- *Corresponding Author:
- Qin Xu
Department of Cardiology and Nephrology,Eastern Theater Air Force Hospital, Nanjing, Jiangsu 210003, China
E-mail: dty20140212345@163.com
| This article was originally published in a special issue,“Exploring the Role of Biomedicine in Pharmaceutical Sciences” |
| Indian J Pharm Sci 2024:86(1) Spl Issue “164-172” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To observe the clinical efficacy of the use of non-invasive ventilator assisted ventilation in combination had morphine in the treatment of patients had acute heart failure. The clinical data of 100 patient had acute heart failure in the department of cardiology of our hospital from 2020 to 2023 were retrospectively collected. They were divided into the experimental group and the control group according to whether morphine treatment was added to the treatment plan, in which the experimental group was treated had non-invasive ventilator-assisted ventilation combined had morphine treatment (n=50), the control group was treated with non-invasive ventilator-assisted ventilation treatment (n=50). Thus, to investigate the therapeutic effect of patient had acute heart failure; non-invasive ventilation had combined with morphine. No significant differences were found between baseline levels in either group, various pre-treatment clinical and heart function indicators (p>0.05). The therapeutic effect of patient in the experimental group was significantly better than that of patient in the control group (p<0.05), the frequency of adverse reactions in patient in the experimental group was lower than in the control group (p<0.05). Comparing the clinical indexes and cardiac function indexes of the two groups, it was found that the clinical indexes of the experimental group included systolic blood pressure, diastolic blood pressure, heart rate, respiration, mean airway pressure, and partial pressure of oxygen, partial pressure carbon dioxide, and oxygen saturation indexes of arterial blood gas analysis in the vital signs of the experimental group, the cardiac function indexes included left ventricular ejection fraction, left ventricular end-diastolic diameter, left ventricular end-diastolic dimension, left ventricular diameter, left ventricular mass index, B-type natriuretic peptide, NT-PP, and NT-PP in the echocardiogram and the heart failure markers of the experimental group, respectively. B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide index levels were better than those of control patient (p<0.05).Treatment of patient with acute heart failure had non-invasive ventilation in combination with morphine is more effective and safer.
Keywords
Acute heart failure, morphine, non-invasive ventilator, therapeutic efficacy, adverse effects
Acute Heart Failure (AHF), referred to as AHF, is a serious and life-threatening disease, mainly due to left ventricular systolic or diastolic dysfunction leading to an increase in preload and afterload, pulmonary congestion with fluid in distribution and retention can lead to systemic congestion, thus insufficient perfusion of the whole organs leading to dysfunction, accompanied by elevated plasma natriuretic peptide levels[1-3]. Currently, AHF is still a disease with a high mortality rate; clinical treatment should be individualized for patient with AHF in order to improve prognostic outcomes.
Treatment goals for AHF patient are based on the severity of their disease. In emergency resuscitation, the main focus is to rapidly stabilize the hemodynamic status, improve symptoms and correct hypoxia. In subsequent stabilization therapy, the main focus is on correcting the triggers and causes of heart failure and preventing thromboembolism[4]. Non-invasive multifunctional electrocardiographic monitoring, necessary condition notification, establishment of venous access are generally given to AHF patient for treatment. However, for patient with AHF-induced dyspnoea with hypoxaemia, oxygen therapy is required, if the condition still deteriorates after active treatment, non-invasive ventilatorassisted ventilation is required[5]. By performing non-invasive ventilator-assisted ventilation, the respiratory distress of patient can be improved more effectively and faster, the risk of tracheal intubation can be reduced, thus improving the survival rate of high-risk patient[6]. At present, the application of non-invasive ventilators in the treatment of AHF is promising, which can improve the symptoms of respiratory distress and improve the survival rate of patient while reducing the complication rate.
In clinical diagnosis and treatment, if AHF patient have symptoms such as irritability, morphine can be used in small doses for slow intravenous injection. In the treatment of AHF patient, morphine cannot only reduce the preload and afterload of the heart, but also effectively improve the symptoms of dyspnoea, chest pain and anxiety in patient[7]. In addition, morphine and other opioids can also be used for sedation, analgesia[8], in the safety guarantee, at the same time, effectively relieve the patient’s irritability and pain symptoms[9]. At present, morphine is an indispensable part of AHF treatment, but how to regulate the use of morphine, and the dosage of reasonable control is the followup clinical work needs to be further studied.
