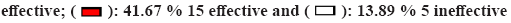- *Corresponding Author:
- J. Liu
Department of Respiratory Medicine, Jiujiang First People’s Hospital, Jiujiang, Jiangxi Province 332008, China
E-mail:juykwbndh76578@163.com
|
This article was originally published in a special issue,“Innovations in Biomedical Research and Drug Development” |
|
Indian J Pharm Sci 2023:85(3) Spl Issue “83-90” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To evaluate the clinical efficacy and safety of glucocorticoids plus immunosuppressive agents for systemic lupus erythematosus. Between March 2018 and March 2022, 72 systemic lupus erythematosus patients were recruited and assigned to receive either glucocorticoids (control group) or immunosuppressive drugs plus glucocorticoids (observation group) via random number table method. Outcome measures included clinical efficacy, safety, systemic lupus erythematosus disease activity index score, immunoglobulin, complement factor C3/C4 levels and N-glycan changes. After the intervention, the systemic lupus erythematosus disease activity index scores and urine protein levels of the observation group were lower than those of the control group immunosuppressive drugs plus glucocorticoids resulted in significantly decreased systemic lupus erythematosus disease activity index scores and urine protein levels and higher treatment efficiency (χ2=1.424, p=0.033) vs. glucocorticoids alone (p<0.05). The difference in the incidence of adverse events between the two groups did not come up to the statistical standard (p>0.05). Patients in the observation group showed higher complement factor C3 levels at 6 mo and 9 mo after therapy and higher levels of complement factor C4 at 3 mo and 6 mo after therapy than those in the control group (p<0.05). There was no significant difference (p>0.05) in antibody protein between the two groups. Immunosuppressive agents plus glucocorticoids provide significant therapeutic benefits for systemic lupus erythematosus management by lowering the serum immune protein levels, improving immunological function and increasing complement factors C3 and C4 levels with consistent safety. Despite the efficacy of this treatment modality, future studies with larger samples are encouraged to provide further high-quality evidence-based evidence.
Keywords
Systemic lupus erythematosus, glucocorticoids, immunosuppressive agents, clinical effect, glucocorticoids
Systemic Lupus Erythematosus (SLE) is an Autoimmune Connective Tissue Disease (AI-CTD) with systemic immune system disorder involving multiple organs. The pathogenesis of SLE is yet unclear[1,2]. Several studies have shown that T lymphocytopenia compromised T suppressor cell function and excessive B cell proliferation results in the secretion of a large number of autoantibodies, particularly antinuclear antibodies, which bind to the corresponding antigens leading to the formation of immune complexes deposited in the body. This process involves organs from numerous systems, including the skin, nerves, blood and respiratory system[3-5]. The autoantibodies also bind to histolytic antigens to cause cell destruction, causing multi- system damage to the body[6]. The complex clinical manifestations and capricious conditions of SLE patients seriously compromise the quality of life of patients[7,8].
The pleiotropic properties of glucocorticoids have been applied in the treatment of several autoimmune diseases with established clinical efficacy and glucocorticoids are the current essential medicine for chronic active SLE[9]. However, the effectiveness and adverse responses of long- term mid and low-dose glucocorticoids during the continuous usage or maintenance therapy phase are concerning in the persistently active or relapsing disease course. Relevant adverse events include obesity, hypertension, diabetes, an increased risk of osteoporosis and immunological function impairment[10]. In the past two decades, glucocorticoids plus immunosuppressive agents have become the primary treatment option for SLE[11].
There is no record of SLE in ancient Traditional Chinese Medical (TCM) texts, but according to its clinical manifestations, it is classified as "warm toxin with spots" and "yin-yang toxin". Insufficient congenital endowment, internal injury from strain and fatigue, and external evil may cause deficiency of kidney yin and internal growth of stasis and toxicity. The treatment is to tonify the kidney and nourish the blood, clear heat and nourish yin. Xijiao Dihuang decoction is a classic TCM decoction for clearing heat and treating the evidence of blood heat.
In this study, 72 patients with SLE were enrolled and retrospectively analyzed to observe the efficacy and safety of glucocorticoids plus immunosuppressive agents in the treatment of SLE. This study aimed to provide theoretical support for treatment plan optimization and prognosis improvement in SLE patients.
