- *Corresponding Author:
- J. Chen
Department of Neurology, The First Hospital of Lanzhou University, Lanzhou City, 730000, Gansu Province, China
E-mail: chenjun_0156@163.com
| This article was originally published in a special issue, "Clinical and Experimental Studies on Drug and Intervention Repurposing in China |
| Indian J Pharm Sci 2019:81(4)spl issue1;21-27 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
In order to study the therapeutic effect and safety of compound cerebral peptide injection in the treatment of Alzheimer’s disease, meta-analysis method based on medical database was adopted to comprehensively evaluate the clinical efficacy of compound cerebral peptide injection in the treatment of senile dementia. Firstly, keywords were searched in the medical database for the included studies, and the inclusion criteria and screening conditions were set. Then, the quality of the included studies was evaluated. Finally, meta-analysis, publication bias and sensitivity analysis were carried out for the included studies. In this study, two enrolled studies and 120 patients were screened. The results of meta-analysis showed that the treatment effect of compound cerebral peptide injection group was significantly improved compared with placebo treatment, and the adverse reaction time of compound cerebral peptide injection group was less than that of placebo treatment. Therefore, in the study, it has certain guiding significance for the research on the treatment of Alzheimer’s disease in the aspect of statistics.
Keywords
Medical database, meta-analysis, compound brain peptide injection, senile dementia, clinical effects
In medicine, senile dementia is known as Alzheimer’s disease, which is the first major disease of human neurodegeneration[1]. With the changes in China’s social and demographic structure, the aging degree is becoming more and more serious in China. The population of the elderly group has increased significantly. The onset age of Alzheimer’s disease is about 50-60 y old. Due to the above reasons, more and more people suffer from Alzheimer's disease in China every year. Alzheimer's disease not only seriously affects the physical and mental health of patients, but also brings a lot of troubles to patients and their families[2,3]. Therefore, it is urgent to study the therapeutic drugs and treatment schemes for Alzheimer's disease in China[4].
Compound brain peptide injection is a sterile aqueous solution based on healthy rabbit extract and pig brain extract, mainly composed of polypeptide (3.2 mg/ml), monosialotetrahexosylganglioside (0.24 mg/ml) and hypoxanthine (0.125 mg/ml)[5]. Relevant studies have shown that monosialotetrahexosylganglioside plays an important role in ganglioside, and it is an important part of nerve cell membrane[6]. And other relevant studies have proved that monosialotetrahexosylganglioside can not only restore the biochemical and morphological indicators of the nervous system to normal, but also repair the injured central nervous system[7]. Other studies have proved that monosialotetrahexosylganglioside can maintain the enzyme activity on the cell membrane, which maintain a balanced state inside and outside the nerve cells, thus protecting the cell membrane and relieving the edema and water shortage of nerve cells[8-10]. Through experimental studies, Ranyang et al. found that monosialotetrahexosylganglioside plays an indispensable role in the division, differentiation and development of nerve cells, which can reconstruct nerve cells and change various behavioral parameters of nerve cells[11]. Stell et al. conducted experiments on monkeys with neurodegenerative behavior, injected single monosialotetrahexosylganglioside into monkeys with neurodegenerative behavior, and found that the degeneration of treated monkeys was slow after continuous treatment for a period of time[12]. Numerous studies have shown that monosialotetrahexosylganglioside has excellent performance in the repair of nerve cells[13-18].
Based on the above mentioned, monosialo tetrahexosylganglioside can be used to treat Alzheimer's disease caused by degeneration of nerve cells. Therefore, through the statistical evaluation method, compound brain peptide injection containing monosia lotetrahexosylganglioside was studied for Alzheimer's disease.
Materials and Methods
Research objective and data collection:
In order to ensure the comprehensiveness of the sample set and the validity of the research results, the objective of this research includes clinically confirmed Alzheimer's disease. The inclusion criteria for Alzheimer's disease in the selection of sample set were as follows. Patients were clinically diagnosed with Alzheimer's disease or Alzheimer's superimposition syndrome, and had two items in motor impairment, quiescent tremor, limb instability, and the myotonia; patients with primary Alzheimer's disease and complications (cerebrovascular disease, dementia, encephalitis); patients with severe heart, lung, and kidney disease and multiple organ failure; patients with malignant tumors, epilepsy, and hematopoietic diseases; patients with long-term use of psychotropic drugs and mental disorders; patients with a history of Guillain-Barre syndrome; Patients with severe adverse reactions and intolerance.
