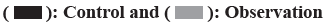- *Corresponding Author:
- Ping Wang
Department of Pediatrics, Clinical College of Huaian Maternal and Child Health Hospital, Xuzhou Medical University, Huaian, Jiangsu 223302, China
E-mail: wp7040@163.com
| This article was originally published in a special issue, “Modern Applications in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(3) Spl Issue “179-185” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The research aimed to explore the clinical effect of atomized budesonide and terbutaline inhalation for acute pediatric asthma. Ninety children with mild or moderate asthma treated in our hospital from January 2019 to December 2019 were selected as study subjects and divided into observation group and control group according using random number table, with 45 patients in each group. On acute attack of asthma, both groups received treatments of fluid rehydration, oxygen inhalation, anti-infection, oral smooth asthma drugs, etc. Both groups were treated with atomized inhalation of budesonide, with the observation group treated with extra atomized inhalation of terbutaline. The therapy lasted for continuous 7 d. The inflammatory factor levels, stress response, lung function and clinical efficacy were compared between the two groups. Forced expiratory volume 1 percentage, peak expiratory flow percentage and forced vital capacity percentage were significantly increased both in control and observation groups (p<0.05). After treatment, both groups showed lower levels of serum nitric oxide and endothelin and higher levels of serum superoxide dismutase than before, with statistically significant differences (p<0.05). There was no significant difference in serum superoxide dismutase, nitric oxide and endothelin levels between the two groups after treatment. Observation group showed no significant differences in the duration of dyspnea, cough and lung rale and hospital stay with control group after treatment (p>0.05). The serum levels of interleukin-6, interleukin-8 and tumor necrosis factor decreased significantly in both groups (p<0.05) with significantly lower levels in observation group than in control group (p<0.05). The total effective rate of observation group was significantly higher than that of control group with significant difference (p<0.05). The treatment of budesonide combined with terbutaline for acute attacks of asthma can significantly improve patients’ lung function, relieve clinical symptoms, reduce inflammation level and ultimately improve the treatment effect, which is worth clinical promotion and application.
Keywords
Pediatric asthma, budesonide, terbutaline, inflammatory factor, oxidative stress
Bronchial asthma is a chronic inflammatory disease of the trachea and bronchial airway, commonly found in early childhood and preschool children. It’s typical characteristics are highly reactive airway and limited reversible airflow[1]. Acute attack of pediatric asthma is a common critical disease. On its attack, the respiratory muscle may continue to spasm, leading to severe obstruction of pulmonary ventilation function. If not handled in time, it will seriously threaten the life safety of children. Budesonide suspension is the most commonly used clinical glucocorticoid drug, which has the effect of efficiently inhibiting the local immune response and reducing the release of allergens such as histamine[2]. Terbutaline is a specific strong Beta (β) 2-receptor agonist that can significantly inhibit the binding of histamine and target cells and inhibit the inflammatory responses[3]. At present, the prospective experimental confirmation of these two drugs are in lack of explored. Therefore, this study aims to investigate the clinical effect of atomized budesonide and terbutaline inhalation for treatment of acute attack of pediatric asthma.
Materials and Methods
General clinical data:
Ninety children with mild or moderate asthma treated in our hospital from January 2019 to December 2019 were selected as study subjects and divided into observation group and control group according using random number table, with 45 patients in each group. In the observation group, there are 28 males and 17 females, aged between 3 y and 6 y old, with mean (4.8±1.6); severity 24 mild and 21 moderate. In the control group, there are 25 males and 20 females, aged between 3 y and 6 y old, with mean (4.6±1.1); severity 23 mild and 22 moderate. The two groups had no significant difference in sex, age and severity, which was comparable. This study was approved by the ethics committee of our hospital, informed and consented by patient's family.
Inclusion criteria: All children met the diagnostic criteria for acute onset of mild to moderate pediatric asthma in the guidelines for diagnosis and treatment of bronchial asthma in children[4]; aged not more than 5, 6 is not limited and no history of glucocorticoids and bronchodilator within 2 w before treatment.
