- *Corresponding Author:
- Ilgin Sinem
Department of Pharmaceutical Toxicology, Faculty of Pharmacy, Anadolu University, Turkey
E-mail: silgin@anadolu.edu.tr
| Date of Received | 26 August 2021 |
| Date of Revision | 22 July 2022 |
| Date of Acceptance | 03 March 2023 |
| Indian J Pharm Sci 2023;85(2):403-411 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Although the correlation between citalopram and cardiovascular pathologies was realized via case studies, there is no experimental study that was performed independently of other risk factors related to cardiotoxicity. In the study reported here, we aimed to identify the cardiotoxic effects of citalopram by evaluating serum cardiac biomarkers, such as serum aspartate transaminase, creatine kinase-myoglobin binding, lactate dehydrogenase and troponin-T levels as well as electrocardiogram parameters, deoxyribonucleic acid damage in cardiomyocytes and histological findings of heart tissue in rats that were administered oral doses of 5, 10, or 20 mg/kg of citalopram for 28 d. Additionally, to investigate possible mechanisms underlying cardiotoxicity, glutathione and malondialdehyde levels in cardiac tissue were determined to evaluate oxidative stress. According to our results, heart rates were increased, PR intervals were prolonged, and T wave amplitudes were significantly decreased in the 20 mg/kg citalopram-administered groups when compared with the control group. Additionally, serum aspartate transaminase, lactate dehydrogenase and troponin-T levels were significantly increased in the 10 and 20 mg/kg citalopram-administered group compared to the control group. Significant deoxyribonucleic acid damages were observed in the 10 and 20 mg/kg citalopram-administered groups. Histopathological investigations revealed degenerative changes in the 10 and 20 mg/kg citalopram-administered groups. In heart tissues, glutathione levels were decreased in the 10 and 20 mg/kg citalopram-administered groups significantly when compared with the control group. These findings indicated the relationship between high-dose and subtherapeutic-dose citalopram administration and cardiac morphological, biochemical and functional toxic effects.
Keywords
Citalopram, cardiac biomarkers, electrocardiogram, deoxyribonucleic acid damage, cardiac histology, oxidative stress
Selective Serotonin Reuptake Inhibitors (SSRIs) are commonly prescribed to treat major depressive disorders, obsessive-compulsive disorder, social phobia and anxiety disorders[1]. In terms of cardiotoxicity, SSRIs are less toxic and more reliable than many first-generation anti- depressants, but there is also evidence that they have adverse cardiovascular system events[2-4]. Reports published in recent years indicated that SSRI administration-induced cardiovascular adverse effects such as prolonged QT interval, arrhythmias and orthostatic hypotension in patients without cardiovascular disorders. Therefore, these reports have caused concern about these drugs safety in the cardiovascular system[5-7].
Citalopram (CTL) is an antidepressant drug of the SSRIs class prescribed for major depression, panic disorder, and obsessive-compulsive disorder[8]. Although reports emphasized that patients well tolerate CTL treatment, bradycardia and Electrocardiogram (ECG) anomalies associated with CTL treatment at high therapeutic and supratherapeutic doses have drawn attention to possible cardiovascular toxic effects. Post- marketing reports of QT interval prolongation emphasized that long QT, which causes torsade de pointes, ventricular tachycardia, and sudden death, occur with CTL treatment, dose-dependently[9-12]. Regarding these reports, the United States Food and Drug Administration (US FDA) published a warning about CTL and advised restricting the use of this drug[13]. On the other hand, clinical studies observed unchanged PQ, QRS, and QT intervals in patients under CTL treatment; it could be thought that CTL did not affect cardiac conduction[14-17].
At this point, it can be emphasized that it is highly required to perform more studies, both experimental and clinical, due to the conflicting results regarding the cardiac effects of CTL administration. Contrary to previous studies, cardiotoxicity was determined by measuring serum cardiac biomarkers, assessing cardiomyocytes' DNA damage and analyzing heart tissue histopathologically in rats exposed to CTL at repeated high-dose. Also, changes in the oxidative status of heart tissue, which has an essential role in drug-induced cardiotoxicity, were determined.
Materials and Methods
Materials:
CTL was a present from the IE Ulagay-Menarini Group (Istanbul, Turkey). Urethane (Sigma-Aldrich, Germany) was used as an anesthetic in animals. The kits were obtained from the following sources: CK- MB Assay Kit (BioCheck, CA, USA), LDH Assay Kit (BioAssay Systems, CA, USA), AST (Cusabio Life Science, Hubei, PR China), Malondialdehyde (MDA) ELISA Kit (Cusabio Life Science, Hubei, PR China), Rat Cardiac Troponin-T ELISA Kit (Cusabio Life Science, Hubei, PR China), Glutathione (GSH) ELISA Kit (Cusabio Life Science, Hubei, PR China).
Animals:
Male Wistar rats (8-10 w old, 200-250 g in weight) were obtained from the Anadolu University Research Center for Animal Experiments were used in the experiments. The animals were placed in a 12 h light/12 h dark cycle room with a regulated temperature (24°) and fed with water and a regular ad libitum diet. The experimental protocol was approved by the Local Ethical Committee on Animal Experimentation of Anadolu University, Eskisehir, Turkey, in adherence to the National Institutes of Health Guidelines for the Use of Laboratory Animals (File Registration Number: 2014-32).
Methods:
Experimental groups: Rats were randomly assigned to a total of 4 groups as follows; Control group, distilled water was administered by oral gavage for 28 d; CTL-5 group, 5 mg/kg/d CTL was administered by oral gavage for 28 d; CTL-10 group, 10 mg/kg/d CTL was administered by oral gavage for 28 d; CTL- 20 group, 20 mg/kg/d CTL was administered by oral gavage for 28 d.