The subjects were divided into experimental group and control group. The experimental group was treated with compound brain peptide injection. The control group was given a placebo and did not limit the duration of the treatment. Efficacy and safety indexes were used to test the therapeutic effect of the subjects. Efficacy index refers to the questionnaire survey of patients in the experimental group after treatment. The internationally used UPDRS scoring scale for Alzheimer's disease was adopted to evaluate the severity of Alzheimer's disease. Safety indicators refer to the occurrence of adverse reactions during treatment.
Retrieval method of medical database:
In this study, existing medical databases were classified and summarized, as shown in Table 1. The search for keywords and topics was performed in the databases listed in Table 1. The key words include compound porcine cerebroside and ganglioside injection, Alzheimer's disease or AD.
| Database | Name | Search time range |
|---|---|---|
| Foreign database | Medline | 1983 to April 2019 |
| Embase | 1983 to April 2019 | |
| Cochrane | 1993 to April 2019 | |
| Web of science | 1965 to April 2019 | |
| Chinese database | Chinese biomedical literature | 1982 to April 2019 |
| VANFUN | 1996 to April 2019 | |
| Cqvip | 1992 to April 2019 | |
| China Journal Full-text Database (CJFD) | 1997 to April 2019 |
Table 1: Currently used medical database and search time range
Literature selection and effective data acquisition:
In this research, the matching of literature titles with abstracts and contents was considered in the literature screening process, and the documents with higher matching were read in the next step. The screening of the adopted literature required two evaluators to determine whether it became the valid information, and if there was a disagreement, it was judged by a third person. The process of screening the literature by two reviewers was as follows: the literature was first evaluated by an evaluator, the main indicators of the evaluation were the efficacy index and the safety index, that is, the mean and standard deviation of UPDRS scores before and after treatment and the number of patients with adverse reactions. Then, another reviewer reviewed the selected literature. If there was a disagreement and still couldn’t reach a consensus after discussion, a third person was required to evaluate it. In case of literature data loss, ambiguity or expression problems in the selection of literature, the third party shall contact the author to verify the literature with data problems. This document was not used without the reply of the author.
Quality evaluation:
The risk of bias criteria provided by the Cochrane Collaboration was used to evaluate the quality of methodologies included in RCT. The evaluation steps are summarized as shown in Table 2.
| Step 1: random method | Low risk bias | According to the order of admission, subjects were coded successively and grouped by random number sequence |
| High risk bias | Subjects were grouped according to admission order, admission number, date of birth and day of week | |
| Not clear | The random method was not described and the original author's literature couldn’t be verified | |
| Step 2: allocation concealment | Low risk bias | The person who assigned the sequence did not participate in the inclusion of cases. The person who assigned the sequence used random numbers and sealed the numerical sequence with an opaque envelope |
| High risk bias | The above method was not used to hide random sequences or not to hide random sequences | |
| Not clear | No mention of allocation concealment | |
| Step 3: blind method | Low risk bias | Double-blind, the test group and control group received the drug with the same appearance, smell, etc. |
| High risk bias | Blindness method was not adopted, or blindness method was improper, and blindness could be broken in advance | |
| Not clear | A literature that didn’t describe the blind method and couldn’t be verified by the original author | |
| Step 4: incomplete data bias | Low risk bias | The failure rate was within 10% and the intension to treat (ITT) analysis was adopted |
| High risk bias | The failure rate was higher than 15% | |
| Moderate risk bias | The failure rate was between 10% and 15% | |
| Step 5: selective reporting bias | Low risk bias | Compared with the experimental plan, the outcome indexes of the two were identical |