Exclusion criteria: Patients with a history of allergy or intolerance of the drugs used in this study; patients who do not cooperate with the doctor during treatment or are receiving other study drug treatment; infectious diseases in the previous month; patients with congenital defects of the immune system and a familial genetic history.
Therapeutic methods:
Both groups received treatments of rehydration, oxygen uptake, anti-infection, oral asthma drugs when acute asthma attacked.
Control group: Budesonide treatment. Atomized budesonide inhalation therapy was performed with 5 mg each dose, 2 times/d and 7 d as one course.
Observation group: Combined treatment of budesonide and terbutaline. Extra terbutaline atomized inhalation was added to the same procedure as control group; 2 times/d, 5 mg/time with 7 d as one course.
Observational indicators:
Comparison of lung function Forced Expiratory Volume 1 Percentage (FEV1 %), Peak Expiratory Flow Percentage (PEF %) and Forced Vital Capacity Percentage (FVC %) were measured before and after treatment using an asthma electronic detector (NK360). After 7 d of treatment, the clinical efficacy of the two groups was determined to be marked effective, effective and ineffective. The criteria[5] were as follows
Complete cure: The clinical symptoms of the child were completely relieved, FEV1 % or PEF % increased by 35 %, PEF % fluctuated by 20 % between day and night.
Marked effective: The clinical symptoms were significantly improved, 25 % ≤FEV1 %, ≤35 % or ≤25 % the increase of PEF % ≤35 % and diurnal fluctuation of PEF % >20 %.
Effective: Clinical symptoms were relieved to a certain extent, 15 % ≤FEV1 % <25 % or the increase of PEF % <25 % and the diurnal fluctuation of PEF % >20 %.
Ineffective: The clinical symptoms were not relieved or even aggravated, and there was no increase in FEV1 % or PEF %
Total response rate=(Complete cure+Marked effective+Effective)/Total cases×100 %
Compare, when the symptoms disappeared in the two groups, including cough, dyspnea and lung rale. The quicker time meant better efficacy. Content determination of serum Superoxide Dismutase (SOD) and Fractional Excretion of Nitric Oxide (FENO). The expression levels of SOD and Endothelin (ET) were measured by radioimmunoassay. The expression levels of Nitric Oxide (NO) were measured by nitrate reductase assay.
Detection of serum inflammatory factors. 5 ml of peripheral venous blood was collected from the two groups before and early morning after treatment. The supernatant was collected by centrifugation and serum Interleukin (IL)-6, IL-8 and Tumor Necrosis Factor Alpha (TNF-α) levels were measured by Enzyme-Linked Immunoassay (ELISA). The kit was provided by Suzhou Jianglai Biological and all operations were performed according to the instructions.
Statistical analysis:
The whole analysis processing was performed using Statistical Package for the Social Sciences (SPSS) 20.0 statistical software. Measurement data were expressed as x±s and analyzed using t-test; count data was expressed as percentage. p<0.05 showed statistical significance.