In this study, we retrospectively collected clinical data of AHF patient, analyzed the clinical efficacy of morphine in the treatment of AHF patient combined had non-invasive ventilation, with a focus on the occurrence of adverse reactions after treatment and the clinical indicators before and after the treatment, including vital signs, arterial blood gas analysis, and cardiac function indicators. We hope that this single-center, small-sample study will provide some reference value for the clinical use of morphine was used in combination had noninvasive ventilation of AHF patient
Materials and Methods
General information:
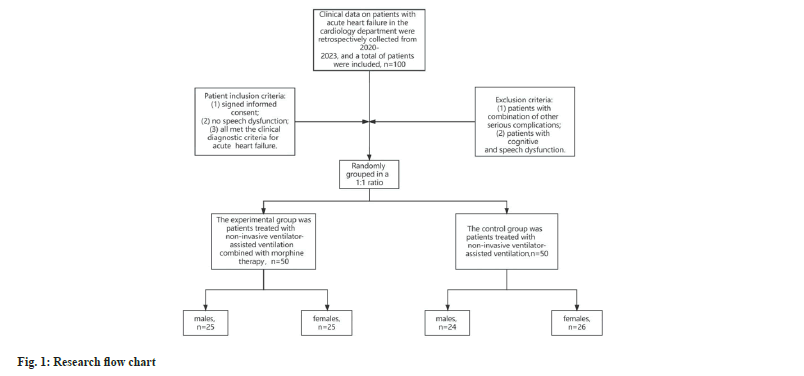
The clinical data of patient had AHF in our hospital between the years 2020 and 2023 were collected retrospectively, a total of 100 patient were included, with 50 cases in the experimental group and 50 cases in the control group. The experimental group consisted of patient who were treated had the use of non-invasive ventilator assisted ventilation in combination had morphine, 25 male cases and 25 female cases, all aged (63.22±4.713) y. The control group was patient treated with the application of non-invasive ventilator-assisted ventilation, 24 males and 26 females, all aged (62.02±4.108) y.
Inclusion criteria: Signed consent form; no speech dysfunction; all met the clinical diagnostic criteria of AHF were included.
Exclusion criteria: Patient with other serious complications; patient with cognitive and speech dysfunction. The flowchart of the study is shown in fig. 1.
Treatment modalities:
Both the groups of patient collected in this study were admitted to the hospital and underwent basic treatment with nasal cannula oxygen, detection of vital signs, cardiotonic, diuretic, and correction of hydroelectrolyte disorders. In the control group, non-invasive ventilator-assisted ventilation was used in the basic treatment, with the parameters of Respiratory Rate (RR) between 12 and 30 breaths/min, the concentration of inhaled oxygen between 30 % and 60 %, and the range of inhaled air pressure between 10 and 16 cm Water (H2O). In the experimental group there were, in the control group, morphine was added to the treatment, and the first step was to give the patient an intravenous injection of morphine 3 to 5 mg, and then pump 5 μg of morphine through the intravenous pump.
Observation indicators:
Criteria for determining the efficacy of treatment: After 24 h of treatment, the clinical symptoms of AHF patient such as dyspnoea, accelerated Heart Rate (HR) and so on were significantly relieved, and the RR and HR tended to be normalized as the effect; after 24 h-48 h of treatment, the clinical symptoms were relieved, and the RR and HR recovered but did not reach the normal range as the effect; after 48 h of treatment, the clinical symptoms and the RR and HR did not improve or even aggravated as ineffective.
Overall effective rate=significant rate+effective rate, Mean Arterial Pressure (MAP)=(systolic blood pressure+2×diastolic blood pressure)/3.
Cardiac function observations:
The data for the B-type Natriuretic Peptide (BNP) are as follows; N-Term B-Type Natriuretic Peptide (NT-PROBNP), echocardiographic Left Ventricle Ejection Fraction (LVEF), Left Ventricle End-Systolic Diameter (LVESD), Left Ventricle End Diastolic Diameter (LVEDD), Left Atrium Diameter (LAD), cardiac Left Ventricle Myocardial Mass Index (LVMI), which were measured before and after the treatment of the patient in both groups, were collected retrospectively by means of a double sandwich enzyme-linked immunosorbent assay.
Statistical method:
Statistical Package for the Social Sciences (SPSS) 26.0 software was used to analyze the data, where qualitative information is presented as mean±standard deviation (x? ±s), and quantitative information was expressed in the form of frequency (percentage) (n, %). To compare the data between the two groups in the measurement data, the t-test was used for independent samples, for count data, the Chi-square (χ2) test was used. Statistical significance indicated by p<0.05.