| High risk bias | Compared with the experimental plan, the outcome indexes of the two were not completely consistent | |
| Not clear | The experimental plan could not be obtained, or the outcome indicators were not mentioned in the experimental plan | |
| Step 6: conflict of interest | Low risk bias | No funding from pilot manufacturers was accepted |
| High risk bias | The funding from pilot manufacturers was accepted | |
| Not clear | No declaration of interest | |
| Step 7: final quality rating | ||
| Step 8: evaluation of outcome indicators | ||
Table 2: Steps to evaluate the quality of the methodology included in RCT by the bias risk evaluation standard
Statistical method:
RevMan5.2 software provided by Cochrane collaboration was used to determine the risk bias of included studies, and then meta-analysis was conducted on the included studies. The mean or standard deviation was chosen for the sample data at 95 % confidence interval. Heterogeneity test was performed by Χ2, and the heterogeneity test was divided into two cases. One was that there was no heterogeneity between the included study groups, i.e., P>0.1, I2<0.5. Therefore, it was necessary to perform fixed-effect model processing on the included studies for meta-analysis; in another case, when inter-group heterogeneity exists, that is, P≤0.1, I2≥0.5, inter-group heterogeneity sources could be analyzed, and subgroup analysis could be conducted on the influencing factors that may generate heterogeneity. If there was statistical heterogeneity between research groups, but no clinical heterogeneity or statistical significance of differences, random effect model analysis could be carried out. If there was clinical heterogeneity, a subgroup analysis was performed. If the heterogeneity of the study was I2≥0.75 or the data can’t be searched, a descriptive study was needed.
Publication bias:
When the number of randomized controlled trials included in the literature was greater than 10, funnel plots were used to evaluate publication bias. Funnel plots verified the size of the bias risk according to the symmetry of the graph. If the risk of bias was small, the funnel plot was symmetric. If the risk of bias was large, the funnel plot was asymmetric.
Sensitivity analysis:
Abnormal results were removed, and then metaanalysis was used again to compare the two results and determine whether the results were stable. If the reobtained results were not significantly different from the previous meta-analysis results and there was no significant change, it could be considered that the literature had a low sensitivity and good stability. If the reobtained results were significantly different from the meta-analysis results or even had the opposite results, the study was considered to be highly sensitive and not stable. Results with high sensitivity and poor stability should be treated with great care, and it should note or indicate the intervention effect and the factors influencing of the intervention effect, because the source of the intervention also needs to be further discussed and explored.
Results and Discussion
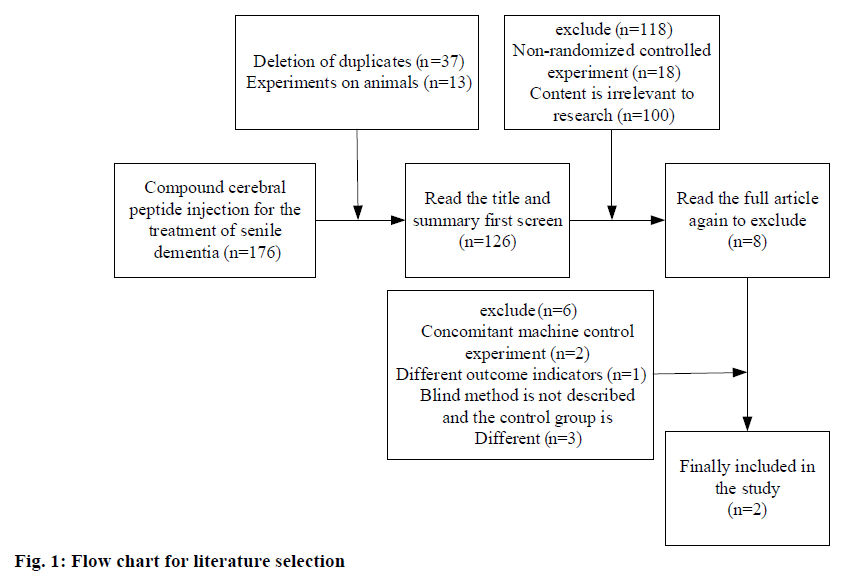
Fig. 1 is the flow chart of literature screening. It can be observed from fig. 1 that 176 relevant reports were searched according to keywords and themes. Among them, there were 76 English reports and 100 Chinese reports. By excluding the repeated reports and irrelevant reports of animal experiments, 126 articles remained. 18 non-randomized controlled trials and 100 irrelevant studies were further excluded, and the remaining 8 reports were included. In addition, 2 reports with randomized controlled trial, 1 article with different outcome indexes and 3 articles with undescribed blind method and different control group were excluded, and a total of 2 reports were finally included in the study. In the three included studies, a total of 120 cases were studied. In the 2 reports included in the study, a total of 120 cases were studied. The intervention measures of the experimental group were injection of compound cerebral peptide, and the control group was placebo treatment, which was taken for an unlimited time. The purpose of the 2 reports was to study the therapeutic effect of compound cerebral peptide injection on Alzheimer's disease, as well as the long-term safety and efficacy sustainability.