Materials and Methods
General data:
From March 2018 to March 2022, 72 eligible patients with SLE were enrolled. The patients were ordered based on the random method. The randomization was carried out using an online web-based randomization tool (http://www.randomizer.org/). For concealment of allocation, the randomization procedure and assignment were managed by an independent research assistant who was not involved in the screening or evaluation of the participants.
The original sample size calculation estimated that 35 patients in each group would be needed to detect a 3 point difference between groups in a 2 sided significance test with a power of 0.8 and an alpha error level of 0.05.
The trial was conducted according to good clinical practice guidelines developed by the international council for harmonisation and in compliance with the trial protocol. The protocol was approved by the institutional review boards or independent ethics committees at each site. All patients provided written informed consent per the declaration of Helsinki principles. An independent data monitoring committee monitored safety and efficacy data. Ethics number: LO-PO20190707.
Patients were enrolled in this study if they met the following criteria diagnosis as SLE by histopathology and imaging meeting H1 classification criteria for SLE patients aged between 18 y and 60 y without the use of glucocorticoids in combination with immunosuppressive agents within 3 mo before enrollment and who had good treatment cooperation and signed informed consent forms.
Patients were excluded if they were experiencing severe mental illness blood Immunoglobulin A (IgA) level seriously below the standard level allergy to the study drugs lactation or pregnancy other concomitant rheumatic diseases or serious dysfunction in liver, kidney and other important organs who have recently received hormonal drugs or immunosuppressive drugs with important organ- related diseases or lesions with serious infectious or infectious diseases with malignant tumors and with mental disorders affecting treatment compliance.
Methods:
Both groups were treated with glucocorticoids. 0.5 g of methylprednisolone was given intravenously for 3 d as a course of treatment, followed by oral prednisone, 1 mg/kg daily, with the maximum dose not exceeding 60 mg per day. The dosage was gradually reduced as the patient's condition improved. The observation group received immunosuppressive agents in addition to glucocorticoids and Cyclophosphamide (CTX) was used in this study. The initial treatment frequency was once a month, 0.5-1/m2 of body surface area/time (i.e. 800-1000 mg/time). After 6 mo, the frequency was increased to once every 3 mo. The treatment was performed continuously for 12 mo and the dose was gradually reduced as the condition improved. The total course was ≥18 mo, during which follow-up was conducted every month. After treatment, the patients were followed up every 6 mo for 1 y. The two groups of patients received Erhuang Yangxue decoction. Radix Rehmannia 15 g, prepared radix Rehmannia 15 g, Corni fructus 15 g, Semen Cuscutae 15 g, Angelica sinensis 15 g, Radix Paeoniae rubra 15 g, Moutan cortex 15 g, buffalo horn 10 g, Oldenlandia diffusa 30 g, Herba Scutellariae barbatae 30 g were decocted with water for 30 min to obtain 300 ml of filtrates, with 150 ml administered in the morning and the other half in the evening. The duration of treatment was 8 w.
Observation indicators:
The SLE Disease Activity Index (SLEDAI) score, 24 h urine protein levels and facial pigmentation were used to evaluate the treatment efficacy. SLEDAI scores were rated based on the degree of disease activity by 24 items such as convulsions, psychiatric symptoms and organic brain syndromes, with 0-4 for no activity, 5-9 for light activity, 10-14 for moderate activity and ≥15 for heavy activity. A markedly effective efficacy refers to the complete disappearance of facial pigmentation and a significant decrease in SLEDAI scores and 24 h urine protein levels. Effective is defined as the partial disappearance of facial pigmentation and increase or no change in SLEDAI scores and 24 h urine protein levels. If no change was observed in facial pigmentation and no decrease or increase was noticed in SLEDAI scores and 24 h urine protein levels, it was considered ineffective.
Total effective rate=(Number of cases with markedly effective+Number of cases with effective)/Total number of cases×100 %
Safety evaluation was performed with the occurrence of adverse events during the whole study. Adverse events include fever, infection, arthralgia, weight gain, oral ulceration, epilepsy, gastrointestinal reactions and eye disorders.
Other indicators include changes in serum inflammatory factors and immune factors. 5 ml of morning fasting venous blood was collected from each subject and centrifuged to obtain the serum. The levels of Immunoglobulin G (IgG) antibody and complement factors C3 and C4 in the two groups were measured by immunoturbidimetry.
Statistical analysis:
The mean difference between the two groups was tested using the student’s t-test for normally distributed variables and the Mann-Whitney U test for non-normal variables.