Results and Discussion
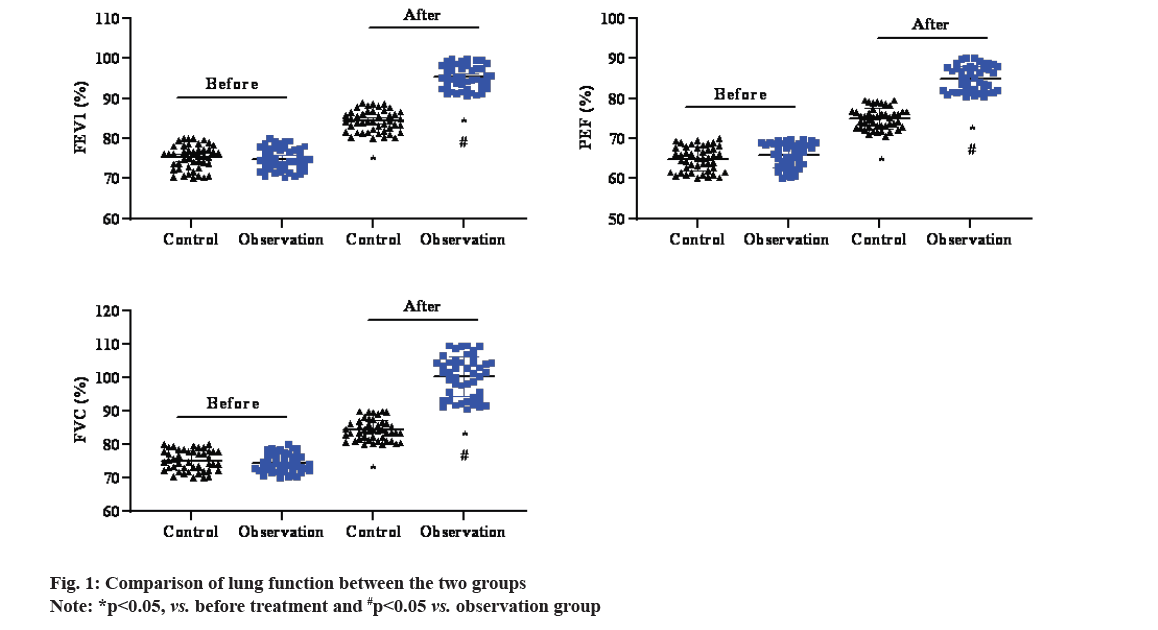
Before treatment, there was no significant difference in corresponding operational indicators between the two groups (p<0.05). FEV1 %, PEF % and FVC % were significantly higher in both control and observation groups (p<0.05), with higher levels in observation group than in control group (p<0.05), as shown in fig. 1.
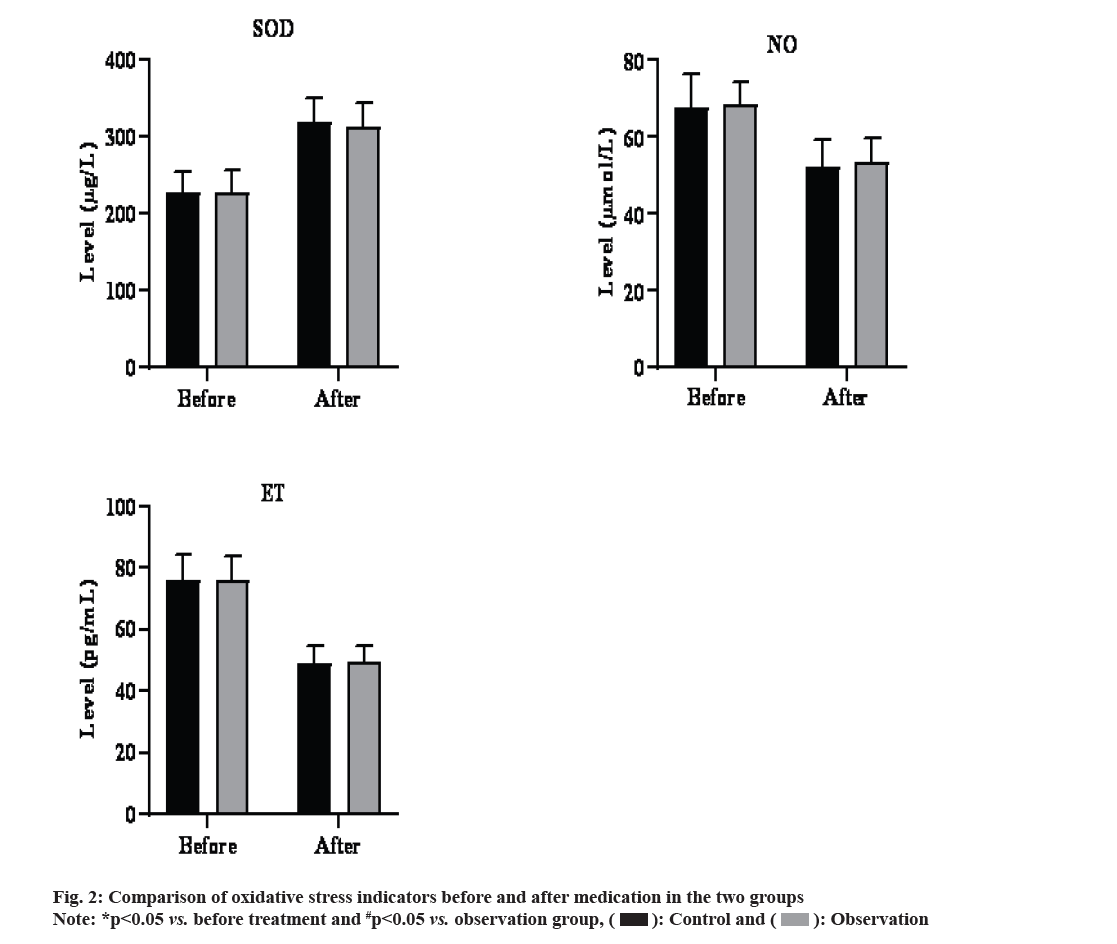
Before treatment, there was no difference in serum SOD, NO and ET levels between the observation group and the control group (p>0.05). After treatment, both groups showed significantly lower serum NO and ET levels and higher SOD level than before (p<0.05).