Statistics were made on the 2 included articles, as shown in Table 3. As can be observed from Table 3, the research objects of the two studies were 50 and 70, respectively. The ratio of male to female in study A was 1.57:1, the age range was 40-80 y old, and the average age was more than 60 y old. The ratio of male to female in study B was 1.63:1, with an age range of 45 to 85 y. The age of the cases in both studies was greater than 60 y old, and both were diagnosed as Alzheimer's disease for more than 5 mo. In addition, the subjects in the two studies included in the experiment group had received compound cerebral peptide injection, or the subjects received compound cerebral peptide injection for more than 1 y in the last treatment.
| Included studies | Patient age | Intervention measures | UPRS scale | Dose / (mg/d) | Course/week | Main solution indicators | Adverse reactions | ||
|---|---|---|---|---|---|---|---|---|---|
| Age range | Number of cases | Experimental group | Control group | ||||||
| Fischhof | 40~80 | 50 | Injection of compound brain peptide | Use of placebo | Yes | 150 | 24 | BRSD behavioral rating scale for dementia | Yes |
| Iwasaki | 45~85 | 70 | Injection of compound brain peptide | Use of placebo | Yes | 150 | 24 | BRSD behavioral rating scale for dementia | Yes |
Table 3: Summary of basic characteristics of the included studies
In the inclusion study with the meta-analysis, the BRSD dementia behavior rating scale was used to conduct a statistical survey of patients who received compound brain peptide injection for Alzheimer's disease, and the scores of the motor function rating scale in the scale were compared.
Both included studies were randomized, double-blind, centrally controlled, and patients were followed up at 12 mo. In the included study, the experimental group was treated with compound brain peptide injection, the control group was given placebo for an unlimited period of time, and treatment methods other than injection of compound brain peptide and placebo were not used. The treatment duration was set at 16 w in Fischhof's[12] study and 22 w in Iwasaki's[13] study. The BRSD dementia behavior rating scale scores were then used to analyze the conditions in pre-treatment, midtreatment and post-treatment stages.
The quality of the included studies needs to be evaluated before the meta-analysis. In this research, the quality of the included studies was evaluated with the bias risk assessment criteria provided by Cochrane collaboration. Random number table was generated by random test machine sequence in the selected included study, and the bias risk of random method could be identified as low risk. Since the information of study subjects and measurers of therapeutic effect included in the study were sealed in opaque envelopes and archived by a designated person, allocation concealment could be identified as a low risk. The subjects in the included study all had lost or disappeared during follow-up. The information on the loss of association and disappearance was described in detail in the study. Therefore, these information were complete for the included studies, and the risk of incomplete data bias in the included studies could be identified as low risk. When determining the risk of selective reporting bias, through analysis of the included studies, it was found that no planned reports were written in the included studies, so the risk of selective reporting bias could be identified as unclear. When identifying the risk of conflict of interest bias, it was necessary to analyze whether there was any conflict of interest with manufacturers and merchants in the included studies. Through the analysis of the included studies, no conflict of interest was found, so the risk of conflict bias could be identified as unclear.