Statistical Package for the Social Sciences (SPSS) 26.0 was used for statistical analysis. Measurement data with normal distribution are expressed as means±standard deviations (x̄ ±s) and non-normal measurement data are expressed as medians. T-tests were performed to compare the measurement data between the two groups. Count data were expressed as percentages and the Chi-square (χ2) test was applied for comparison between the two groups. Mapping was performed using GraphPad Prism 9. A p<0.05 indicate statistical significance between the two groups.
Results and Discussion
There were 3 males and 33 females in the observation group, aged 14-71 y (36.61±13.84) y. The control group had 4 males and 32 females, aged 12-79 y (34.31±15.06) y. The baseline patient profiles of the two groups were comparable (p>0.05) as shown in Table 1.
| Item | Observation group (n=36) | Control group (n=36) | t/χ2 | p |
|---|---|---|---|---|
| Gender | ||||
| Male/female | 12114 | 11780 | 6.871 | 0.657 |
| Age (years) | 9.851 | 0.673 | ||
| Range | 14-71 | 12-79 | ||
| Mean age | 36.61±13.84 | 34.31±15.06 | ||
| Mean disease course (months) | 12.01±2.42 | 12.30±2.51 | 7.254 | 0.869 |
| SLEDAI score (points) | 16.39±4.82 | 16.41±3.18 | 5.424 | 0.084 |
| SDI score (points) | 0.63±1.04 | 0.65±1.01 | 3.537 | 0.091 |
| ANA positive | 35 | 35 | 1.448 | >0.05 |
| Anti-dsDNA positive (n) | 10 | 11 | 0.331 | >0.05 |
| Abnormal (low) complement factor C3 (n) | 11 | 10 | 0.287 | >0.05 |
| Abnormal (low) complement factor C4 (n) | 9 | 10 | 0.928 | >0.05 |
| Urine protein level (g) | 3.92±0.94 | 3.95±0.97 | 1.572 | 0.384 |
Table 1: Comparison of Patient Data Between the two Groups
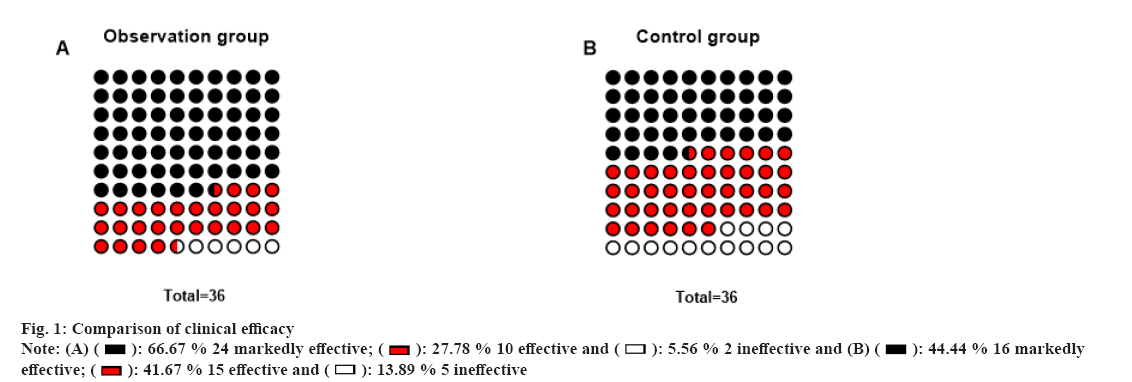
As shown in Table 2 and fig. 1, there was no significant difference in SLEDAI scores and urine protein levels between the two groups before intervention (p>0.05). The SLEDAI scores and urine protein levels of the two groups were considerably lower after the intervention and the observation group had significantly lower results than the control group. There were 24 cases with markedly effective efficacy, 10 with effective efficacy and 2 with ineffective efficacy, with a total effective rate of 94.44 % in the observation group (34 cases) (p<0.05). In the control group, 16 cases showed markedly effective efficacy, 15 cases showed effective efficacy and 5 cases showed ineffective efficacy, with a total effective rate of 86.11 % (31 cases). Glucocorticoids plus immunosuppressive agents resulted in higher treatment effectiveness vs. glucocorticoids (χ2=1.424, p=0.033).