There was no difference in serum SOD, NO and ET levels between the 2 groups after treatment (p<0.05). The results were shown in fig. 2.
There was no statistically significant difference in the duration of dyspnea, cough and lung rales and the hospital stay between the 2 groups after treatment (p<0.05), as shown in Table 1.
| Group | n | Dyspnea (min) | Cough (min) | Lung rales (min) | Asthma (d) | Hospital stay (d) |
|---|---|---|---|---|---|---|
| Observation | 45 | 1.34±0.80 | 0.68±0.06 | 1.14±0.17 | 5.22±1.21 | 6.81±1.79 |
| Control | 45 | 1.26±0.15 | 0.71±0.13 | 1.17±0.13 | 5.14±1.33 | 6.87±1.65 |
| t | 0.331 | 1.314 | 0.752 | 0.415 | 0.184 | |
| p | 0.374 | 0.087 | 0.218 | 0.331 | 0.412 |
Table 1: Comparison of time indicators between the two Groups
Before treatment, there was no significant difference in corresponding indicators between the observation and control groups (p<0.05). After treatment, the serum levels of IL-6, IL-8 and TNF-α decreased significantly in both groups (p<0.05) with lower levels in observation group than in control group (p<0.05). The results are shown in fig. 3.
The total effective rate of the patients in the observation group was remarkably higher than that in the control group with statistically significant difference (p<0.05), as shown in Table 2.
| Group | n | Complete cure | Marked effective | Effective | Ineffective | Ineffective |
|---|---|---|---|---|---|---|
| Observation | 45 | 9 (20.00) | 12 (26.67) | 15 (33.33) | 9 (20.00) | 80 |
| Control | 45 | 16 (35.56) | 14 (31.11) | 11 (24.44) | 4 (8.89) | 91.11 |
| t | - | 9.973 | ||||
| p | - | <0.001 | ||||
Table 2: Comparison of the clinical efficacy between the two Groups
In recent years, the incidence of childhood asthma increases by year, which has drawn global attention as a public health problem. Children account for about 3 % of the asthma population[6,7]. Childhood is the stage of rapid physical and psychological growth. Repeated attacks of asthma not only seriously threaten the life safety of children, but also may have a serious impact on their psychology. The pathogenesis of childhood asthma is complex, diverse and not completely clear. The mainstream view is that pediatric asthma is a chronic inflammatory response associated with multiple cells and components of the airway[8-10]. The acute attack of asthma is caused by airway hypersensitivity resulted from this abnormal immune response[11-13]. Repeated attacks of asthma can not only lead to hypoxia and asphyxia in children in the short term, but also lead to airway fibrosis in the long term, affecting ventilation and gas exchange[14,15].