According to the previous studies[1,18-22], we determined CTL doses that investigated the dose-dependent antidepressant and anxiolytic effects of CTL in rats. Drugs were administered in 1 ml/100 g of distilled water. After 28 d exposure, 1.5 g/kg urethane was administered, i.p, for the anesthesia protocol[23].
ECG recordings: A six-channel electrocardiograph (Biopac Systems, Santa Barbara, CA) was used. All procedures were performed in a quiet room to minimize stress. All ECGs were performed and analyzed by the Biopac MP36 data acquisition system. Rodents were placed in a dorsal recumbence position, and four needle electrodes were attached to the skin on the right wrist, left wrist, right ankle, and left ankle (lead II). The parameters evaluated were Heart Rate (HR) and cardiac rhythm, duration of the P wave, QRS complex (QRS), PR and QT intervals, and amplitude of P waves, R waves, and T waves. The corrected QT interval (QTc) was determined by the normalized Bazett formula QTc=QT/(RR/f)1/2[24].
Determination of serum cardiac biomarker levels:
Aspartate transaminase (AST), Creatine Kinase-Myoglobin Binding (CK-MB), Lactate Dehydrogenase (LDH), and troponin-T levels were determined in blood samples collected by cardiac puncture. The samples were centrifuged at 1000 g for 15 min at 4°; serum was separated from the blood, frozen, and stored at -20° until analysis. Levels of AST, LDH, CK-MB, and troponin-T were evaluated by following the kit's supplier's directions.
The animals were euthanized by the withdrawal of high volumes of blood from the heart. Heart tissue was collected and cleaned in a Phosphate Buffer Solution (PBS) (composition: NaCl: 8 g/l, KCl: 0.2 g/l, KH2PO4: 0.2 g/l and Na2HPO4: 1.14 g/l at pH: 7.4). The cardiac tissues were kept at -80° until the analysis of GSH and MDA levels.
Determination of DNA damage in cardiomyocytes:
Heart tissues were homogenized in ice-cold Merchant’s medium (pH 7.4) using a homogenizer (Potter Elvehjem). Cell suspensions were then rapidly placed in agarose and the assay was performed according to the study by Ilgın et al.[25]. The Tail Moment (TM) was calculated for 40 randomly selected cells from each sample according to the following formula: extent TM=tail length×tail DNA %/100[26].
Histological analysis of heart tissue:
The heart tissues were fixed in paraformaldehyde (4 %) in phosphate buffer pH 7.2 at 20 to 22° for 2 h. Then, they were transferred to a graduated sequence of alcohols and treated and embedded in LR White (Electron Microscopy Sciences, FT Washington PA) and 70 % ethanol (2:1) (v:v) for 1 h at 20 to 22°. Samples were sliced at 700 nm (0.7 microns) by Leica EM UC7 ultramicrotome and stained with 1 % toluidine blue/borax (pH 8.4) for a 2 min staining procedure. Samples were investigated under the Leica DM 750 light microscope[27].
Determination of GSH and MDA levels in heart tissue:
Lysis buffer (50 mM MES, pH 6-7, containing 1 mM EDTA) was added to the tissue (1/20, weight/ volume), and the mixture was homogenized using a homogenizer. Then, the lysate was centrifuged at 10000 xg 15 min at +4°. The supernatants were used to measure the total GSH. Measurement of MDA levels was carried out by adding cold buffer (0.25 M sodium phosphate buffer, pH 7.4, containing 0.05 M sucrose) to the tissue samples (1/20, weight/volume), and the mixture was homogenized. After that, the lysate was centrifuged at 10000 xg 15 min at +4°. The supernatants were used to measure MDA.
Statistical analysis:
SPSS software package (IBM SPSS Statistics) was used for the statistical analysis of data. Data were analyzed using a one-way ANOVA, followed by the Tukey post-hoc test. A p-value less than 0.05 (p≤0.05) was accepted as statistically significant.
Results and Discussion
According to the groups ECG recordings, CTL administration increased the heart rate, but increased heart rate was only statistically significant in the CTL-20 group. Similar to heart rate, the PR interval was increased in the CTL-administered groups, but the increased PR interval was only statistically significant in the CTL-20 group. On the other hand, CTL administration decreased the QTc value in the groups according to the control, but these differences were not statistically significant. Additionally, the T-wave amplitudes were decreased in the CTL- administered groups compared with the control. There were no significant differences between CTL groups in terms of the ECG parameters (Table 1).
