- *Corresponding Author:
- Junling Zhang
Department of Dermatology, Tianjin Institute of Integrative Dermatology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Hongqiao, Tianjin 300120, China
E-mail: 13920301679@126.com
| This article was originally published in a special issue, “New Research Outcomes in Drug and Health Sciences” |
| Indian J Pharm Sci 2023:85(6) Spl Issue “204-209” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To investigate the clinical efficacy of dupilumab injection in combination with systemic low-dose hormones in the treatment of bullous pemphigoid. 60 patients with bullous pemphigoid were included and the treatment plan is determined by the patient's condition, fully informed to the patient, and jointly decided upon with the consent of the patient and family. 35 patients were treated with conventional systemic low-dose hormone therapy and were classified as the control group and 25 patients were treated with dupilumab injection in combination with systemic low-dose hormone therapy and were classified as the study group. Patients in both groups received topical wet compresses on the rash area and topical hormone-based creams during their hospitalization. The time to control disease activity, the dose of the hormones at disease control, the cumulative dose of the hormones at disease control, laboratory indices, pruritus level score, bullous pemphigoid activity level score and incidence of adverse reactions were compared between the two groups. The time to control disease activity was shorter in study group than in control group (p<0.05). The dose of the hormones at disease control and the cumulative dose of the hormones at disease control in study group were smaller than in control group (p<0.05). After 4 w of treatment, the peripheral blood eosinophil count, serum anti-bullous pemphigoid 180 antibody concentration, serum interleukin-4 and interleukin-13 levels, total serum immunoglobulin E concentrations, bullous pemphigoid activity score and pruritus level score were significantly lower in study group than in control group (p<0.05). The incidence of adverse reactions was lower in study group than in control group (p<0.05). Dupilumab injection in combination with systemic low-dose hormones is safe and effective in the treatment of bullous pemphigoid, and it can provide more options for the clinical management of patients with bullous pemphigoid.
Keywords
Bullous pemphigoid, dupilumab, hormone, interleukin-4, interleukin-13, blister
A frequent autoimmune sub epidermal blistering illness known as Bullous Pemphigoid (BP) is characterized by the development of tight blisters on edematous erythema and severe itching[1]. The primary pathogenic characteristic is the BP180 and BP230 sub epidermal structural protein’s breakdown, which results in the development of tense blisters[2,3]. As the world's population ages, BP prevalence is largely observed in elderly people aged 66 y to 83 y, and it is increasing yearly. Annual increases in the prevalence of BP are being caused by the aging of the global population[4]. The current incidence of BP is about 6 to 43 cases per million population per year, and the prognosis is poor for patients of advanced age, with concomitant neurological disorders and longterm use of high-dose corticosteroids[5]. Currently, systemic glucocorticoids are the main clinical regimen for BP, with the addition of immunosuppressant’s or intravenous immunoglobulin if necessary[6] , but this regimen has significant side effects, poor efficacy in preventing relapse, and limited overall efficacy[7]. The annual mortality rates for BP have been reported to be 11 %-23 %, 13 %-41 % and 12 %-27 % in the United States of America (USA), Europe and Asia respectively, with the most common cause of death being opportunistic infections due to longterm medical immunosuppression[8]. It is therefore essential to find a safe and effective treatment with long-term viability.
According to earlier studies, inflammatory reactions mediated by Interleukin (IL)-4 and IL-13, notably stimulation of T helper 2 cells (Th2), are crucial in the pathophysiology of BP. Type 2 inflammatory responses are involved in the pathogenesis of BP[9]. High expression of IL-4 and IL-13, two types 2 pro-inflammatory cytokines, in blister fluid or skin biopsies from patients with BP[10]. Therefore, a potential therapeutic approach for BP could involve inhibiting the activity of IL-4 and IL-13. Dupilumab, a human monoclonal antibody that suppresses IL-4 and IL-13 signaling and stops the inflammatory cascade, targets the alpha component of the IL-4 receptor (IL-4r) [11]. As a biologic agent, dupilumab may be a safer and more effective treatment for BP[12]. According to reports, dupilumab has recently played a key role in the management of BP[13,14,11], but this conclusion is not supported by valid clinical data in China.
Based on this, we performed a real-world trial to look at the effects of systemic low-dose hormones combined with dupilumab injection for the treatment of BP, with the aim of providing a valid reference for the clinical management of BP.