| Group | SLEDAI score (points) | Urine protein level (g) | ||
|---|---|---|---|---|
| Before intervention | After intervention | Before intervention | After intervention | |
| Observation group | 16.39±4.82 | 8.18±2.36 | 3.92±0.94 | 1.40±0.55 |
| Control group | 16.41±3.18 | 11.74±3.49 | 3.95±0.97 | 2.61±0.57 |
| t | 5.424 | 8.349 | 1.572 | 5.714 |
| p | 0.084 | 0.023 | 0.384 | 0.037 |
Table 2: Changes In SLEDAI Scores and Urine Protein Levels before and after Intervention (x̄±s)
A total of 12 cases of adverse reactions occurred in the observation group and 11 in the control group, while no significant differences were observed (p>0.05) as shown in Table 3. As shown in fig. 2A, the mean level of complement factor C3 increased in both groups compared with that before treatment during the continuous treatment (p<0.05), with the observation group starting to show a decrease in complement factor C3 at 6 mo of continuous treatment, followed by a rise and a significant decrease at 6 mo and 12 mo of follow-up after the termination of treatment. Once 12 mo of continuous therapy, the control group had a fall in the mean level of complement factor C3, followed by a rise and then a decrease after treatment was stopped. According to fig. 2B, the mean levels of complement factor C4 in both groups rose during the continuous therapy compared to before treatment (p<0.05). After 3 mo of therapy, the observation group exhibited a quick increase, followed by a steady and sustained increase and a drop after 12 mo of continuous treatment. Following treatment continuance, a modest rise was noted, followed by a drop following treatment discontinuation. The control group followed the same path as the observation group. The number of anti-double stranded Deoxyribonucleic Acid (dsDNA) antibody proteins in the two groups decreased significantly after treatment, as shown in fig. 2C after 9 mo of continuous treatment, the IgG level began to rise and then fell again and after 6 mo of treatment termination, the follow-up results showed a significant increase. And after 12 mo of treatment termination, the degree of increase was significantly reduced.
| Group | Fever | Infection | Arthralgia | Weight gain | Oral ulceration | Epilepsy | Gastrointestinal reactions | Eye disorders | Total occurrence |
|---|---|---|---|---|---|---|---|---|---|
| Observation group | 2 | 1 | 2 | 5 | 1 | 0 | 1 | 0 | 12 (33.33 %) |
| Control group | 3 | 2 | 2 | 2 | 1 | 0 | 1 | 0 | 11 (30.56 %) |
| χ2 | 0.064 | ||||||||
| p | 0.8 |
Table 3: Comparison of all Adverse Reactions (n %)
Fig. 2A and fig. 2B show the mean levels of complement factors C3 (A) and C4 (B) over time in the two groups. Fig. 2C depicts the mean levels in anti-dsDNA antibody-positive patients as determined by IgG Enzyme Immunoassay (IgGEIA). *p<0.05 compared with the control group.
SLE is a chronic, multilayered and complex autoimmune disease and its clinical manifestations, progression and prognosis vary greatly[12,13]. It has been reported that the prevalence of SLE in China is about 0.03 % and it is more common in young and middle-aged women[14]. In recent years, thanks to the ongoing advancements in pathogenesis research and the development of new treatment options, the life expectancy of SLE patients has been extended, with the 5 y mean survival rate as high as 82 % to 90 %. However, the clinical response rate and complete response rate of SLE patients were lower than 3.5 %[15,16]. Medications are currently the mainstay of SLE management. With the rapid development of the medical field, SLE-related drugs have evolved from chemical drugs to biological agents and then to small molecule chemical agents. Significant enrichment in the diagnosis and treatment of SLE has been obtained[17]. The ongoing emergence of novel targeted drugs offers new alternatives for the clinical management of SLE. These targeted drugs deserve further study given their advantages such as easy administration, few side effects, noticeable effects, fast metabolism and high specificity[18]. The long-term use of high-dose glucocorticoids, however, elevates the risk of adverse drug reactions and drug dependence. The reduction of dosage or discontinuation of treatment may lead to disease recurrence, resulting in poorly managed primary lesions accompanied by secondary hormone-dependent dermatitis, which complicates further treatment[19].