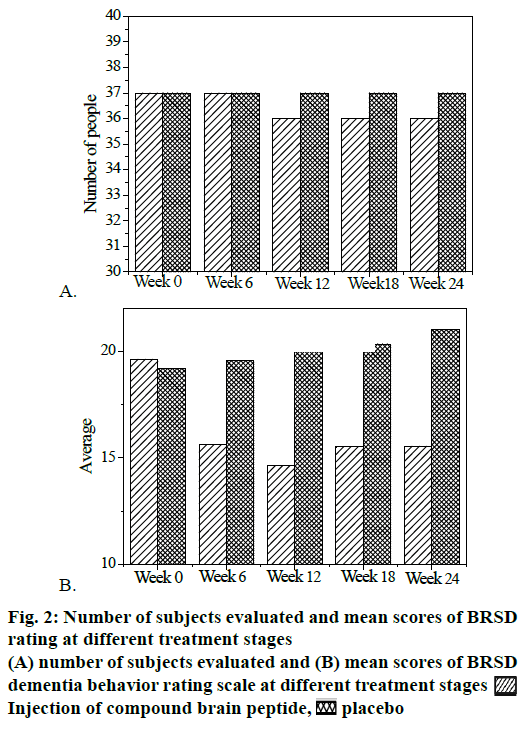
The meta-software was used to analyze the therapeutic effect of the experiment group and the control group, and the results were shown in fig. 2. According to fig. 2, the experiment group with compound cerebral peptide injection had significant statistical significance in the treatment of Alzheimer's disease.
The two included studies indicated the mean and standard deviation of the BRSD dementia behavior rating scale 10 w after the treatment. In the two studies, the heterogeneity test P=0.46 and I2=0%, so there was no statistical significance between the two groups. By combining effect size with fixed effect model, it was concluded that MD=–5.41 and CI= [–5.96, –4.91], and the hypothesis result of overall effect was p<0.000001, so MD was statistically significant. Fig. 2 shows the number of subjects evaluated with the BRSD dementia behavior rating scale at different treatment stages.
Fig. 3 shows the mean and standard deviation of the BRSD dementia behavior rating scale at each stage of treatment in Iwasaki’s (2017) study. As can be concluded from the figure, compared with the group treated with compound cerebral peptide injection, the score of BRSD dementia behavior rating scale was significantly improved compared with that before treatment. In the experiment group, nearly 81 % of the Alzheimer's patients had more than a 25 % improvement in their scale scores, and motor function scores on the scale did not deteriorate or stagnate before the end of treatment (up to w 16). There was no significant change in the BRSD dementia behavior rating scale in the control group treated with placebo, and even nearly 55 % of the patients had lower scores than before treatment.
The results of the statistical F test showed that p≤0.0002, which meant that the differences in the mean scores of the BRSD dementia behavior rating scale at the 4th, 8th, 12th, and 16th w were statistically significant. The effect of compound brain peptide injection was better than that of placebo, and the motor function of compound brain peptide injection group was improved significantly.
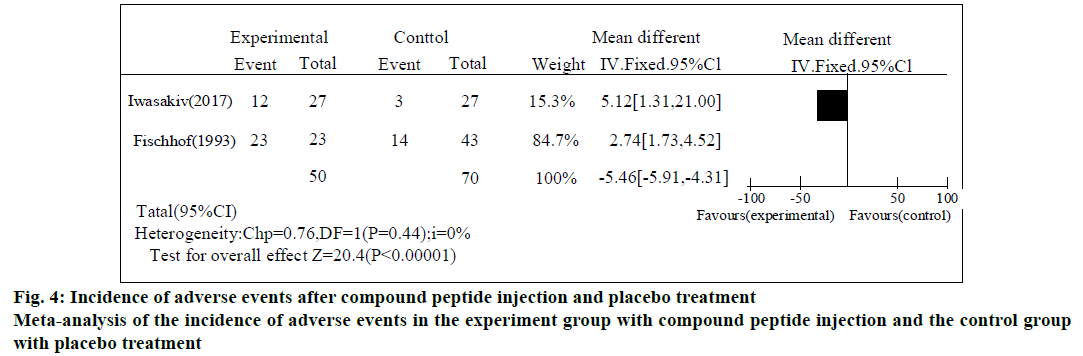
In the literature included in the study, adverse reactions in the experiment group treated with compound cerebral peptide injection and the control group treated with placebo were mainly reflected in the degree of allergy and pain tolerance at the injection site. Therefore, meta-analysis of related events at the injection site was performed in the experiment group treated with compound cerebral peptide injection and the control group treated with placebo. Fig. 4 shows the results of meta-analysis. According to the results of fig. 4, the adverse reaction events at the injection site in the experiment group treated with compound cerebral peptide injection and the control group treated with placebo were statistically significant (p<0.00001).