Results and Discussion
Comparison of the clinical data of the experimental group with those of the control group, the results showed that there was no significant difference in the general clinical data of the two groups of patients before treatment (p>0.05), as shown in Table 1 and Table 2.
| Experimental group (n=50) | Control group (n=50) | t | p | |
|---|---|---|---|---|
| Gender | 0.04 | 0.841 | ||
| Male | 25 (50 %) | 24 (48 %) | ||
| Female | 25 (50 %) | 26 (52 %) | ||
| Drinking History | 0.041 | 0.84 | ||
| Yes | 22 (44 %) | 21 (42 %) | ||
| No | 28 (56 %) | 29 (58 %) | ||
| Smoking History | 3.241 | 0.072 | ||
| Yes | 21 (42 %) | 30 (60 %) | ||
| No | 29 (58 %) | 20 (40 %) | ||
| Coronary heart disease | 0.932 | 0.334 | ||
| Yes | 13 (26 %) | 9 (18 %) | ||
| No | 37 (74 %) | 41 (82 %) | ||
| Hypertensive | 1.98 | 0.159 | ||
| Yes | 19 (38 %) | 26 (52 %) | ||
| No | 31 (62 %) | 24 (48 %) | ||
| Hyperlipidemia | 2.627 | 0.105 | ||
| Yes | 25 (50 %) | 33 (66 %) | ||
| No | 25 (50 %) | 17 (34 %) | ||
| Diabetes | 0.176 | 0.677 | ||
| Yes | 17 (34 %) | 19 (38 %) | ||
| No | 33 (66 %) | 31 (62 %) | ||
| Pulmonary embolism | 2.102 | 0.147 | ||
| Yes | 22 (44 %) | 15 (30 %) | ||
| No | 28 (56 %) | 35 (70 %) | ||
| COPD | 1.099 | 0.295 | ||
| Yes | 15 (30 %) | 20 (40 %) | ||
| No | 35 (70 %) | 30 (60 %) | ||
| Myocardial infarction | 0.361 | 0.548 | ||
| Yes | 22 (44 %) | 25 (50 %) | ||
| No | 28 (56 %) | 25 (50 %) | ||
| Heart valve disease | 1.144 | 0.229 | ||
| Yes | 20 (40 %) | 26 (52 %) | ||
| No | 30 (60 %) | 24 (48 %) | ||
| Surgical history | 4.482 | 0.028 | ||
| Yes | 19 (38 %) | 30 (60 %) | ||
| No | 31 (62 %) | 20 (40 %) | ||
| Heart function classification | 3.331 | 0.345 | ||
| 1 | 12 (24 %) | 8 (16 %) | ||
| 2 | 16 (32 %) | 11 (22 %) | ||
| 3 | 12 (24 %) | 18 (36 %) | ||
| 4 | 10 (20 %) | 13 (26 %) | ||
| Age (year) | 63.22±4.713 | 62.02±4.108 | 1.357 | 0.151 |
| BMI (kg/m2) | 22.84±1.390 | 23.16±1.405 | -1.145 | 0.758 |
Table 1: General Baseline
| Experimental group (n=50) | Control group (n=50) | t | p | |
|---|---|---|---|---|
| WBC (109/l) | 6.86±2.1 | 7.2±2.080 | -0.813 | 0.58 |
| RBC (1012/l) | 4.342±0.486 | 4.44±0.527 | -0.439 | 0.727 |
| TP (g/l) | 69.52±6.139 | 71.34±5.539 | -1.557 | 0.333 |
| Seroglobulin A (g/l) | 24.86±2.548 | 24.46±2.435 | 0.803 | 0.917 |
| Hemoglobin (g/l) | 123.72±17.551 | 127.90±17.415 | -1.195 | 0.281 |
| Serum ferritin (ng/ml) | 129.82±12.895 | 131.12±14.175 | -0.48 | 0.576 |
| Serum potassium (mmol/l) | 4.286±0.468 | 4.354±0.437 | -0.75 | 0.442 |
| Serum sodium (mmol/l) | 140±2.901 | 140.86±3.136 | -0.563 | 0.539 |
| Hematocrit (%) | 38.0±4.456 | 37.57±5.36 | 0.429 | 0.389 |
| Alanine transaminase (mg/l) | 14.97±1.539 | 15.64±1.668 | -2.087 | 0.351 |
| Aspartate aminotransferase (mg/l) | 31.24±5.738 | 30.72±6.493 | 0.424 | 0.307 |
| Alkaline phosphatase (mg/l) | 67.84±3.232 | 68.98±3.512 | -1.683 | 0.527 |
| Blood urea nitrogen (mg/dl) | 17.40±1.726 | 17.30±1.619 | 0.299 | 0.527 |
| Serum creatinine (µmol/l) | 126.28±3.902 | 126.32±3.862 | -0.052 | 0.637 |
Table 2: Comparison of laboratory data
It was found that there was no difference in the vital signs of the two groups before the start of treatment, includes systolic blood pressure, diastolic blood pressure, blood pressure, HR, respiration, MAP, LVEF, the levels of Partial Pressures for Oxygen (PaO2), Partial Pressures Carbon Dioxide (PaCO2), Oximeter Saturation (SpO2) in arterial blood gas analysis (p>0.05); after treatment, the vital signs of the experimental group, including systolic blood pressure, diastolic blood pressure, HR, respiration, MAP, LVEF, and the levels of PaO2, PaCO2, and SpO2 in arterial blood gas analysis were all better than those of the control group (p<0.05). PaCO2, SpO2 levels were better than the control group (p<0.05) as shown in Table 3 and Table 4.