Glucocorticoid is a hormone synthesized and secreted by adrenal zona fasciculata cells. Glucocorticoid Receptors (GRs) in the cytoplasm by reducing the permeability of endothelial cells. After a series of complex processes, it enters the nucleus and performs transcriptional inhibition and activation. It acts as an anti-inflammatory, diuretic, edema reducer and urine protein reducer by interfering with the function of proinflammatory transcription factors and downregulating the production of proinflammatory protein. Following glucocorticoid binding to certain gene sequences, a significant number of regulatory proteins are generated, resulting in undesirable effects such as gluconeogenesis, skin shrinkage and bone formation inhibition, which emphasize the need for co-administration of drugs. Immunosuppressive agents are routinely used to treat rheumatic illnesses. They can control the cellular and humoral immune response in vivo by inhibiting the abnormal immune expression of the body and reduce the immune damage of tissues, resulting in compromised immunity of patients[20,21].
In this study, patients in the observation group had rapid symptom relief and their SLEDAI scores and urine protein levels also decreased significantly. No significant adverse reactions were observed. It is suggested that the combination therapy of two drugs quickly alleviates the symptoms of SLE. Moreover, co-administration potentiates the treatment efficiency of monotherapy[22]. The mechanisms of autoimmunity in SLE and the mechanisms of interactions between multiple pathogenic factors during the onset and progression of the disease are poorly understood. The main known internal factors are genetic and immune factors, which are collectively, termed non-interventional factors and external factors are environmental and hormonal use, which are considered interventional factors and are the target direction of clinical treatment. Immune damage is the main pathogenic role of SLE and is closely associated with the development of numerous chronic diseases[23,24]. The preferred therapeutic agent for SLE is glucocorticoids, which are rapidly and well effective in relieving clinical symptoms. The efficacy of glucocorticoids depends largely on the GRs[25], which are members of the nuclear hormone receptor superfamily and can alter target genes, thereby modifying the effects of target cells and organs. In clinical treatment, many conditions require high-dose glucocorticoid therapy for effective control of disease progression, especially in critically ill patients, such as severe pneumonia, sepsis and other serious illnesses. However, the use of high doses of glucocorticoids will reduce the intestinal absorption of vitamin D3 and accelerate the catabolism of 1,25-Dihydroxyvitamin D3 (1,25 (OH)D3)[26,27].
The determination of complement factors C3 and C4 levels during treatment and subsequent follow- up contributes to assessing the disease condition of SLE patients. Significantly low blood complement factor C3 and C4 levels indicate active disease and necessitate vigorous medication therapy and constant monitoring[28]. The levels of complement components C3 and C4 were greatly improved following therapy in the present trial, and their decline in the middle may be attributed to the low medication compliance of the patients. Zucchi[29] also reported comorbid autoimmune diseases in SLE patients with considerably higher immunoglobulin levels than those in healthy individuals[30]. The combination of the drugs effectively inhibited the abnormal proliferation of B lymphocytes and non-specific removal of antigen-sensitive small lymphocytes, thereby down-regulating IgG, IgM and IgA levels and promoting the recovery of immunity[31].
In addition, immunosuppressive agents plus methylprednisolone tablets reduced the use of hormone drugs and the occurrence of adverse reactions, thereby enhancing the recovery of patients to the maximum extent[32]. The Asia Pacific League of Associations for Rheumatology (APLAR) developed the first consensus on the management of SLE more suitable for Asian populations and recommended high and medium-dose of glucocorticoids (including methylprednisolone pulse therapy) in combination with CTX as the first- line treatment of SLE, which provides insights for the standard treatment of SLE patients in China[33].
In addition, TCM treatment was combined with glucocorticoids in this study. In TCM, SLE patients have a deficiency of kidney essence, deficiency of kidney water, internal growth of deficiency fire and yin deficiency fire. Hormonal drugs are pure yang products, which deplete the yin essence, resulting in internal deficiency of yin in the kidney. The treatment is to tonify the kidney and nourish the blood, clear heat and nourish yin. Xijiao Dihuang decoction is composed of Rhinoceros horn (buffalo horn alternative), Radix Rehmannia, Paeonia lactiflora and Moutan cortex, which are effective in clearing the toxins and cooling the blood to disperse blood stasis, but weak in nourishing kidney yin. In this study, this formula was supplemented with products that nourish kidney-Yin to obtain the Erhuang Yangxue decoction. In this formula, radix Rehmannia and prepared radix Rehmannia nourish Yin and clear heat, cool the blood and tonify the blood, Corni fructus and Semen Cuscutae tonify the liver and kidneys, astringent essence, flatten the liver and kidneys, nourish Yin and Yang, Angelica sinensis activates blood stasis, nourishes blood and harmonizes Ying, Radix Paeoniae rubra, Moutan cortex and buffalo horn clear heat and cool blood, Oldenlandia diffusa clear heat and detoxify blood and Herba Scutellariae barbatae activate blood circulation and remove blood stasis.