Materials and Methods
Study design:
Sixty patients with BP who received inpatient treatment in a tertiary hospital in China from January 2021 to January 2022 were included in the study. The treatment plan is determined by the patient's condition, fully informed to the patient, and jointly decided upon with the consent of the patient and family. 35 patients were assigned to the Control group (group C) and received systemic low-dose hormones treatment, while 25 patients were treated with dupilumab injection in combination with systemic low-dose hormones and were classified as the Study group (group S). All research participants gave their informed consent for this study. The Medical Ethics Committee of our hospital supported this study.
Inclusion and exclusion criteria:
Inclusion criteria: Age ≥18 y; meeting the diagnostic criteria for BP as a patient who has not been treated after diagnosis or who is in complete remission after stopping medication but has recently relapsed and is not receiving treatment[6,15] and moderate to severe severity of the disease were included[16].
Exclusion criteria: Minor lesions that are isolated or treatable with topical medication or short-term systemic therapy and treatment with systemic glucocorticoids, immunosuppressive agents or biologics within 12 w before study entry were excluded.
Treatment:
Patients in the control group receive simple systemic low-dose hormones at a standard dose of 0.4 mg/ kg/d of methylprednisolone for 7 d, and then the dose may be increased to 1 mg/kg/d if the disease is not effectively controlled. If the disease is not controlled despite the increase in dose, then adjuvant therapy such as immunosuppressive drugs and intravenous immunoglobulin may be added. Maintain the dose until blister formation ceases, crusting and erosions disappear, and the lesion begins to re-epithelialize. The dose is then reduced sequentially until 16 mg/d is reached, then by 4 mg every 2 w until 8 mg/d. Subsequently, the dose is gradually reduced by 2 mg every 2 w until it reaches 0.
Dupilumab was administered to patients in the S group in addition to the C group. The initial dose was 600 mg subcutaneously, followed by 300 mg subcutaneously every 2 w, and then gradually reduced to 300 mg every 3 w and 300 mg every 4 w until discontinuation, depending on disease control.
Primary outcomes:
Disease control occurs when no new lesions develop, when those that already present start to heal, and when the amount of itching starts to decrease and the dose of hormone at which the lesion is fully controlled. The cumulative dose of the hormone used from baseline to the time of disease control.
Secondary outcomes:
Laboratory indicators: Peripheral blood eosinophil count, serum anti-BP180 antibody concentration, serum IL-4 and IL13 levels and serum total Immunoglobulin E (IgE) concentration.
Pruritus level: The level of pruritus was assessed in both groups using the Numerical Rating Scale (NRS). The scale scores range from 0 to 10, corresponding to no itching to unbearable itching. Higher scores indicate more severe itching.
BP activity level: Assessed using the BP Disease Area Index (BPDAI) activity score. Skin-vesicles/ blisters; <15 as mild, 15-34 as moderate and >34 as severe. Skin-erythema/erythema; <20 is considered mild, 20-34 is moderate and >34 is severe. Mucous membranes-vesicles/blisters; <10 as mild, 10 to 24 as moderate and >24 as severe [16,17].
Adverse reactions: Record the occurrence of adverse reactions such as conjunctivitis and calculate the incidence.
Statistical analysis:
The data were analyzed using the social sciences statistical program Statistical Package for the Social Sciences (SPSS) 21.0. Statistics were given as [n (%)], and the Chi-square (χ2) test was performed to compare the data. The Kolmogorov-Smirnov test was used to determine if the data were normally distributed, and the Mann-Whitney U test was employed to compare between groups. Measures that were not normally distributed were provided as median and quartiles. Measures that conformed to the normal distribution were expressed as (x? ±s), and comparisons were made using the t-test, Analysis of Variance (ANOVA) and Least Significant Difference (LSD) test. At p<0.05, differences were declared statistically significant.