| C | CTL-5 | CTL-10 | CTL-20 | |
|---|---|---|---|---|
| Heart rate (Beats/min) | 300.88±15.17 | 375.63±23.62 | 380.5±27.66 | 390±22.63(*) |
| PR interval (msec) | 57±1.23 | 59.86±1.78 | 61.14±1.22 | 63±1.56(*) |
| QT interval (msec) | 60.86±0.88 | 61.29±0.71 | 61.25±1.14 | 60.5±0.36 |
| QTc interval | 43.21±1.4 | 39.12±1.49 | 39.32±2.66 | 38.09±1.44 |
| QRS amplitude (mV) | 1.15±0.03 | 0.99±0.01 | 1.17±0.03 | 1.07±0.02 |
| QRS duration (msec) | 13.14±0.40 | 13.29±0.87 | 12.57±0.72 | 15.14±1.01 |
| T-wave amplitude (mV) | 0.32±0.008 | 0.24±0.019(**) | 0.24±0.007(**) | 0.26±0.011(*) |
Note: C: Control rats; CTL-5: 5 mg/kg citalopram-administered rats for 28 d group; CTL-10: 10 mg/kg citalopram-administered rats for 28 d group; CTL-20: 20 mg/kg citalopram-administered rats for 28 d group. All data were expressed as mean±standard error; *Significant differences when compared with the control group (p<0.05); **Significant differences when compared with the control group (p<0.01)
Table 1: Effects of Citalopram Administration on ECG Parameters
AST levels of the CTL-10 and CTL-20 groups were significantly higher than the control group. There were no significant differences between groups. Serum LDH levels of CTL-administered groups were significantly higher compared to the control group. No significant differences were obtained among the CTL-administered groups. CK-MB levels of CTL groups remained unchanged compared with the controls. The serum cTn-T level of the CTL-20 group was significantly higher compared to the control group. Also, a significant increase was observed in the CTL-20 group compared to the CTL-5 and CTL- 10 groups. These results were shown in Table 2.
| C | CTL-5 | CTL-10 | CTL-20 | |
|---|---|---|---|---|
| AST | 197.27±20.11 | 252.43±24.20 | 306.82±24.22(*) | 285.23±21.52(*) |
| LDH (mU/ml) | 22±2.22 | 46.72±2.60(**) | 46.03±4.57(**) | 58.7±3.15(**) |
| CK-MB (ng/ml) | 4.65±0.36 | 4.13±0.21 | 3.63±0.33 | 4.32±0.26 |
| cTn-T (pg/ml) | 0.68±0.05 | 0.75±0.04 | 0.83±0.05 | 3.61±0.27(***, +, #) |
Note: AST: aspartate transaminase; CK-MB: creatine kinase-myocardial band; LDH: lactate dehydrogenase; cTn-T: cardiac troponin T; C: Control rats; CTL-5: 5 mg/kg citalopram-administered rats for 28 d group; CTL-10: 10 mg/kg citalopram-administered rats for 28 d group; CTL-20: 20 mg/kg citalopram-administered rats for 28 d group. All data were expressed as mean±standard error; * Significant differences when compared with control group (p<0.05); ** Significant differences when compared with control group (p<0.01); *** Significant differences when compared with control group (p<0.001); +Significant differences when compared with CTL-5 (p<0.01); #Significant differences when compared with CTL-10 (p<0.01)
Table 2: Effects of Citalopram Administration on Cardiac Biomarkers
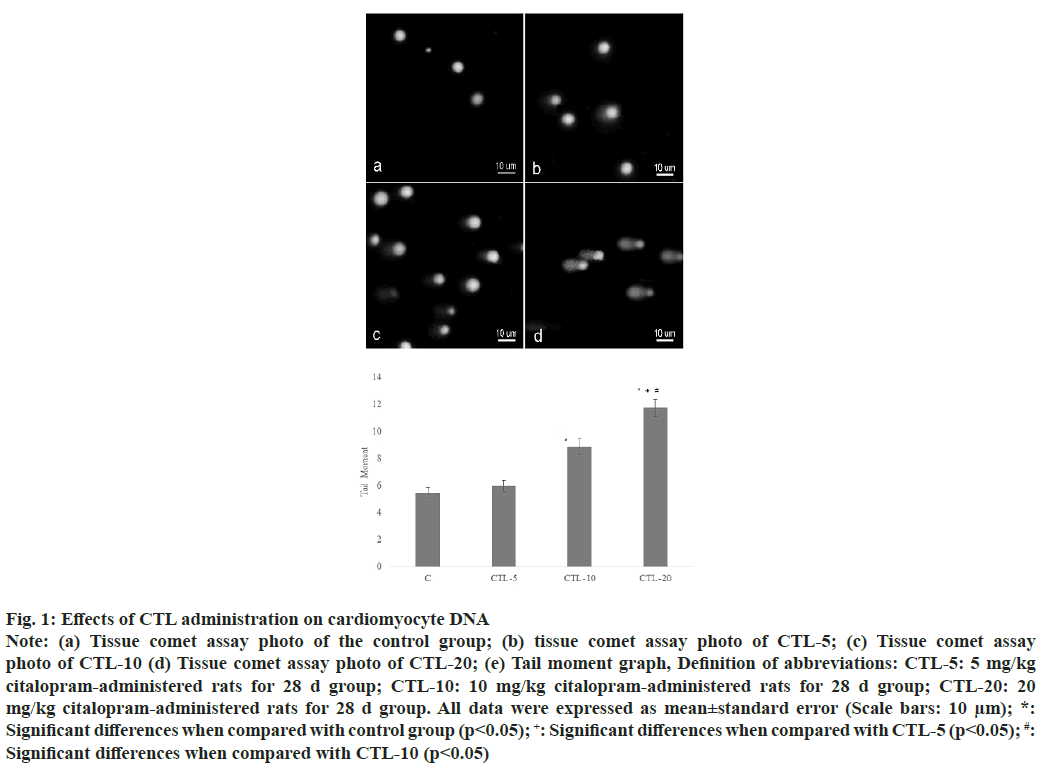
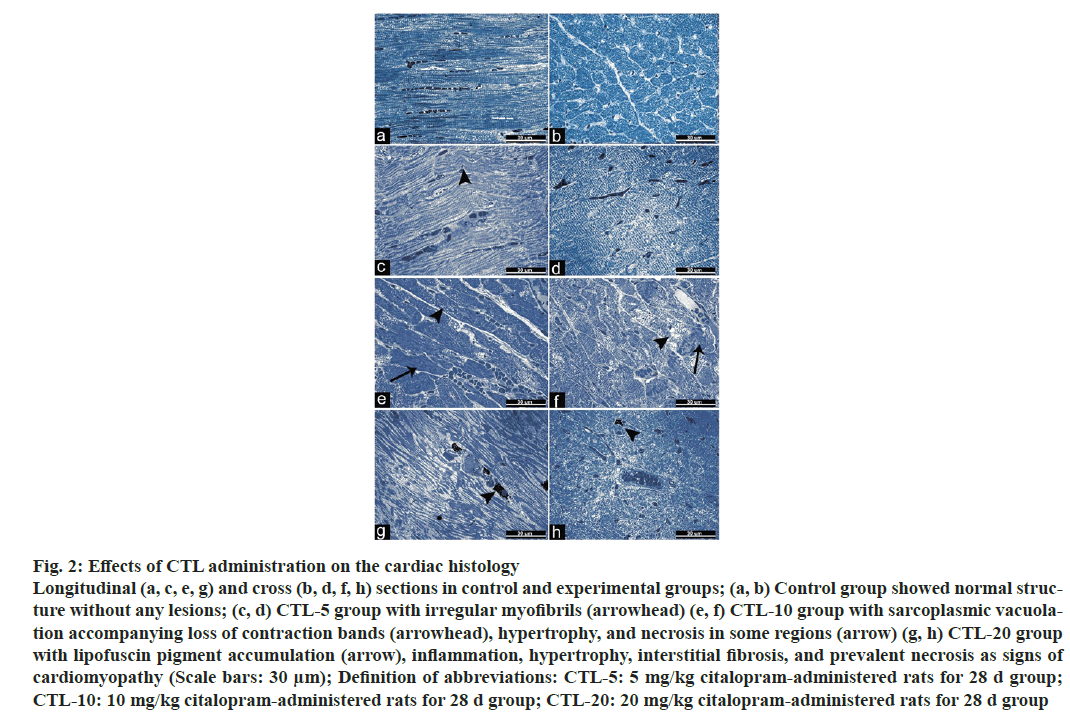
