- *Corresponding Author:
- Junling Zhang
Department of Dermatology, Tianjin Institute of Integrative Dermatology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Hongqiao, Tianjin 300120, China
E-mail: 13920301679@126.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “284-289” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the clinical effects of tazarotene betamethasone cream combined with compounded allantoin cream in the treatment of chronic hand eczema. 95 patients with chronic hand eczema were randomly divided into a study group (n=48) and a control group (n=47). Study group received tazarotene betamethasone cream in combination with compounded allantoin cream and control group received tretinoin acetate 1 % cream in combination with compounded allantoin cream. The clinical efficacy, lesion degree score and pruritus score, incidence of adverse reactions and quality of life score of the two groups were compared. Study group had a higher treatment efficiency than control group (p<0.05). After treatment, study group had lower lesion extent score, pruritus score and dermatological life quality index than control group (p<0.05). There was no difference in the incidence of adverse reactions between the two groups (p<0.05). Tazarotene betamethasone cream combined with compounded allantoin cream is clinically effective and safe in the treatment of chronic hand eczema, and has a significant positive effect on the improvement of patients' quality of life.
Keywords
Chronic hand eczema, tazarotene betamethasone, adverse reactions, anxiety, depression
Chronic Hand Eczema (CHE) is a common inflammatory skin disease that occurs mainly on the hands and wrists, and is heterogeneous. It is characterized by itching, pain and dry skin[1,2]. The prevalence is higher in women than in men[3,4]. The duration of the disease is ≥3 mo or at least 2 episodes per year. Exogenous factors such as skin irritants, proteins or contact allergens and endogenous factors such as immune dysfunction and dysbiosis of the skin microbiome can all contribute to CHE or cause an exacerbation of CHE[5-7]. Patients with CHE often experience pain, itching and burning sensations that interfere with activities such as daily life and work, and cause sleep disturbances and concern about the aesthetics of the hands[8]. Due to the variety of causes and complexity of the pathogenesis, there is no standard treatment for CHE[9]. Currently, treatment of CHE is based on topical symptomatic management, such as topical corticosteroids and calcium phosphatase inhibitors, but also includes systemic treatment such as oral antihistamines and immunosuppressant’s when necessary. However, these options do not reduce the recurrence of CHE and the prolonged use of drugs such as corticosteroids can lead to atrophy and damage to the skin barrier[6]. Current treatment options are still limited and the management of CHE remains difficult and unsatisfactory[10,11].
Tazarotene betamethasone cream, a new fixed formulation consisting of 0.05 % tazarotene and 0.05 % betamethasone dipropionate, has been formalized as having a significant role in the treatment of psoriasis[12-14]. Previous studies have shown that Tazarotene Inducible Gene Protein 2 (TIG2) correlates with the severity of eczema, with the more severe the patient’s condition, the higher the TIG2 protein expression[15]. A Chinese study showed that tazarotene was effective in the treatment of chronic hypertrophic eczema[16]. Allantoin, a naturally occurring biologically active metabolic intermediate derived from animals, bacteria and plants, is the main ingredient in allantoin cream. It has been widely used to promote wound healing, treat skin ulcers and other skin defects[17,18] and is also effective in the treatment of eczema[19]. However, the effect of tazarotene betamethasone cream in combination with compounded allantoin cream in CHE has not been reported in studies. We believe that the use of tazarotene betamethasone cream in conjunction with allantoin cream is crucial for the treatment of CHE. Based on this, we conducted a randomized controlled study to analyses the clinical effects of tazarotene betamethasone cream in combination with compounded allantoin cream in the treatment of CHE, with the aim of providing an effective and feasible reference for the clinical management of CHE.