Results and Discussion
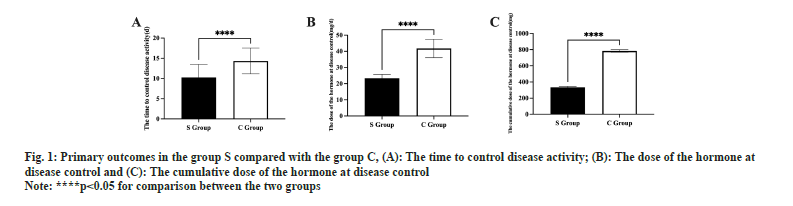
Information from the baseline did not differ between the two groups (p<0.05, Table 1). The duration of disease control was shorter in S group than in C group (p<0.05, fig. 1A). The effective control dose of hormones and the cumulative effective control dosage of hormones were smaller in S group than in C group (p<0.05, fig. 1B and fig. 1C).
| Characteristic | Group S (n=25) | Group C (n=35) | χ2/t | p value |
|---|---|---|---|---|
| Age (years, mean±SD) | 65.35±1.25 | 65.41±1.39 | 0.172 | 0.864 |
| Male/female n, (%) | 14 (56.00)/11 (44.00) | 19 (54.29)/16 (45.71) | 0.017 | 0.895 |
| Weight (kg, mean±SD) | 67.32±3.27 | 67.55±3.24 | 0.270 | 0.788 |
| BP duration | 14.35±3.22 | 14.48±3.12 | 0.157 | 0.876 |
| Coexisting conditions | ||||
| Hypertension, n (%) | 7 (28.00) | 11 (31.43) | 0.082 | 0.755 |
| Cardiovascular disease, n (%) | 4 (16.00) | 6 (17.14) | 0.014 | 0.907 |
| Diabetes mellitus | 4 (16.00) | 7 (20.00) | 0.156 | 0.693 |
| Chronic renal insufficiency, n (%) | 4 (16.00) | 6 (17.14) | 0.014 | 0.907 |
| Neurologic disorder, n (%) | 3 (12.00) | 2 (5.71) | 0.754 | 0.385 |
| Interstitial lung disease, n (%) | 2 (8.00) | 2 (5.71) | 0.122 | 0.726 |
| Tumor, n (%) | 1 (4.00) | 1 (2.86) | 0.059 | 0.808 |
Table1: Baseline Information
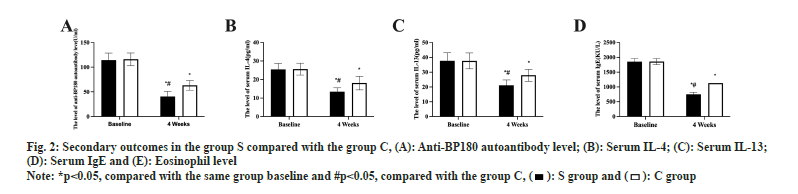
Eosinophil, serum anti-BP180 antibody concentration, serum IL-4 and IL13 levels, and serum total IgE concentration did not change between the two groups prior to therapy (p>0.05). Both groups experienced a statistically significant drop in the levels of the aforementioned indicators after 4 w of therapy, with S group experiencing a greater drop than C group (p<0.05, fig. 2A-fig. 2E).
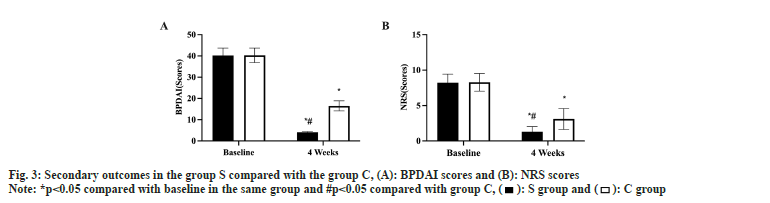
Prior to treatment, there was no difference between the two groups in the BP activity level score or the pruritus level score (p>0.05). Both groups experienced a statistically significant decline in these scores after the first 4 w of treatment, with S group experiencing a greater decline than C group (p<0.05, fig. 3A and fig. 3B). There was no difference in the incidence of adverse reactions between the two groups (p>0.05, Table 2).
| Characteristic | Group S (n=25) | Group C (n=35) | χ2 | p value |
|---|---|---|---|---|
| Infection | 0 (0.00) | 0 (0.00) | ||
| Hepatic disorder | 0 (0.00) | 1 (2.86) | ||
| Gastritis | 0 (0.00) | 1 (2.86) | ||
| Gastrointestinal bleeding | 0 (0.00) | 1 (2.86) | ||
| Glaucoma | 0 (0.00) | 1 (2.86) | ||
| Eosinophilia | 1 (4.00) | 0 (0.00) | ||
| Conjunctivitis of the eye | 1 (4.00) | 0 (0.00) | ||
| Total | 2 (8.00) | 4 (11.43) | 0.191 | 0.663 |
Table 2: Adverse effects in The group S compared with the group C
According to our research, dupilumab can help treat BP that ranges from moderate to severe, primarily in terms of reducing the cumulative dose of the hormone at disease control and the time to control disease activity, as well as reducing clinical symptoms and optimizing laboratory parameters.