This trial has the following limitations, including the small sample size and short follow-up. Despite the shortcomings, this treatment protocol is feasible for the routine treatment of SLE patients in various medical institutions. Future studies are expected to provide more reliable data.
In conclusion, immunosuppressive agents plus glucocorticoids provide significant therapeutic benefits for SLE management by lowering the serum immune protein levels, improving immunological function and increasing complement factors C3 and C4 levels with consistent safety. Despite the efficacy of this treatment modality, future studies with larger samples are encouraged to provide further high-quality evidence- based evidence.
Conflict of interests:
The authors declared no conflict of interests.
References
- di Battista M, Marcucci E, Elefante E, Tripoli A, Governato G, Zucchi D, et al. One year in review 2018: Systemic lupus erythematosus. Clin Exp Rheumatol 2018;36(5):763-77.
[Google Scholar] [PubMed]
- Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019;393(10188):2332-43.
[Crossref] [Google Scholar] [PubMed]
- Fan Y, Hao YJ, Zhang ZL. Systemic lupus erythematosus: Year in review 2019. Chin Med J 2020;133(18):2189-96.
[Google Scholar] [PubMed]
- Fischer-Betz R, Schneider M. Deescalation and glucocorticoid-free treatment in SLE. Z Rheumatol 2021;80:332-8.
- Gergianaki I, Bortoluzzi A, Bertsias G. Update on the epidemiology, risk factors and disease outcomes of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2018;32(2):188-205.
[Crossref] [Google Scholar] [PubMed]
- Gile JJ, Sara JD, Mueller MR. Systemic lupus erythematosus multiorgan flare with quiescent serologic markers. BMJ Case Rep 2021;14(3):e239048.
[Crossref] [Google Scholar] [PubMed]
- Goulielmos GN, Zervou MI, Vazgiourakis VM, Ghodke-Puranik Y, Garyfallos A, Niewold TB. The genetics and molecular pathogenesis of systemic lupus erythematosus (SLE) in populations of different ancestry. Gene 2018;668:59-72.
[Crossref] [Google Scholar] [PubMed]
- Ichinose K. Unmet needs in systemic lupus erythematosus. Japan J Clin Immunol 2017;40(6):396-407.
- Liu N, Ma ZZ, Yan HF, Li Q, Lyu XQ, Kang WL, et al. Clinical effect of double filtration plasmapheresis combined with glucocorticoid and immunosuppressant in treatment of children with severe Henoch-Schönlein purpura nephritis. Zhongguo Dang Dai Er Ke Za Zhi 2019;21(10):955-9.
[Crossref] [Google Scholar] [PubMed]
- Qiu KY, Liao XY, Guo SY, Qi HN, Lan JJ, Fang JP, et al. Efficacy and safety of MSC infusion in treatment of children with refractory LOHC: A clinical study. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018;26(3):900-4.
[Google Scholar] [PubMed]
- Li Y, Wu T. Proteomic approaches for novel systemic lupus erythematosus (SLE) drug discovery. Expert Opin Drug Discov 2018;13(8):765-77.
[Crossref] [Google Scholar] [PubMed]
- Narvaez J. Systemic lupus erythematosus 2020. Med Clin 2020;155(11):494-501.
[Crossref] [Google Scholar] [PubMed]
- Oku K, Atsumi T. Systemic lupus erythematosus: Nothing stale her infinite variety. Mod Rheumatol 2018;28(5):758-65.
[Crossref] [Google Scholar] [PubMed]
- Rivas-Larrauri F, Yamazaki-Nakashimada MA. Systemic lupus erythematosus: Is it one disease? Reumatol Clin 2016;12(5):274-81.
[Crossref] [Google Scholar] [PubMed]
- Saidane HA, Gozalov A, Cvetkovic VV. Intracranial hypertension secondary to systemic lupus erythematosus. Ugeskr Laeger 2021;183(5):V10200782.
[Google Scholar] [PubMed]
- Sebastiani GD, Prevete I, Iuliano A, Minisola G. The importance of an early diagnosis in systemic lupus erythematosus. Isr Med Assoc J 2016;18(3-4):212-5.