Materials and Methods
Study design:
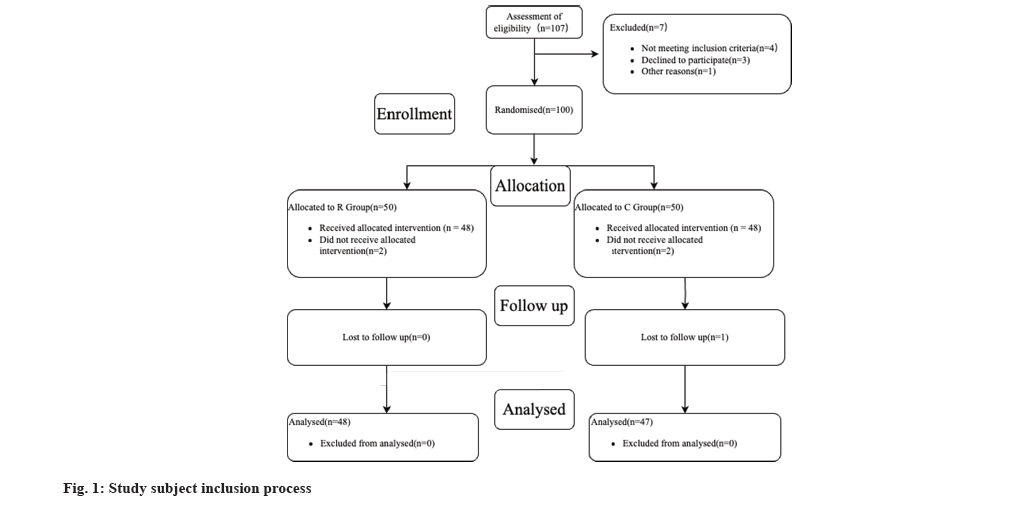
Ninety-five patients with CHE treated at the Affiliated Hospital of Tianjin Academy of Chinese Medicine between January 2020 and December 2021 were divided into a Study group (group S) (n=48) and a Control group (group C) (n=47) using a random number table. Group S was treated with tazarotene betamethasone cream combined with clotrimazole cream and group C received tretinoin acetate 1 % cream combined with compounded allantoin cream. All participating patients signed an informed consent form and the medical ethics committee of the affiliated hospital of Tianjin Academy of traditional Chinese medicine supported the study as shown in fig. 1.
Inclusion and exclusion criteria:
Inclusion criteria: Meeting the diagnostic criteria for eczema, negative local fungal microscopy of lesions, confirmed by local dermoscopy and diagnosis of complex inflammatory dermatoses; lesions limited to the hands, duration of disease ≥3 mo or recurrence ≥2 times per year; age ≥18 y and Hand Eczema Severity Index (HECSI) score 0-27[20].
Exclusion criteria: Combination of cardiac, hepatic, renal and other vital organ dysfunction; combination of malignancy; combination of immune system disorders; application of other drugs within 2 w before the start of the study at the site of the rupture; long-term systemic application of hormones or immunosuppressive drugs; and pregnant or lactating women.
Treatment:
Patients in the group C were treated with 1 % tretinoin acetate cream in combination with allantoin cream. 1 % tretinoin acetate cream was applied to the lesions and rubbed gently until the drug was completely absorbed, 2-3 times/d. Allantoin cream was applied in the same way as 1 % tretinoin acetate cream.
Patients in the group S were treated with tazarotene betamethasone cream in combination with compounded allantoin cream. The compounded allantoin cream was applied as in the control group. After washing the affected area and allowing the skin to dry, apply an appropriate amount of tazarotene betamethasone cream evenly to the affected area, avoiding contact with normal skin, and wash hands with soap and water after application. Apply once daily, at bedtime. The total area of each application should not exceed 20 % of the body surface area. The total weekly dosage should not exceed 45 g. Patients in both groups were treated continuously for 2 w.
Outcomes:
Primary outcomes: Clinical efficacy is assessed by the rate of skin lesion clearance. In HESCI scoring method, each hand is divided into 5 areas; fingertips, fingers (except fingertips), palm, back of hand and wrist, and the extent of involvement in each area is scored as 0 for no involvement, 1 for 1 %-25 %, 2 for 26 %-50 %, 3 for 51 %-75 % and 4 for 76 %-100 %. The combination of the total score and the extent of engagement score for each area yields the overall HESCI score, which has a point range of 0-360. Scores between 12 to 27 are regarded as moderate, while scores over 28 are regarded as severe[20,21]. Healing is defined as 90 %-100 % clearance of lesions; efficacy is defined as 60 %-89 % clearance of lesions; improvement is defined as 20 %-59 % clearance of lesions; and ineffectiveness is defined as 0 %-19 % clearance of lesions.
Treatment efficiency=(cured+efficacious)/total×100 %
Extent of lesions score: The extent of lesions was scored for both groups based on mossiness, chancroid, keratinisation and infiltrative hypertrophy. A 4-point scale from 0 to 3 was used, with a score of 3 indicating marked mossiness and infiltrative hypertrophy; a score of 2 indicating mossiness and infiltrative hypertrophy but not marked; a score of 1 indicating mild infiltration and no mossiness; and a score of 0 indicating no lesions or residual hyperpigmentation. Lower scores indicate milder symptoms.