We used the time to control disease activity as the primary observation in this study and showed that dupilumab rapidly prevented the appearance of new lesions and promoted the healing of primary lesions, resulting in more rapid control of BP compared to hormone therapy alone. Also, our study showed that when comparing the effective duration of hormone use between the two groups, the addition of dupilumab to hormone use showed that dupilumab allowed BP to progress in less time and at a lower hormone dose (both the effective control dose and cumulative dose of hormones were lower in S group than in C group). Previous studies have shown that long-term use of methylprednisolone can affect the function of multiple systems in the body and has a higher number of side effects[18]. Dupilumab has a high safety profile in the treatment of BP by effectively reducing the use of hormones while reducing the incidence of adverse effects.
Our study showed that IL-4 and IL-13 were abundantly expressed in the serum of BP patients, further confirming the important role of IL-4 and IL- 13 mediated type 2 inflammation in the development of BP. The levels of IL-4 and IL-13 were significantly lower in BP patients after 4 w of dupilumab treatment, significantly lower than those of patients receiving hormone therapy alone, confirming that dupilumab can reduce the type 2 inflammatory response by inhibiting the expression of IL-4 and IL-13, thus hindering the progression of BP. Additionally, it was discovered that individuals with BP had significant levels of IgE, eosinophils and anti-BP180 antibodies. By inducing the IL-4/IL-13 inflammatory pathway and changing the Th1/Th2 balance, IgE subtype antibodies govern Th2 cells, favoring the Th2 cell population for immune response[19]. The stimulation of type 2 cells promotes the development of autoantibodies against BP180 and BP230 as well as the proliferation of B cells. These autoantibody/ self-antigen combinations increase the inflammatory pathway’s level of activation, encourage mast cell degranulation, and draw in eosinophils. By blocking the type 2 inflammatory pathway, dupilumab can achieve effective regulation of IgE, eosinophils and other substances, thus optimizing the body’s immune status and achieving BP control.
Pruritus, which is primarily induced by Th2 cytokines like IL-13 via the peripheral itch sensory neuron signaling pathway, is one of the most common clinical symptoms of BP[20]. By directly blocking the IL-4/ IL-13 pathway and suppressing the activity of Th2 cells, the targeted biologic medication dupilumab can considerably reduce pruritus and improve the lesion state. The findings of this study confirmed the efficacy of dupilumab in reducing the clinical symptoms of BP patients and enhancing the standard of care by demonstrating that after 4 w of dupilumab treatment, the pruritus scores and BP activity in S group were significantly lower.
In terms of safety, this study found that patients with BP treated with hormones alone experienced adverse reactions such as abnormal liver function and gastric upset, which did not occur in patients treated with dupilumab. There was one case of ocular conjunctivitis and one case of eosinophilia in patients treated with dupilumab. The overall incidence of adverse reactions was not significantly different between the two groups, indicating that the addition of dupilumab to hormone therapy for BP does not increase the risks associated with the use of the drug and has a significant safety profile. However, the potential for eosinophilia and ocular conjunctivitis as a result of dupilumab treatment needs to be formalized in a larger clinical data set and solutions actively explored to further enhance the safety of dupilumab in the treatment of BP.
This study is a real-world, non-randomized controlled study, which does not allow for more effective control of some of the variables in the patients, which may affect the conclusions of the study to some extent. The long-term prognostic result of the patients in this trial could not be determined because they were not followed up on. Patient's quality of life and comfort with treatment were not assessed in this study, and it is not possible to know whether dupilumab may provide benefits to patients in terms of quality of life.
The clinical efficacy of dupilumab injection in combination with systemic low-dose hormone therapy for BP is remarkable. It can effectively reduce the type 2 inflammatory response of the body, shorten the duration of disease control, reduce the dose of hormone use, improve pruritus and other clinical symptoms, reduce adverse reactions, and have a significant safety profile. However, more clinical data are needed to verify whether it can be widely promoted and applied in clinical practice.
Conflict of interests:
The authors declared no conflict of interests.
References
- Bolognia JL, Jorizzo JL, Rapini RP. Chapter 30-pemphigoid group. In: Bolognia JL, Jorizzo JL, Rapini RP, editors. Dermatology. 3rd ed. Mosby Elsevier; 2012. p. 475-82.
