zhang Q, Wen Q, Zheng Y, Lazarovici P, Jiang H, et al. Proline-rich Akt substrate of 40 kDa (PRAS40): A novel downstream target of PI3k/Akt signaling pathway. Cell Signal 2012;24(1):17-24.
[Crossref] [Google Scholar] [PubMed]
- Malla R, Ashby Jr CR, Narayanan NK, Narayanan B, Faridi JS, Tiwari AK. Proline-rich AKT substrate of 40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys Res Commun 2015;463(3):161-6.
[Crossref] [Google Scholar] [PubMed]
- Koenigsberg C, Ondrey FG. Genomic database analysis for head and neck cancer prevention targets: MTOR signal transduction pathway. Anticancer Res 2020;40(10):5417-21.
[Crossref] [Google Scholar] [PubMed]
- Konishi T, Yoshidome H, Shida T, Furukawa K, Takayashiki T, Kuboki S, et al. Phosphorylated mTOR expression as a predictor of survival after liver resection for colorectal liver metastases. J Surg Oncol 2021;124(4):598-606.
[Crossref] [Google Scholar] [PubMed]
- Cayo A, Segovia R, Venturini W, Moore-Carrasco R, Valenzuela C, Brown N. mTOR activity and autophagy in senescent cells, a complex partnership. Int J Mol Sci 2021;22(15):8149.
[Crossref] [Google Scholar] [PubMed]
- Anandapadamanaban M, Masson GR, Perisic O, Berndt A, Kaufman J, Johnson CM, et al. Architecture of human Rag GTPase heterodimers and their complex with mTORC1. Science 2019;366(6462):203-10.
[Crossref] [Google Scholar] [PubMed]
- Fromm SA, Lawrence RE, Hurley JH. Structural mechanism for amino acid-dependent Rag GTPase nucleotide state switching by SLC38A9. Nat Struct Mol Biol 2020;27(11):1017-23.
[Crossref] [Google Scholar] [PubMed]