Pruritus score: A 4-point scale from 0 to 3 is used, with 3 indicating severe, 2 indicating moderate, 1 indicating mild and O indicating none. Higher scores indicate more severe itching.
Secondary outcomes: Record the adverse reactions that occurred during and after treatment in both groups. Quality of life was assessed in both groups using the Dermatological Life Quality Index (DLQI) [22,23]. Each of the 10 entries on the scale is given a score on a 4-point scale, with 0–3 denoting none–very significant and a total score of 0–30 indicating a lower quality of life.
Statistical analysis:
The Statistical Package for the Social Sciences (SPSS) 21.0 statistical software for social sciences was used to analyze the data. The Chi-square (χ²) test was used to compare data, and statistics were presented as [n (%)]. The Mann-Whitney U test was used to compare between groups, and the Kolmogorov-Smirnov test was performed to determine if the data were normally distributed. Non-normally distributed measures were reported as median and quartiles. Measures that conformed to the normal distribution were expressed as (x̄±s), and comparisons were made using the t-test, Analysis of Variance (ANOVA) and Least Significant Difference (LSD) test. At p<0.05, differences were declared statistically significant.
Results and Discussion
Between the two groups, there were no variations in the demographic and baseline data (p>0.05, Table 1). Group S had a higher treatment efficiency than group C (p<0.05, Table 2).
| Group S (n=48) | Group C (n=47) | χ²/t | p value | |
|---|---|---|---|---|
| Age (years, mean±SD) | 43.52±3.48 | 43.61±3.24 | 0.13 | 0.897 |
| Female/male, n (%) | 30 (62.50)/18 (37.50) | 31(65.96)/16 (34.04) | 0.124 | 0.725 |
| Duration of CHE (months, mean±SD) | 13.26±2.31 | 13.29±2.25 | 0.064 | 0.949 |
| CHE subtypes (main diagnosis), n (%) | ||||
| Allergic contact dermatitis | 5 (10.42) | 4 (8.51) | 0.101 | 0.751 |
| Irritant contact dermatitis | 14 (29.17) | 16 (34.04) | 0.261 | 0.609 |
| Contact urticaria/protein contact dermatitis | 0 (0.00) | 0 (0.00) | 0 | 1 |
| Atopic hand eczema | 18 (37.50) | 18 (38.30) | 0.006 | 0.936 |
| Pompholyx | 5 (10.42) | 5 (10.64) | 0.001 | 0.972 |
| Hyperkeratotic eczema | 6 (12.50) | 4 (8.51) | 0.401 | 526 |
| HECSI score, n (%) | ||||
| 0~11 | 25 (52.08) | 23 (48.94) | 0.094 | 0.759 |
| 12~27 | 20 (41.67) | 22 (46.81) | 0.255 | 0.614 |
| ≥28 | 3 (6.25) | 2 (4.26) | 0.19 | 0.663 |
Table 1: Demographics and baseline information for both groups.
| Group S (n=48) | Group C (n=47) | χ² | p value | |
|---|---|---|---|---|
| Cured | 5 (10.42) | 3 (6,38) | ||
| Effective | 37 (77.08) | 30 (63.83) | ||
| Improved | 4 (8.33) | 8 (17.02) | ||
| Ineffective | 2 (4.17) | 6 (12.77) | ||
| Cured+effective | 42 (87.50) | 33 (70.21) | 4.27 | 0.039 |
Table 2: Clinical outcomes [n (%)].
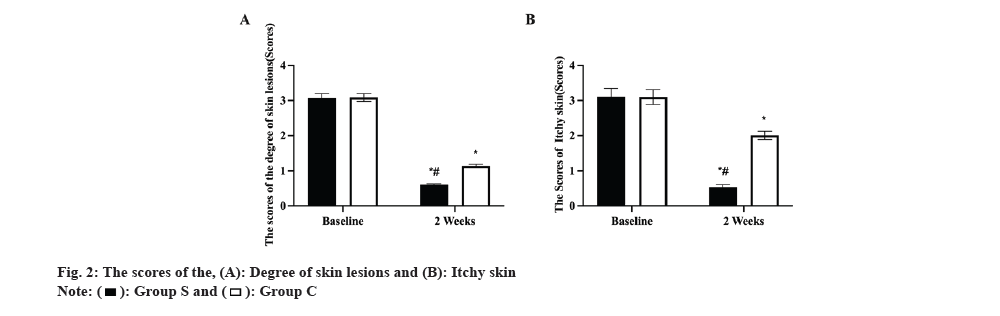
Prior to therapy, there was no difference between the two groups in the amount of lesion score or pruritus score (p>0.05). Lesion extent and pruritus scores decreased after treatment in both groups, with group S having a lower score than group C (p<0.05, fig. 2A and fig. 2B).