The two included studies described the number of patients with Alzheimer's who had adverse reactions to the treatment with the compound peptide injection. According to the analysis, in the heterogeneity test of the two included studies, P=0.36 and I2=0%, and the heterogeneity test results showed that there was no statistical heterogeneity between the injection site and adverse reactions. Therefore, the fixed effect model was used to combine the effect size, and RR=3.14, 95 % CI=[1.94, 4.95]. The overall hypothesis results of the effect test were p<0.00001, indicating that the overall RR was statistically significant and the risk of adverse reactions at the injection site caused by compound peptide injection was statistically significant compared with that caused by placebo.
The number of included studies in this research was small, according to the requirements, bias analysis could only be carried out when the included studies were more than 10, so no bias analysis was carried out for the included studies.
In the results of the meta-analysis, it was found that there was no significant heterogeneity in this study, and the overall difference of results could be obtained by combining the random fixed effect model, which indicated that the therapeutic effect and safety indicators were reliable.
In this study, meta-analysis method based on medical database was adopted to comprehensively evaluate the clinical efficacy of compound cerebral peptide injection in the treatment of senile dementia. Firstly, the medical database was used to search the literature of the included studies by keywords, the inclusion conditions and screening conditions were set, and two included studies and 120 patients were obtained. Then, quality evaluation was conducted on the included studies, and it was concluded that the two included studies were of low risk in the bias risk by random methods, low risk in the allocation of hidden data, low risk in the bias risk with incomplete data, unclear risk in the bias risk by selective reporting, and unclear risk in the conflict bias risk. Finally, meta-analysis was conducted on the included studies. The results of meta-analysis showed that the treatment effect of compound cerebral peptide injection group was significantly improved compared with placebo treatment, and the adverse reaction time of compound cerebral peptide injection group was less than that of placebo treatment.
In this research, the medical database was used to analyze the clinical effect of Alzheimer's disease. The results obtained have certain guiding significance for the clinical treatment of Alzheimer's disease. Therefore, based on the results of this study, more diseases can be combined with statistical methods, which will promote the development of medical and health care to some extent in the future.
References
- Parfenov VА, Kamchatnov PR, Vorobyova ОV, Gustov АV, Glushkov КS, Doronina ОB. Results of multicenter study of efficacy and safety of divaza in the treatment of the asthenic and mild to moderate cognitive disorders in elderly and senile subjects. Zh Nevrol Psikhiatr Im S S Korsakova 2017;117(9):43-50.
- Izumi R, Negi O, Suzuki T, Tominaga M, Kamo A, Suga Y, et al. Efficacy of an emollient containing diethylene glycol/dilinoleic acid copolymer for the treatment of dry skin and pruritus in patients with senile xerosis. J Cosmet Dermatol 2017;16(4):15-17.
- Zeng Y, Bi D, Yi Z, Lu J, Zhong F, Jiang B. Clinical study of medicinal-cake-separated moxibustion for senile osteoporosis. Zhongguo Zhen Jiu 2017;37(5):473.
- Lapid MI, Kuntz KM, Mason SS, Aakre JA, Lundt ES, Kremers W, et al. Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with lewy bodies: a pilot study. Dement Geriatr Cogn Disord 2017;43(5-6):269.
- Pakdaman H, Harandi AA, Abbasi M, Kasmaei HD, Ashrafi F, Gharagozli K, et al. Efficacy and safety of MLC601 in the treatment of mild cognitive impairment: a pilot, randomized, double-blind, placebo-controlled study. Dement Geriatr Cogn Dis Extra 2017;7(1):136-42.