Budesonide is a glucocorticoid that can inhibit the immune response by enhancing the stability of endothelial cells and lysosomes membrane in children, reduce the secretion of allergic active mediators such as histamine and various inflammatory secretions, inhibit smooth muscle contraction, dilate the trachea, reduce respiratory tract obstruction and relieve respiratory spasm[16]. Terbutaline is a β2-receptor agonist for the treatment of acute asthma attacks, which relieves dyspnea in a short period of time and maintains efficacy for 6 h[17]. Terbutaline can promote the synthesis of internal cyclic adenosine phosphate, effectively reduce the concentration of calcium ions in the patient’s body[18] and then effectively inhibit the release of inflammatory factors to a certain extent, reduce edema caused by cytokines[19], inhibit tracheal smooth muscle contraction, relieve symptoms of shortness of breath and improve lung function[20]. Pulmonary function is a common clinical evaluation index for the treatment of children with asthma. In this study, budesonide and terbutaline were used to treat pediatric patients with mild to moderate asthma and it was found higher FEV1 %, PEF % and FVC % in observation group than in control group (p<0.05). The total clinical effective rate of observation group was 94.83 %, also significantly better than that of control group (p<0.05), suggesting that the combination of terbutaline and budesonide can indeed improve lung function, relieve clinical symptoms and improve clinical efficiency in children with asthma.
In this study, serum IL-6, IL-8 and TNF-α decreased significantly in both groups, with significant lower levels in observation group than control group (p<0.05). IL-6, IL-8 and TNF-α are inflammatory factors expressed downstream of Toll-like Receptor 4 (TLR4) and Signal Transducer and Activator of Transcription 1 (STATI) messenger Ribonucleic Acid (mRNA), which can directly or indirectly participate in, amplify or suppress the immune response. IL-6, a marker of inflammatory response secreted by activated T cells, amplifies immune inflammatory response and is significantly increased in various inflammation-related diseases; IL-8 has characteristic of chemotaxis besides mediating inflammatory responses. TNF-α is mainly secreted by activated mononuclear macrophages, which can promote the proliferation and differentiation of macrophages and exert anti-infection effects and is an important inflammatory factor[21]. Analysis showed ciliary activity distributed on bronchial mucosa helps discharge inflammatory secretions in airway and reduce airway resistance[13] Meanwhile, TNF-α and IL in asthma patients can cooperate with Granulocyte-Colony Stimulating Factor (G-CSF) to accelerate the synthesis and release of histamine. The rapid growth of histamine will aggravate the downstream immune response, stimulate the secretion of TNF-α and IL and aggravate the inflammatory damage[22]. Terbutaline attenuates the inflammatory response mediated by IL-6, IL-8 and TNF-α secreted by strengthening the ciliary activity in the bronchial mucosa[23]. The reduction of IL-6, IL-8 and TNF content can feedback to regulate and reduce the generation of inflammatory secretions, so as to reduce the airway inflammatory response in children with asthma.
In this study, there was no significant difference (p>0.05) in the PEFR % and FEV1 % between the 2 groups before treatment (p<0.05) and observation group showed better indicators (PEFR % and FEV1 %) than control group after treatment; wheezing, dyspnea and cough were shorter than (p<0.05) and it was higher than the control group (p<0.05). The observation group showed shorter duration of wheezing, dyspnea and cough (p<0.05) and higher total effective rate than control group (p<0.05). Meanwhile, after treatment, the serum NO and ET levels of the two groups were significantly lower than before, while there was no significant difference between the groups. After analysis, budesonide is an anti-inflammatory glucocorticoid, mainly used in the treatment of bronchial asthma as well as asthma chronic bronchitis disease. This drug can improve the stability of smooth muscle cells and endothelial cells to inhibit the immune response, reduce the composition of antibodies, release of allergic activity and the enzymatic reaction, prevent the release and synthesis of bronchial substances and reduce the smooth muscle contraction reaction. Its pharmacological effects are promoting the synthesis of lipocortin, inhibiting phospholipase and reducing the release and synthesis of platelet activation factors and prostaglandins. Meanwhile, it accelerates the synthesis of angiotensin 2, promotes adrenaline receptor, increases ET production, inhibits oxidative NO synthase, reduces the production of oxidative NO, shrinks blood vessels, improves receptor sensitivity and has immunosuppressive effect. However, the effect of this drug on acute asthma is not effective enough with high untoward effect incidence. Terbutaline is a selective receptor agonist for bronchial asthma and wheezing bronchitis and also for patients with coronary heart disease, hypertension and asthma. It dilates bronchi and has high selectivity for smooth muscle, but has no central nervous effect. Its pharmacological effect was high selection of airway receptor and bronchodilation; the inter hydroxyphenol ring replaces catechol with hydrogen atoms replaced on the ethanol side chain and the structure is not easily inactivated by sulfate kinase thus to create long-lasting effect. It can also stimulate the myometrium receptor to inhibit uterine contraction. The combination of this product with budesonide can increase the treatment effect, reduce the incidence of adverse reactions, promote the recovery speed, improve clinical symptoms and mucosal edema, relieve smooth muscle spasm, airway obstruction, promote the growth and development of children, improve the quality of life[24].