The extent of the tail moment was significantly increased in the CTL-10, and CTL-20 groups compared to the control group. Also, a significant increase was observed in the CTL-20 group compared to the CTL-5 and CTL-10 groups (fig. 1). Additionally, values were as the following; 5.44±0.40, 5.7±0.40, 8.85±0.57, 11.72±0.63 (mean±standard error) for the control group, CTL-5, CTL-10, and CTL-20 groups, respectively. The microscopic examination of heart tissues from the control group revealed typical histological architecture and cardiac muscle structure. In the CTL-5 group, mild pathologic findings such as irregular myofibrils were observed. On the other hand, heart tissues from the CTL-10 group showed sarcoplasmic vacuolation, loss of contraction bands, and hypertrophy accompanying necrosis in some regions. Degenerative changes, including lipofuscin pigment accumulation, inflammation, hypertrophy, interstitial fibrosis, and prevalent necrosis of the cardiac tissue, were observed in the CTL-20 group (fig. 2).
Fig. 1: Effects of CTL administration on the cardiac histology
Longitudinal (a, c, e, g) and cross (b, d, f, h) sections in control and experimental groups; (a, b) Control group showed normal structure
without any lesions; (c, d) CTL-5 group with irregular myofibrils (arrowhead) (e, f) CTL-10 group with sarcoplasmic vacuolation
accompanying loss of contraction bands (arrowhead), hypertrophy, and necrosis in some regions (arrow) (g, h) CTL-20 group
with lipofuscin pigment accumulation (arrow), inflammation, hypertrophy, interstitial fibrosis, and prevalent necrosis as signs of
cardiomyopathy (Scale bars: 30 μm); Definition of abbreviations: CTL-5: 5 mg/kg citalopram-administered rats for 28 d group;
CTL-10: 10 mg/kg citalopram-administered rats for 28 d group; CTL-20: 20 mg/kg citalopram-administered rats for 28 d group
Fig. 2: Effects of CTL administration on the cardiac histology
Longitudinal (a, c, e, g) and cross (b, d, f, h) sections in control and experimental groups; (a, b) Control group showed normal structure
without any lesions; (c, d) CTL-5 group with irregular myofibrils (arrowhead) (e, f) CTL-10 group with sarcoplasmic vacuolation
accompanying loss of contraction bands (arrowhead), hypertrophy, and necrosis in some regions (arrow) (g, h) CTL-20 group
with lipofuscin pigment accumulation (arrow), inflammation, hypertrophy, interstitial fibrosis, and prevalent necrosis as signs of
cardiomyopathy (Scale bars: 30 μm); Definition of abbreviations: CTL-5: 5 mg/kg citalopram-administered rats for 28 d group;
CTL-10: 10 mg/kg citalopram-administered rats for 28 d group; CTL-20: 20 mg/kg citalopram-administered rats for 28 d group
GSH levels of heart tissues were decreased significantly in the CTL-10 and CTL-20 groups compared to the control group. A significant decrease was observed in the CTL-10 and CTL-20 groups compared to the CTL-5 group. When MDA levels in the heart tissues of each group were compared, there were no significant differences among the groups (Table 3).