- Deng YZ, Xu LG, Chen L, Zhou D, Liu Y. Effectiveness of acupuncture in the management of cervical spondylosis: a meta-analysis. J Biol Regul Homeost Agents 2017;31(4):1017-22.
- Fomin VV, Svistunov AA, Napalkov DA, Sokolova AA, Gabitova MA. Direct oral anticoagulants in atrial fibrillation patients aged 75 years or older: efficacy and safety balance. Terapevticheskii Arkhiv 2017;89(4):4-6.
- Cheng L, Liu Y, Sun XB. The clinical efficacy of yindanxinnaotong soft capsule in the treatment of stroke and angina pectoris: a meta-analysis. Evid Based Complement Alternat Med 2017;2017(6):1-14.
- Hupert M, Elfgen A, Schartmann E, Schemmert S, Buscher B, Kutzsche J, et al. Development and validation of an uhplc-esi-qtof-ms method for quantification of the highly hydrophilic amyloid-β oligomer eliminating all-d-enantiomeric peptide rd2 in mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2017;1073:123.
- Lu JX, Dong XQ, Zhang JJ. Solid-state structure of a-beta (aβ) in alzheimer's disease. Protein Pept Lett 2017;24(4):322.
- Ranyang MA, Hong YU, Wang X. Evaluation on clinical efficacy of hyperbaric oxygenation in treatment of sudden deafness:A Meta-analysis. J Jilin Univ 2017;43(2):298-305.
- Stephens M, Kim DI, Shepherd B, Gustafson S, Thomas P. Intense uptake in amyloidosis of the seminal vesicles on 68ga-psma pet mimicking locally advanced prostate cancer. Clin Nucl Med 2017;42(2):147-8.
- Tang M, Nishi K, Yamamoto T. Analysis of fluctuation in cerebral venous oxygenation using mr imaging: quantitative evaluation of vasomotor function of arterioles. Magn Reson Med Sci 2017;16(1):45-53.
- Fischhof PK, Saletu B, Rüther E, Litschauer G, Möslinger-Gehmayr R, Herrmann WM. Therapeutic efficacy of pyritinol in patients with senile dementia of the Alzheimer type (SDAT) and multi-infarct dementia (MID). Neuropsychobiology 1992;26(1-2):65-70.
- Iwasaki Y, Deguchi A, Mori K, Ito M, Mimuro M, Yoshida M. An autopsy case of a centenarian with the pathology of senile dementia of the neurofibrillary tangle type. Psychogeriatrics 2017;17(2):126-9.
- Tavares RS, Martins S, Almeida-Santos T, Sousa AP, Ramalho-Santos J, da Cruz E Silva OA. Alzheimer's disease-related amyloid-β1-42 peptide induces the loss of human sperm function. Cell Tissue Res 2017;369(3):1-5.
- Sako, H, Miyazaki M, Suematsu Y, Koyoshi R, Shiga Y, Kuwano T, et al. A case of multifaceted assessment in an elderly patient with acute decompensated heart failure. Cardiol Res 2017;8(6):339-343.
- Khalaji N, Sarkissian J, Chavushyan V, Sarkisian V. Protective effects of proline–rich peptide in a rat model of alzheimer disease: an electrophysiological study. Basic Clin Neurosci 2017;8(1):5-12.
 Injection of compound brain peptide,
Injection of compound brain peptide,  placebo
placebo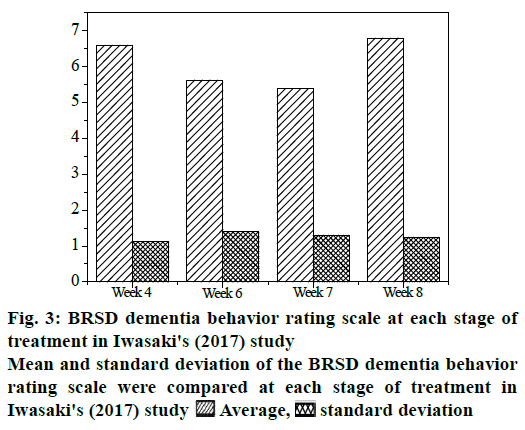
 Average,
Average,  standard deviation
standard deviation