Group S patients did not experience any adverse reactions during and after treatment, with an incidence of 0 %; group C patients experienced one case of hyperpigmentation and one case of pain, with an incidence of 4.26 %. The difference between the two groups was not statistically significant (χ²=2.087, p=0.149).
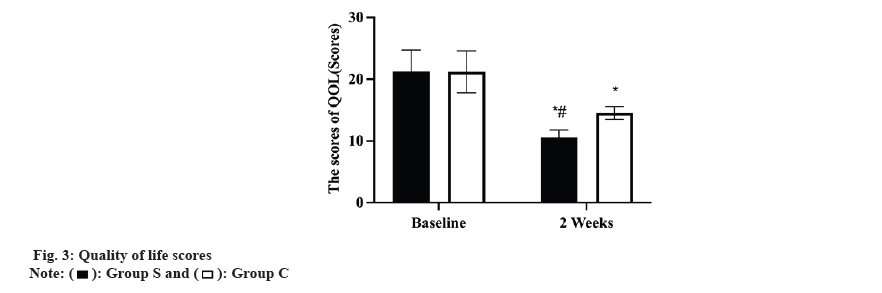
Prior to therapy, there was no difference between the two group’s DLQI ratings (p>0.05). Both groups experienced a decline in DLQI ratings following therapy, with group S experiencing a greater decline than group C (p<0.05, fig. 3).
In this study, daily treatment with tazarotene betamethasone cream in combination with compounded allantoin cream for 2 w in patients with mild to severe CHE showed better therapeutic efficacy without causing any adverse effects and a high safety profile.
Compared to group C, group S has a treatment efficacy rate of 87.50 % after 2 w of treatment. Tazarotene is a third generation vitamin A derivative that selectively acts on the beta and gamma groups of the retinoic acid receptor, resulting in significant anti-proliferative, anti-inflammatory and differentiation normalizing effects[24,25]. Previous studies have shown that tazarotene is safe and effective for treating psoriasis[26,27]. CHE and psoriasis are also inflammatory skin diseases, so we applied tazarotene to the treatment of CHE based on the theory of synergistic effects and showed that tazarotene betamethasone cream combined with compounded allantoin cream was more effective than tretinoin acetate 1 % combined with compounded allantoin cream, tazarotene betamethasone cream achieved significant results in the treatment of CHE as expected, validating the hypothesis.
Tazarotene regulates the mitosis of keratin-forming cells, inhibits the proliferation, degeneration and terminal differentiation of keratin-forming cells, inhibits the production of keratin and thus inhibits the proliferation and differentiation of the skin at the site of the CHE lesion, resulting in a gradual thinning of the thickened skin and a gradual return to normal. Local pruritus, one of the most prevalent CHE symptoms, has a significant detrimental effect on patient’s quality of life[2]. The anti-inflammatory effect of tazarotene is effective in reducing the irritation of the skin by inflammatory factors, thus reducing the pruritus at the lesions. According to the study’s findings, group S post-treatment lesion extent and pruritus scores were lower than those of group C, confirming the good synergistic effect of tazarotene betamethasone cream in combination with compounded allantoin cream in improving the clinical symptoms of CHE.
In terms of safety, no adverse reactions occurred in group S patients, which is considered to be due to the fact that tazarotene betamethasone cream, when applied topically, has low transdermal absorption and the absorbed blood portion is rapidly metabolized to hydrophilic tazarotene acid which is excreted without accumulation and therefore induces adverse reactions and has a high safety profile.