| C | CTL-5 | CTL-10 | CTL-20 | |
|---|---|---|---|---|
| MDA (nmol/ml) | 1276.31±56.86 | 1161.29±33.24 | 1115.27±58.38 | 1152.92±46.68 |
| GSH (µM) | 50.33±2.17 | 49.83±4.54 | 31.08±3.29(*,+) | 39.52±4.27(*) |
GSH: glutathione; MDA: malondialdehyde; C: Control rats; CTL-5: 5 mg/kg citalopram-administered rats for 28 d group; CTL-10: 10 mg/kg citalopram-administered rats for 28 d group; CTL-20: 20 mg/kg citalopram-administered rats for 28 d group; All data were expressed as mean±standard error; *Significant differences when compared with control group (p<0.05); +Significant differences when compared with CTL-5 (p<0.05)
Table 3: Effects of Citalopram Administration on GSH and MDA Levels in Cardiac Tissue
Our study, independent of other risk factors associated with cardiotoxic effects, indicated that CTL administration at repeated high-dose might cause cardiotoxic effects. As known, the cardiac cycle, which includes the phase of relaxation diastole and the phase of contraction systole, refers to the sequence of mechanical and electrical events that repeats with every heart rate[28]. Firstly, it should be expressed that heart rate was increased in the CTL-administered groups; however, these increases were only statistically significant in the high-dose. At this point, we emphasized that the therapeutic doses of CTL are between 10 to 60 mg/d[29]. While the 5 and 10 mg/kg doses we have chosen also were animal doses extrapolated from human doses, 20 mg/kg was twice the maximum dose following the guideline[30]. Conversely, studies showed that CTL/ escitalopram administration affected heart rate, but it decreased heart rate. In these studies, decreased heart rate was associated with the elevation of plasma serotonin levels with CTL administration[31]. On the other hand, there are also case reports that showed CTL administration might cause monomorphic ventricular tachycardia and sinus tachycardia even at therapeutic doses when used with drugs metabolized by the same Cytochrome P450 (CYP) enzyme family and in case of overdose[32]. Additionally, FDA safety reports emphasized that CTL treatment might induce tachycardia in patients[13]. As mentioned above, it is known that ECG is the most critical indicator routinely used in the determination of cardiac function and myocardial pathologies. Drugs affect the myocardium's electrical potential, resulting in functional adverse effects, which are determined by ECG[33,34]. PR interval that reflects the conduction velocity through the Atrioventricular (AV) node[35] was increased with the high-dose administration of CTL. Generally, the prolonged PR interval known as the first atrioventricular block arises from AV conduction delay time[36,37]. Ischemia, heart failure, and inflammation may cause prolongation of PR interval[38]. Agents that block β-adrenergic receptors and calcium channels in the heart may also induce PR interval prolongation[34]. At this point, it can be emphasized that the study was shown that CTL inhibited L-type calcium-channel current[3,39], which might induce PR interval prolongation. Additionally, it is well known that prolongation of PR interval may be associated with structural changes in the heart[40]. Structural changes such as hypertrophy, cardiac necrosis, and cardiac inflammation induced with high-dose administration of CTL might have caused PR interval prolongation. Also, T-wave amplitude that reflects ventricular repolarization[41] was decreased after CTL administration in our study. The causes of decreased T-wave amplitude are similar to prolonged PR intervals. Ischemia is one of the most important reasons that induce decreased T-wave amplitude[42]. Additionally, previous studies indicated that β-adrenergic and sodium channel blocking agents also caused reductions in the T-wave amplitudes[43,44]. It is also known that tachycardia may cause a decrease in T-wave amplitude by shortening the interbeat interval[45,46]. Structural changes in the heart, including hypertrophy, cardiac necrosis, and cardiac inflammation[47] may lead to decreases in T waves. Therefore, it can be emphasized that the structural changes observed in the cardiac tissue of the 10 and 20 mg/kg CTL groups might be the reason for the decreased T-wave amplitude. These histopathologic findings such as hypertrophy, interstitial fibrosis, and necrosis induced with high- dose administration of CTL also indicated myocardial ischemia.
Before structural changes in heart tissue, biochemical markers play an important role in detecting myocardial ischemia. As known, myocardial ischemia occurs due not to receiving enough oxygen for heart function. Biomarkers of myocardial ischemia such as AST, CK-MB, LDH, and troponins are released from myocytes into the blood; so, an increase of these biomarkers in blood indicates ischemia[48,49]. AST is no specific biomarker for cardiac toxicity; however, high levels of AST attract attention after myocardial ischemia[50]. Similar to AST, the specificity of LDH is low for myocardial ischemia; it also increases in different pathologies[51]. Consequently, cardiac enzymes such as LDH, AST, and CKMB are of little value for determining cardiac ischemia. Conversely to these biomarkers, troponins are recommended to diagnose myocardial ischemia because troponins are more expressed in the heart than in other tissues and are more sensitive biomarkers than other conventional biomarkers for determining ischemia[48,52-53]. In our study, increases in AST, LDH, and troponin T levels observed with the high-dose administration of CTL could be considered another one of the ischemic findings.
DNA damage has been considered one of the potential etiologies in various cardiac pathologies[54,55]. Essentially, it has been expressed to coexist oxidative stress and DNA damage in many different heart disease forms[55,56]. Many heart diseases are associated with excess production of Reactive Oxygen Species (ROS)[57]. If the balance between ROS production and the antioxidant system is disrupted in ROS production, oxidative stress occurs, which triggers several deleterious events, including DNA damage[57-59]. Also, insufficient antioxidant defense systems in the heart make it vulnerable to ROS- mediated cellular damage[60,61]. GSH is an essential cellular antioxidant to protect the heart against ROS-mediated injury, and therefore, decreased cellular GSH content could be observed after cardiac pathologies, including ischemia[51]. It is known that CYP2C19, CYP2D6, and CYP3A4 are responsible for the metabolism of CTL, which is converted to its reactive metabolites via oxidation pathways[62]. GSH has critical importance in detoxifying oxidative metabolites such as di-desmethyl CTL, which is the reactive metabolite of CTL. Therefore, decreases in GSH levels in hearts of CTL-administered groups could be associated with reactive metabolites' detoxification. At this point, we have to emphasize that GSH depletion renders the heart vulnerable to oxidant-mediated injury, consequently, may also lead to cardiotoxicity[63-65]. DNA damage observed with CTL administration could be a consequence of oxidative stress. Xenobiotic-induced oxidative stress in cardiac tissue may also cause morphological changes such as loss of myofibril and myocardial cells' vacuolization[59]. In cardiac tissue, the abnormal morphological structure such as accumulation of lipofuscin pigment, hypertrophy, fibrosis, and a marker of irreversible damage, might be associated with the oxidative stress determined after CTL administration.