In conclusion, the treatment of budesonide combined with terbutaline for acute attacks of asthma can significantly improve patients’ lung function, relieve clinical symptoms, reduce inflammation level and ultimately improve the treatment effect, which is worth clinical promotion and application.
Acknowledgements:
This work was supported by scientific research project of Huaian Science and Technology Bureau of Jiangsu Province (No. HAS2015010) and Subject of Young Talents by Science and Education of Jiangsu Provincial Health Committee (No. QNRC2016441).
Conflict of interests:
The authors declared no conflict of interests.
References
- O’Byrne PM, FitzGerald JM, Zhong N, Bateman E, Barnes PJ, Keen C, et al. The SYGMA programme of phase 3 trials to evaluate the efficacy and safety of budesonide/formoterol given ‘as needed’ in mild asthma: Study protocols for two randomised controlled trials. Trials 2017;18(1):12.
[Crossref] [Google Scholar] [Pub Med]
- Bisgaard H, Le Roux P, Bjamer D, Dymek A, Vermeulen JH, Hultquist C. Budesonide/formoterol maintenance plus reliever therapy: A new strategy in pediatric asthma. Chest 2006;130(6):1733-43.
[Crossref] [Google Scholar] [Pub Med]
- Waalkens HJ, Gerritsen J, Koeter GH, Krouwels FH, van AAlderen WM, Knol K. Budesonide and terbutaline or terbutaline alone in children with mild asthma: Effects on bronchial hyper responsiveness and diurnal variation in peak flow. Thorax 1991;46(7):499-503.
[Crossref] [Google Scholar] [Pub Med]
- Fuglsang G, Agertoft L, Vikre-Jorgensen J, Pedersen S. Influence of budesonide on the response to inhaled terbutaline in children with mild asthma. Pediatr Allergy Immunol 1995;6(2):103-8.
[Crossref] [Google Scholar] [Pub Med]
- Grover S, Jindal A, Bansal A, Singhi SC. Acute bronchial asthma. Indian J Pediatr 2011;78(11):1388-95.
[Crossref] [Google Scholar] [Pub Med]
- Bush A, Saglani S. Management of severe asthma in children. Lancet 2010;376(9743):814-25.
[Crossref] [Google Scholar] [Pub Med]
- Volovitz B, Bilavsky E, Nussinovitch M. Effectiveness of high repeated doses of inhaled budesonide or fluticasone in controlling acute asthma exacerbations in young children. J Asthma 2008;45(7):561-7.
[Crossref] [Google Scholar] [Pub Med]
- Nuho?lu Y, Bahçeciler NN, Barlan IB, Ba?aran MM. The effectiveness of high-dose inhaled budesonide therapy in the treatment of acute asthma exacerbations in children. Ann Allergy Asthma Immunol 2001;86(3):318-22.
[Crossref] [Google Scholar] [Pub Med]
- Fuglsang G, Vikre-Jørgensen J, Agertoft L, Pedersen S. Effect of salmeterol treatment on nitric oxide level in exhaled air and dose–response to terbutaline in children with mild asthma. Pediatr Pulmonol 1998;25(5):314-21.