Our study further analyzed the effects of tazarotene on negative emotions and quality of life in patients with CHE. The symptoms of peeling, redness and blistering caused by CHE are detrimental to the appearance of the hands and can trigger emotions such as nervousness and embarrassment, which can lead to avoidance of activities involving the hands and, to a certain extent, social isolation and reduced quality of life[8]. Yu et al.[28] developed that patients with eczema have higher levels of anxiety and depression levels and a tendency to develop other physical and psychological symptoms. Lahfa suggests that emotional factors can influence the development and treatment of CHE[29,30]. Studies have shown that 80 % of patients report some degree of disruption to their work and life at the start of CHE, and that over a third of patients experience sleep problems and leisure activities over the next 15 y, with the impact of CHE increasing over the course of the disease. This indicates that psychological elements are crucial in the emergence of CHE. The study’s findings demonstrated that after therapy, the DLQI scores of group S patients were all significantly better than those of group C after treatment, suggesting that tazarotene betamethasone cream combined with compounded allantoin cream helped to improve the quality of life of CHE patients, presumably for reasons related to the excellent therapeutic effect of tazarotene on CHE.
The study’s findings may not have been sufficiently representative of the population at large due to the study's small number of cases. Moreover, laboratory indicators such as inflammatory factors were not measured in the study, and the effect of tazarotene betamethasone cream combined with compounded allantoin cream on laboratory indicators such as CHE could not be known. Finally, no follow-up was carried out in this study to know the prognosis and long-term results and rash recurrence of tazarotene betamethasone cream combined with compounded allantoin cream in the treatment of CHE.
Tazarotene betamethasone cream combined with allantoin cream is effective in the treatment of CHE, reducing clinical symptoms such as itching and pain, improving the quality of life of patients with skin lesions, without causing adverse reactions. The once-a-day dosing frequency also greatly reduces the frequency of dosing and improves patient compliance. However, more clinical data are needed to support whether tazarotene betamethasone cream in combination with compounded allantoin cream can be widely used and promoted in the clinical setting for the treatment of CHE.
Conflict of interests:
The authors declared no conflict of interests.
References
- Quaade AS, Simonsen AB, Halling AS, Thyssen JP, Johansen JD. Prevalence, incidence and severity of hand eczema in the general population: A systematic review and meta-analysis. Contact Dermatitis 2021;84(6):361-74.
[Google Scholar] [PubMed] [Crossref]
- Grant L, Seiding Larsen L, Burrows K, Belsito DV, Weisshaar E, Diepgen T, et al. Development of a conceptual model of Chronic Hand Eczema (CHE) based on qualitative interviews with patients and expert dermatologists. Adv Ther 2020;37(2):692-706.
[Google Scholar] [PubMed] [Crossref]
- Vindenes HK, Svanes C, Lygre SH, Hollund BE, Langhammer A, Bertelsen RJ. Prevalence of, and work-related risk factors for, hand eczema in a Norwegian general population (the HUNT study). Contact Dermatitis 2017;77(4):214-23.
[Google Scholar] [PubMed] [Crossref]
- Johannisson A, Pontén A, Svensson A. Prevalence, incidence and predictive factors for hand eczema in young adults-a follow-up study. BMC Dermatol 2013;13:14.
[Google Scholar] [PubMed] [Crossref]
- Silverberg JI, Guttman-Yassky E, Agner T, Bissonnette R, Cohen DE, Simpson E, et al. Chronic hand eczema guidelines from an expert panel of the international eczema council. Dermatitis 2021;32(5):319-26.
[Google Scholar] [PubMed] [Crossref]
- Diepgen TL, Andersen KE, Chosidow O, Coenraads PJ, Elsner P, English J, et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges 2015;13(1):e1-22.
[Google Scholar] [PubMed] [Crossref]
- Suarez-Fariñas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Non-lesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 2011;127(4):954-64.
[Google Scholar] [PubMed] [Crossref]
- Moberg C, Alderling M, Meding B. Hand eczema and quality of life: A population-based study. Br J Dermatol 2009;161(2):397-403.
[Google Scholar] [PubMed] [Crossref]
- Lee GR, Maarouf M, Hendricks AK, Lee DE, Shi VY. Current and emerging therapies for hand eczema. Dermatol Ther 2019;32(3):e12840.
[Google Scholar] [PubMed] [Crossref]
- Barati Sedeh F, Ebbehoj NE, Agner T, Caroe TK. Systemic therapy and the use of complementary and alternative medicine in patients with recognized occupational hand eczema in Denmark: A cross-sectional questionnaire-based study. Contact Dermatitis 2020;82(5):272-8.
[Google Scholar] [PubMed] [Crossref]
- Elsner P, Agner T. Hand eczema: A ‘neglected’ disease. J Eur Acad Dermatol Venereol 2020;34(1):3.