In conclusion, our findings showed the toxic effects of repeated high-dose administration of CTL on cardiac function and morphology. Perhaps, the toxic effects observed in the high-dose group can be interpreted as a result of the supra-therapeutic dose of the CTL calculated as out of the therapeutic index according to the guideline. In our study, the contribution of oxidative stress to cardiac pathology is indisputable. However, could the ECG abnormalities be related to the effect of CTL on cardiac ion channels? As mentioned above, the inhibitory effect of CTL on cardiac ion channels has been shown in several studies. However, their effects on ion channels that may cause cardiac functional abnormalities are not clear. Perhaps these studies should be detailed for safe drug treatment. On the other hand, although CTL treatment is considered safe in patients, poor metabolizers of CYP2D6 and CYP2C19, which are roles in CTL metabolism, should be considered for the potential of CTL-related cardiotoxicity. Accordingly, CTL doses should be adjusted in the patients mentioned above. Additionally, doctors should pay attention to patients with risk factors for cardiac toxic effects, such as gender, age, pre- existing cardiovascular disorders, and electrolyte abnormalities, and patients should be monitored during treatment.
Conflict of interests:
Authors declared that there is no conflict of interest.
References
- Sekar S, Verhoye M, van Audekerke J, Vanhoutte G, Lowe AS, Blamire AM, et al. Neuroadaptive responses to citalopram in rats using pharmacological magnetic resonance imaging. Psychopharmacology 2011;213(2-3):521-31.
[Crossref] [Google Scholar] [PubMed]
- Catalano G, Catalano MC, Epstein MA, Tsambiras PE. QTc interval prolongation associated with citalopram overdose: A case report and literature review. Clin Neuropharmacol 2001;24(3):158-62.
[Crossref] [Google Scholar] [PubMed]
- Hamplova-Peichlova J, Krusek J, Paclt I, Slavicek J, Lisa V, Vyskocil F. Citalopram inhibits L-type calcium channel current in rat cardiomyocytes in culture. Physiol Res 2002;51(3):317-21.
[Google Scholar] [PubMed]
- Bruggeman C, O'Day CS. Selective serotonin reuptake inhibitor toxicity. InStatPearls; 2022.
[Google Scholar] [PubMed]
- de la Torre BR, Dreher J, Malevany I, Bagli M, Kolbinger M, Omran H, et al. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Ther Drug Monit 2001;23(4):435-40.
[Crossref] [Google Scholar] [PubMed]
- Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: New drugs, old concerns? Curr Pharm Des 2004;10(20):2463-75.
[Crossref] [Google Scholar] [PubMed]
- Nose M, Barbui C. Do antidepressants prolong the QT interval? Epidemiol Psychiatr Sci 2014;23(1):19-20.
[Crossref] [Google Scholar] [PubMed]
- Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, et al. Citalopram versus other anti‐depressive agents for depression. Cochrane Database Sys Rev 2012;11(7):CD006534.
[Crossref] [Google Scholar] [PubMed]
- Grundemar L, Wohlfart B, Lagerstedt C, Bengtsson F, Eklundh G. Symptoms and signs of severe citalopram overdose. Lancet 1997;349(9065):1602.
[Crossref] [Google Scholar] [PubMed]
- Meuleman C, Jourdain P, Bellorini M, Sadeg N, Loiret J, Guillard N, et al. Citalopram and torsades de pointes: A case report. Arch Mal Coeur Vaiss 2001;94(9):1021-4.
[Google Scholar] [PubMed]
- Luchini D, Morabito G, Centini F. Case report of a fatal intoxication by citalopram. Am J Forensic Med Pathol 2005;26(4):352-4.
[Crossref] [Google Scholar] [PubMed]
- Kanjanauthai S, Kanluen T, Chareonthaitawee P. Citalopram induced torsade de pointes, a rare life-threatening side effect. Int J Cardiol 2008;131(1):e33-4.
[Crossref] [Google Scholar] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. US Food and Drug Administration; 2012.
- Rasmussen SL, Overø KF, Tanghøj P. Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol 1999;19(5):407-15.
[Crossref] [Google Scholar] [PubMed]
- Pogosova GV, Zhidko NI, Mikheeva TG, IKh B. Clinical effectiveness and safety of citalopram in patients with depression after myocardial infarction. Kardiologiia 2003;43(1):24-9.
[Google Scholar] [PubMed]
- Howland RH. A critical evaluation of the cardiac toxicity of citalopram: Part 2. J Psychosoc Nurs Ment Health Serv 2011;49(12):13-6.
[Crossref] [Google Scholar] [PubMed]
- Vieweg WV, Hasnain M, Howland RH, Hettema JM, Kogut C, Wood MA, et al. Citalopram, QTc interval prolongation, and torsade de pointes. How should we apply the recent FDA ruling? Am J Med 2012;125(9):859-68.