[Google Scholar] [PubMed]
- Thomas DC, Kohli D, Chen N, Peleg H, Almoznino G. Orofacial manifestations of rheumatoid arthritis and systemic lupus erythematosus: A narrative review. Quintessence Int 2021;52(5):454-66.
[Crossref] [Google Scholar] [PubMed]
- Ugarte-Gil MF, González LA, Alarcón GS. Lupus: The new epidemic. Lupus 2019;28(9):1031-50.
[Crossref] [Google Scholar] [PubMed]
- Yasuda S. Emerging targets for the treatment of lupus erythematosus: There is no royal road to treating lupus. Mod Rheumatol 2019;29(1):60-9.
[Crossref] [Google Scholar] [PubMed]
- Fassio A, Adami G, Giollo A, Viapiana O, Malavolta N, Saviola G, et al. Acute effects of glucocorticoid treatment, TNFα or IL-6R blockade on bone turnover markers and Wnt inhibitors in early rheumatoid arthritis: A pilot study. Calcified Tissue Int 2020;106:371-7.
[Crossref] [Google Scholar] [PubMed]
- Fassio A, Adami G, Idolazzi L, Giollo A, Viapiana O, Vantaggiato E, et al. Wnt inhibitors and bone turnover markers in patients with polymyalgia rheumatica and acute effects of glucocorticoid treatment. Front Med 2020;7:551.
[Crossref] [Google Scholar] [PubMed]
- Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. Association of intravenous immunoglobulins plus methylprednisolone vs.immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021;325(9):855-64.
[Crossref] [Google Scholar] [PubMed]
- Abe K, Ishikawa Y, Kita Y, Yajima N, Inoue E, Sada KE, et al. Association of low-dose glucocorticoid use and infection occurrence in systemic lupus erythematosus patients: A prospective cohort study. Arthritis Res Ther 2022;24(1):179.
[Crossref] [Google Scholar] [PubMed]
- Sprow G, Afarideh M, Dan J, Hedberg ML, Werth VP. Bullous systemic lupus erythematosus in females. Int J Women Dermatol 2022;8(3):e034.
- Hao S, Zhang J, Huang B, Feng D, Niu X, Huang W. Bone remodeling serum markers in children with systemic lupus erythematosus. Pediatr Rheumatol 2022;20(1):54.
[Crossref] [Google Scholar] [PubMed]
- Hammam N, Evans M, Bell CF, Gairy K, Yazdany J, Schmajuk G. Evaluating the use of glucocorticoids among belimumab-treated patients with systemic lupus erythematosus in real-world settings using the rheumatology informatics system for effectiveness registry. ACR Open Rheumatol 2022;4(10):883-9.
[Crossref] [Google Scholar] [PubMed]
- Ko D, Forrest N, Mai Q, Pawlowski A, Balsley K, Chung A, et al. Electronic health record data use in the assessment of quality indicators for glucocorticoid osteoporosis screening in systemic lupus erythematosus. Lupus 2022;31(12):1516-22.
[Crossref] [Google Scholar] [PubMed]
- Yu H, Nagafuchi Y, Fujio K. Clinical and immunological biomarkers for systemic lupus erythematosus. Biomolecules 2021;11(7):928.
[Crossref] [Google Scholar] [PubMed]
- Zucchi D, Elefante E, Schilirò D, Signorini V, Trentin F, Bortoluzzi A, et al. One year in review 2022: Systemic lupus erythematosus. Clin Exp Rheumatol 2022;40(1):4-14.
[Crossref] [Google Scholar] [PubMed]
- Porta S, Danza A, Arias Saavedra M, Carlomagno A, Goizueta MC, Vivero F, et al. Glucocorticoids in systemic lupus erythematosus. Ten questions and some issues. J Clin Med 2020;9(9):2709.
[Crossref] [Google Scholar] [PubMed]
- Zucchi D, Elefante E, Calabresi E, Signorini V, Bortoluzzi A, Tani C. One year in review 2019: Systemic lupus erythematosus. Clin Exp Rheumatol 2019;37(5):715-22.
[Google Scholar] [PubMed]
- Torrelo A. Methyl prednisolone aceponate for atopic dermatitis. Int J Dermatol 2017;56(6):691-7.
[Crossref] [Google Scholar] [PubMed]
- Ruiz-Irastorza G, Bertsias G. Treating systemic lupus erythematosus in the 21st century: New drugs and new perspectives on old drugs. Rheumatology 2020;59(5):v69-81.
[Crossref] [Google Scholar] [PubMed]