[Google Scholar] [PubMed] [Crossref]
- Wu JJ, Hansen JB, Patel DS, Nyholm N, Veverka KA, Swensen AR. Effectiveness comparison and incremental cost-per-responder analysis of calcipotriene 0.005 %/betamethasone dipropionate 0.064 % foam vs. halobetasol 0.01 %/tazarotene 0.045 % lotion for plaque psoriasis: A matching-adjusted indirect comparative analysis. J Med Econ 2020;23(6):641-9.
[Google Scholar] [PubMed] [Crossref]
- Chen H, Sun J, Yang H, Sun Q, Zhang J, Gu J, et al. Fixed combination of tazarotene and betamethasone dipropionate for treatment of psoriasis vulgaris: The result of a phase 3, multicenter, randomized controlled trial. J Dermatol 2020;47(7):728-34.
[Google Scholar] [PubMed] [Crossref]
- Tanghetti E, Lebwohl M. Tazarotene revisited: Safety and efficacy in plaque psoriasis and its emerging role in treatment strategy. J Drugs Dermatol 2018;17(12):1280-7.
[Google Scholar] [PubMed]
- Chen Y. Analysis of the expression and clinical significance of tazarotene induced gene protein and interleukin 23 and 17 in patients with eczema. J Pract Hosp Clin 2020;17(5):207-10.
- Yuan SY, Qiao L, Zheng W. Efficacy and safety analysis of tazarotene ointment in the treatment of chronic hypertrophic eczema. Chin J Leprosy Dermatol 2010;26(7):528-9.
- Valle KZ, Saucedo Acuna RA, Rios Arana JV, Lobo N, Rodriguez C, Cuevas-Gonzalez JC, et al. Natural film based on pectin and allantoin for wound healing: Obtaining, characterization and rat model. Biomed Res Int 2020:6897497.
[Google Scholar] [PubMed] [Crossref]
- Sakthiguru N, Sithique MA. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int J Biol Macromol 2020;152:873-83.
[Google Scholar] [PubMed] [Crossref]
- Sun AY, Tang WB. Clinical observation of 25 cases of palmoplantar keratotic eczema treated with avium combined with compound tretinoin topical and allantoin encapsulation. Chin J Dermatol Venereol 2013;27(8):858-9.
- Held E, Skoet R, Johansen JD, Agner T. The Hand Eczema Severity Index (HECSI): A scoring system for clinical assessment of hand eczema. A study of inter-and intra-observer reliability. Br J Dermatol 2005;152(2):302-7.
[Google Scholar] [PubMed] [Crossref]
- Agner T, Elsner P. Hand eczema: Epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol 2020;34(1):4-12.
[Google Scholar] [PubMed] [Crossref]
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)-A simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19(3):210-6.
[Google Scholar] [PubMed] [Crossref]
- Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: What do dermatology life quality index scores mean? J Invest Dermatol 2005;125(4):659-64.
[Google Scholar] [PubMed] [Crossref]
- Duvic M, Nagpal S, Asano AT, Chandraratna RA. Molecular mechanisms of tazarotene action in psoriasis. J Am Acad Dermatol 1997;37(2):S18-24.
[Google Scholar] [PubMed] [Crossref]
- Marks R. The role of tazarotene in the treatment of psoriasis. Br J Dermatol 1999;140(54):24-8.
[Google Scholar] [PubMed] [Crossref]
- He C, Jin H, Liu X, Hu F, Zhang L, Zhang S, et al. Tazarotene/betamethasone dipropionate cream in patients with plaque psoriasis: Results of a prospective, multicenter, observational study. Dermatology 2021;237(4):603-10.
[Google Scholar] [PubMed] [Crossref]
- Heath MS, Sahni DR, Curry ZA, Feldman SR. Pharmacokinetics of tazarotene and acitretin in psoriasis. Expert Opin Drug Metab Toxicol 2018;14(9):919-27.
[Google Scholar] [PubMed] [Crossref]
- Yu SH, Silverberg JI. Association between atopic dermatitis and depression in US adults. J Invest Dermatol 2015;135(12):3183-6.
[Google Scholar] [PubMed] [Crossref]
- Lahfa M. Definition and psychopathology of chronic hand dermatitis. Ann Dermatol VenereoI 2014;141(1):S106-110.
[Google Scholar] [PubMed] [Crossref]
- Meding B, Wrangsjö K, Jarvholm B. Fifteen-year follow-up of hand eczema: Persistence and consequences. Br J Dermatol 2005;152(5):975-80.
[Google Scholar] [PubMed] [Crossref]
 .
.
 .
.