[Crossref] [Google Scholar] [Pub Med]
- Vikre-Jørgensen J, Agertoft L, Pedersen S. Dose titration of nebulized budesonide in young children. Pediatr Pulmonol 1997;23(4):270-7.
[Crossref] [Google Scholar] [Pub Med]
- Tsai YG, Lee MY, Yang KD, Chu DM, Yuh YS, Hung CH. A single dose of nebulized budesonide decreases exhaled nitric oxide in children with acute asthma. J Pediatr 2001;139(3):433-7.
[Crossref] [Google Scholar] [Pub Med]
- Aagaard L, Hansen EH. Paediatric adverse drug reactions following use of asthma medications in Europe from 2007 to 2011. Int J Clin Pharm 2014;36(6):1222-9.
[Crossref] [Google Scholar] [Pub Med]
- Buchvald F, Bisgaard H. FeNO measured at fixed exhalation flow rate during controlled tidal breathing in children from the age of 2 yr. Am J Respir Crit Care Med 2001;163(3):699-704.
[Crossref] [Google Scholar] [Pub Med]
- Volovitz B, Bentur L, Finkelstein Y, Mansour Y, Shalitin S, Nussinovitch M, et al. Effectiveness and safety of inhaled corticosteroids in controlling acute asthma attacks in children who were treated in the emergency department: A controlled comparative study with oral prednisolone. J Allergy Clin Immunol 1998;102(4):605-9.
[Crossref] [Google Scholar] [Pub Med]
- Sethi S, Kerwin E, Watz H, Ferguson GT, Mroz RM, Segarra R, et al. AMPLIFY: A randomized, phase III study evaluating the efficacy and safety of aclidinium/formoterol vs. monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J Chron Obstruct Pulmon Dis 2019;14:667-82.
[Crossref] [Google Scholar] [Pub Med]
- Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten AM, et al. Efficacy and safety of aclidinium/formoterol vs. salmeterol/fluticasone: A phase 3 COPD study. Eur Respir J 2016;48(4):1030-9.
[Crossref] [Google Scholar] [Pub Med]
- Mogayzel Jr PJ, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Cystic fibrosis pulmonary guidelines: Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187(7):680-9.
[Crossref] [Google Scholar] [Pub Med]
- Wood ME, Stockwell RE, Johnson GR, Ramsay KA, Sherrard LJ, Kidd TJ, et al. Cystic fibrosis pathogens survive for extended periods within cough-generated droplet nuclei. Thorax 2019;74(1):87-90.
[Crossref] [Google Scholar] [Pub Med]
- Knibbs LD, Johnson GR, Kidd TJ, Cheney J, Grimwood K, Kattenbelt JA, et al. Viability of Pseudomonas aeruginosa in cough aerosols generated by persons with cystic fibrosis. Thorax 2014;69(8):740-5.
[Crossref] [Google Scholar] [Pub Med]
- Wood ME, Stockwell RE, Johnson GR, Ramsay KA, Sherrard LJ, Jabbour N, et al. Face masks and cough etiquette reduce the cough aerosol concentration of Pseudomonas aeruginosa in people with cystic fibrosis. Am J Respir Crit Care Med 2018;197(3):348-55.
[Crossref] [Google Scholar] [Pub Med]
- Cullen KA, Ambrose BK, Gentzke AS, Apelberg BJ, Jamal A, King BA. Notes from the field: Use of electronic cigarettes and any tobacco product among middle and high school students-United States, 2011-2018. Morb Mortal Wkly Rep 2018;67(45):1276-7.
[Crossref] [Google Scholar] [Pub Med]
- Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, et al. e-Cigarette use among youth in the United States, 2019. JAMA 2019;322(21):2095-103.
[Crossref] [Google Scholar] [Pub Med]
- Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, et al. Pathology of vaping-associated lung injury. N Engl J Med 2019;381(18):1780-1.
[Crossref] [Google Scholar] [Pub Med]
- Bayly JE, Bernat D, Porter L, Choi K. Secondhand exposure to aerosols from electronic nicotine delivery systems and asthma exacerbations among youth with asthma. Chest 2019;155(1):88-93.
[Crossref] [Google Scholar] [Pub Med]