[Crossref] [Google Scholar] [PubMed]
- Vermoesen K, Massie A, Smolders I, Clinckers R. The antidepressants citalopram and reboxetine reduce seizure frequency in rats with chronic epilepsy. Epilepsia 2012;53(5):870-8.
[Crossref] [Google Scholar] [PubMed]
- Flores-Serrano AG, Vila-Luna ML, Álvarez-Cervera FJ, Heredia-López FJ, Góngora-Alfaro JL, Pineda JC. Clinical doses of citalopram or reboxetine differentially modulate passive and active behaviors of female Wistar rats with high or low immobility time in the forced swimming test. Pharmaco Biochem Behav 2013;110:89-97.
[Crossref] [Google Scholar] [PubMed]
- Karlsson L, Carlsson B, Hiemke C, Ahlner J, Bengtsson F, Schmitt U, et al. Altered brain concentrations of citalopram and escitalopram in P-glycoprotein deficient mice after acute and chronic treatment. Eur Neuropsychopharmacol 2013;23(11):1636-44.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Dennis KA, Darling RD, Alzghoul L, Paul IA, Simpson KL, et al. Neonatal citalopram exposure decreases serotonergic fiber density in the olfactory bulb of male but not female adult rats. Front Cell Neurosci 2013;7:67.
[Crossref] [Google Scholar] [PubMed]
- Vega Rivera NM, Gallardo Tenorio A, Fernández-Guasti A, Estrada Camarena E. The post-ovariectomy interval affects the antidepressant-like action of citalopram combined with ethynyl-estradiol in the forced swim test in middle aged rats. Pharmaceuticals 2016;9(2):21.
[Crossref] [Google Scholar] [PubMed]
- Takeuchi K, Takayama S, Hashimoto E, Itayama M, Amagase K, Izuhara C. Effect of rebamipide on gastric bleeding and ulcerogenic responses induced by aspirin plus clopidogrel under stimulation of acid secretion in rats. J Gastroenterol Hepatol 2014;29:37-46.
[Crossref] [Google Scholar] [PubMed]
- Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol 2010;641(2-3):187-92.
[Crossref] [Google Scholar] [PubMed]
- Ilgin S, Kilic V, Baysal M, Aydogan-Kilic G, Ucarcan S, Dermenci B, et al. Evidence for cardiotoxicity associated with sertraline in rats. Toxicol Res 2018;7(5):817-25.
[Crossref] [Google scholar] [PubMed]
- Lee E, Oh E, Lee J, Sul D, Lee J. Use of the tail moment of the lymphocytes to evaluate DNA damage in human biomonitoring studies. Toxicol Sci 2004;81(1):121-32.
[Crossref] [Google Scholar] [PubMed]
- Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. Elsevier health sciences; 2008.
- Calvert JW, Lefer DJ. Overview of cardiac muscle physiology. In: Hill JA, Olson EN, editors. Muscle: fundamental biology and mechanisms of disease. 1th ed. London : Academic Press; 2012. p. 57-66.
- Bech P, Tanghøj P, Andersen H, Overø K. Citalopram dose-response revisited using an alternative psychometric approach to evaluate clinical effects of four fixed citalopram doses compared to placebo in patients with major depression. Psychopharmacology 2002;163:20-5.
[Crossref] [Google Scholar] [PubMed]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers; 2005.
- Veríssimo LF, Volpini VL, Estrada VB, Matsubara NK, Gomes MV, Resstel LB, et al. Treatment with escitalopram modulates cardiovascular function in rats. Eur J Pharmacol 2018;824:120-7.
[Crossref] [Google Scholar] [PubMed]
- Maddry JK, Breyer K, Cook KM, Heard K. Monomorphic ventricular tachycardia after intentional citalopram overdose. Am J Emerg Med 2013;2(31):447-e5.
[Crossref] [Google Scholar] [PubMed]
- Hazari MS, Haykal-Coates N, Winsett DW, Costa DL, Farraj AK. Continuous electrocardiogram reveals differences in the short-term cardiotoxic response of Wistar-Kyoto and spontaneously hypertensive rats to doxorubicin. Toxicol Sci 2009;110(1):224-34.
[Crossref] [Google Scholar] [PubMed]
- Yates C, F Manini A. Utility of the electrocardiogram in drug overdose and poisoning: theoretical considerations and clinical implications. Curr Cardiol Rev 2012;8(2):137-51.
[Crossref] [Google Scholar] [PubMed]
- Lorsheyd A, De Lange DW, Hijmering ML, Cramer MJ, Van De Wiel A. PR and OTc interval prolongation on the electrocardiogram after binge drinking in healthy individuals. Neth J Med 2005;63(2):59-63.
[Google Scholar] [PubMed]
- Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. Jama 2009;301(24):2571-7.
[Crossref] [Google Scholar] [PubMed]
- Cheng M, Lu X, Huang J, Zhang S, Gu D. Electrocardiographic PR prolongation and atrial fibrillation risk: A meta‐analysis of prospective cohort studies. J Cardiovasc Electrophysiol 2015;26(1):36-41.
[Crossref] [Google Scholar] [PubMed]
- Crisel RK, Farzaneh-Far R, Na B, Whooley MA. First-degree atrioventricular block is associated with heart failure and death in persons with stable coronary artery disease: Data from the Heart and Soul Study. Eur Heart J 2011;32(15):1875-80.
[Crossref] [Google Scholar] [PubMed]
- Gambassi G, Incalzi RA, Gemma A. Atrioventricular blocks associated with citalopram. Am J Geriatr Psychiatry 2005;13(10):918-9.