- Miyamoto D, Santi CG, Aoki V, Maruta CW. Bullous pemphigoid. Bras Dermatol 2019;94:133-46.
- Amber KT, Murrell DF, Schmidt E, Joly P, Borradori L. Autoimmune subepidermal bullous diseases of the skin and mucosa: Clinical features, diagnosis and management. Clin Rev Allergy Immunol 2018;54:26-51.
[Crossref] [Google Scholar] [PubMed]
- Kridin K, Ludwig RJ. The growing incidence of bullous pemphigoid: Overview and potential explanations. Front Med 2018;5:220.
[Crossref] [Google Scholar] [PubMed]
- Liu YD, Wang YH, Ye YC, Zhao WL, Li L. Prognostic factors for mortality in patients with bullous pemphigoid: A meta-analysis. Arch Dermatol Res 2017;309:335-47.
- Feliciani C, Joly P, Jonkman MF, Zambruno G, Zillikens D, Ioannides D, et al. Management of bullous pemphigoid: The European dermatology forum consensus in collaboration with the European academy of dermatology and venereology. Br J Dermatol 2015;172(4):867-77.
[Crossref] [Google Scholar] [PubMed]
- Suárez-Fernández R, España-Alonso A, Herrero-González JE, Mascaró-Galy JM. Practical management of the most common autoimmune bullous diseases. Actas Dermosifiliogr 2008;99(6):441-55.
[Crossref] [Google Scholar] [PubMed]
- Phoon YW, Fook-Chong SM, Koh HY, Thirumoorthy T, Pang SM, Lee HY. Infectious complications in bullous pemphigoid: An analysis of risk factors. J Am Acad Dermatol 2015;72(5):834-9.
[Crossref] [Google Scholar] [PubMed]
- Geller S. Interleukin 4 and interleukin 13 inhibition: A promising therapeutic approach in bullous pemphigoid. J Am Acad Dermatol 2020;83(1):37-8.
[Crossref] [Google Scholar] [PubMed]
- Büdinger L, Borradori L, Yee C, Eming R, Ferencik S, Grosse-Wilde H, et al. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest 1998;102(12):2082-9.
[Crossref] [Google Scholar] [PubMed]
- Abdat R, Waldman RA, de Bedout V, Czernik A, Mcleod M, King B, et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J Am Acad Dermatol 2020;83(1):46-52.
[Crossref] [Google Scholar] [PubMed]
- Cao P, Xu W, Zhang L. Rituximab, omalizumab and dupilumab treatment outcomes in bullous pemphigoid: A systematic review. Front Immunol 2022;13:928621.
[Crossref] [Google Scholar] [PubMed]
- Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatol 2018;154(10):1225-6.
[Crossref] [Google Scholar] [PubMed]
- Russo R, Cozzani E, Gasparini G, Parodi A. Targeting interleukin 4 receptor a: A new approach to the treatment of cutaneous autoimmune bullous diseases? Dermatol Ther 2020;33(1):e13190.
[Crossref] [Google Scholar] [PubMed]
- Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G, Hughes JR, et al. British Association of Dermatologists’ guidelines for the management of bullous pemphigoid 2012. Br J Dermatol 2012;167(6):1200-14.
[Crossref] [Google Scholar] [PubMed]
- Murrell DF, Daniel BS, Joly P, Borradori L, Amagai M, Hashimoto T, et al. Definitions and outcome measures for bullous pemphigoid: Recommendations by an international panel of experts. J Am Acad Dermatol 2012;66(3):479-85.
[Crossref] [Google Scholar] [PubMed]
- Ujiie H, Iwata H, Yamagami J, Nakama T, Aoyama Y, Ikeda S, et al. Japanese guidelines for the management of pemphigoid (including epidermolysis bullosa acquisita). J Dermatol 2019;46(12):1102-35.
[Crossref] [Google Scholar] [PubMed]
- Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Exp Opin Drug Safety 2016;15(4):457-65.
[Crossref] [Google Scholar] [PubMed]
- Moyle M, Cevikbas F, Harden JL, Guttman-Yassky E. Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Exp Dermatol 2019;28(7):756-68.
[Crossref] [Google Scholar] [PubMed]
- Rico, Benning, Weingart, Streilein. Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. Br J Dermatol 1999;140(6):1079-86.
[Crossref] [Google Scholar] [PubMed]