[Crossref] [Google Scholar] [PubMed]
- Xiao HB, Roy C, Fujimoto S, Gibson DG. Natural history of abnormal conduction and its relation to prognosis in patients with dilated cardiomyopathy. Int J Cardiol 1996;53(2):163-70.
[Crossref] [Google Scholar] [PubMed]
- Dilaveris P, Gialafos E, Poloniecki J, Hnatkova K, Richter D, Andrikopoulos G, et al. Changes of the T‐wave amplitude and angle: An early marker of altered ventricular repolarization in hypertension. Clin Cardiol 2000;23(8):600-6.
[Crossref] [Google Scholar] [PubMed]
- Gambill CL, Wilkins ML, Haisty Jr WK, Anderson ST, Maynard C, Wagner NB, et al. T wave amplitudes in normal populations: Variation with ECG lead, sex, and age. J Electrocardiol 1995;28(3):191-7.
[Crossref] [Google Scholar] [PubMed]
- Morita H, Morita ST, Nagase S, Banba K, Nishii N, Tani Y, et al. Ventricular arrhythmia induced by sodium channel blocker in patients with Brugada syndrome. J Am Coll Cardiol 2003;42(9):1624-31.
[Crossref] [Google Scholar] [PubMed]
- Tada T, Kusano KF, Nagase S, Banba K, Miura D, Nishii N, et al. Clinical significance of macroscopic T‐wave alternans after sodium channel blocker administration in patients with Brugada syndrome. J Cardiovasc Electrophysiol 2008;19(1):56-61.
[Crossref] [Google Scholar] [PubMed]
- Annila P, Yli-Hankala A, Lindgren L. Effect of atropine on the QT interval and T-wave amplitude in healthy volunteers. Br J Anaesth 1993;71(5):736-7.
[Crossref] [Google Scholar] [PubMed]
- Kline KP, Ginsburg GP, Johnston JR. T-wave amplitude: relationships to phasic RSA and heart period changes. Int J Psychophysiol 1998;29(3):291-301.
[Crossref] [Google Scholar] [PubMed]
- Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 1995;146(1):3.
[Google Scholar] [PubMed]
- Kemp M, Donovan J, Higham H, Hooper J. Biochemical markers of myocardial injury. Br J Anaesth 2004;93(1):63-73.
[Crossref] [Google Scholar] [PubMed]
- Bodor GS. Biochemical markers of myocardial damage. Ejifcc 2016;27(2):95.
[Google Scholar] [PubMed]
- Aydin S, Ugur K, Aydin S, Sahin İ, Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc Health Risk Manag 2019:1-0.
[Crossref] [Google Scholar] [PubMed]
- Priscilla DH, Prince PS. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem Biol Interact 2009;179(2-3):118-24.
[Crossref] [Google Scholar] [PubMed]
- Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It’s time for a change to a troponin standard. Circulation 2000;102(11):1216-20.
[Crossref] [Google Scholar] [PubMed]
- Wolfe Barry JA, Barth JH, Howell SJ. Cardiac troponins: their use and relevance in anaesthesia and critical care medicine. CEACCP 2008;8(2):62-6.
- Ishida T, Ishida M, Tashiro S, Yoshizumi M, Kihara Y. Role of DNA damage in cardiovascular disease. Circ J 2014;78(1):42-50.
[Crossref] [Google Scholar] [PubMed]
- Bautista-Niño PK, Portilla-Fernandez E, Vaughan DE, Danser AJ, Roks AJ. DNA damage: A main determinant of vascular aging. Int J Mol Sci 2016;17(5):748.
[Crossref] [Google Scholar] [PubMed]
- Vakonaki E, Tsarouhas K, Spandidos DA, Tsatsakis AM. Complex interplay of DNA damage, DNA repair genes, and oxidative stress in coronary artery disease. Anatol J Cardiol 2016;16(12):939.
[Crossref] [Google Scholar] [PubMed]
- Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients 2019;11(9):2090.
[Crossref] [Google Scholar] [PubMed]
- Zhu H. Doxorubicin-Induced Cardiotoxicity. In: Tan WY, editor. Cardiotoxicity, London: Intech. Open; 2018.
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int 2014;2014:761264.
[Crossref] [Google Scholar] [PubMed]
- Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: A novel treatment target in psychiatry. Trends Pharmacol Sci 2008;29(7):346-51.
[Crossref] [Google Scholar] [PubMed]
- Ferreira AL, Matsubara LS, Matsubara BB. Anthracycline-induced cardiotoxicity. Cardiovasc Hematol Agents Med Chem 2008;6(4):278-81.
[Crossref] [Google Scholar] [PubMed]
- Sangkuhl K, Klein TE, Altman RB. PharmGKB summary: Citalopram pharmacokinetics pathway. Pharmacogenet Genomics 2011;21(11):769.
[Crossref] [Google Scholar] [PubMed]
- Usal A, Acartürk E, Yüregir Gt, Ünlükurt I, Demirci C, Kurt HI, et al. Decreased glutathione levels in acute myocardial infarction. Jp Heart J 1996;37(2):177-82.
[Crossref] [Google Scholar] [PubMed]
- Damy T, Kirsch M, Khouzami L, Caramelle P, le Corvoisier P, Roudot-Thoraval F, et al. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PloS one 2009;4(3):e4871.
[Crossref] [Google Scholar] [PubMed]
- Lin Z, Will Y. Evaluation of drugs with specific organ toxicities in organ-specific cell lines. Toxicol Sci 2012;126(1):114-27.
[Crossref] [Google Scholar] [PubMed]