| Experimental group (n=50) | Control group (n=50) | t | p | |
|---|---|---|---|---|
| Pre-treatment systolic blood pressure (mmHg) | 152.62±7.059 | 153.74±7.134 | -0.789 | 0.95 |
| Post-treatment systolic blood pressure (mmHg) | 120.18±3.385 | 130.38±0.256 | -11.537 | 0.002 |
| Pre-treatment diastolic blood pressure (mmHg) | 102.38±4.290 | 102.80±4.690 | -0.467 | 0.222 |
| Post-treatment diastolic blood pressure (mmHg) | 73.50±4.722 | 84.46±2.779 | -14.145 | 0.000 |
| Pre-treatment HR (times/min) | 123.20±6.207 | 125.12±5.706 | -1.61 | 0.287 |
| Post-treatment HR (times/min) | 86.46±6.491 | 99.98±5.016 | -11.654 | 0.041 |
| Pre-treatment respiration (times/min) | 27.42±3.104 | 27.50±3.570 | -0.12 | 0.36 |
| Post-treatment respiration (times/min) | 15.34±1.624 | 20.28±1.126 | -17.679 | 0.003 |
| Pre-treatment MAP (mmHg) | 119.13±3.673 | 119.78±3.652 | -0.89 | 0.69 |
| Post-treatment MAP (mmHg) | 89.06±3.280 | 99.77±2.331 | -18.813 | 0.00 |
Table 3: Vital signs indicators of The two groups
| Experimental group (n=50) | Control group (n=50) | t | p | |
|---|---|---|---|---|
| Pre-treatment PaO2 (mmHg) | 54.74±2.940 | 54.56±2.727 | 0.317 | 0.301 |
| Post-treatment PaO2 (mmHg) | 96.54±1.328 | 90.74±2.813 | 13.186 | 0.000 |
| Pre-treatment PaCO2 (mmHg) | 60.96±4.160 | 62.16±4.90 | -1.32 | 0.139 |
| Post-treatment PaCO2 (mmHg) | 34.42±2.081 | 43.52±3.228 | -16.756 | 0.001 |
| Pre-treatment SpO2 (%) | 88.24±4.069 | 87.88±4.114 | 0.44 | 0.944 |
| Post-treatment SpO2 (%) | 95.32±2.351 | 92.48±1.741 | 6.684 | 0.009 |
Table 4: Comparison of arterial blood gas analysis between The two groups
Comparison found that before the treatment of the two groups of patients, there was no significant difference in all cardiac function indexes (p>0.05), through the treatment, the experimental group of patient cardiac function indexes, echocardiography including LVEF, LVESD, LVEDD, LAD, LVMI, and the level of heart failure markers including BNP, NT-PROBNP indexes were significantly better than the control group (p<0.05) as shown in Table 5.
| Experimental group (n=50) | Control group (n=50) | t/z/χ2 | p | |
|---|---|---|---|---|
| Cardiac enzymes before treatment | ||||
| AST (U/L) | 27.52±11.11 | 29.22±12.31 | -0.727 | 0.362 |
| CPK (U/L) | 111.17±87.72 | 111.45±92.02 | -0.016 | 0.581 |
| Α-HBDH (U/L) | 161.04±71.76 | 173.98±73.87 | -0.088 | 0.725 |
| HDL (U/L) | 198.90±74.44 | 197.30±67.446 | 0.113 | 0.764 |
| Echocardiography | ||||
| LVEF before treatment (%) | 36.14±3.010 | 35.42±2.836 | 1.231 | 0.719 |
| LVEF after treatment (%) | 56.18±3.837 | 42.22±2.477 | 21.615 | 0.02 |
| LVESD before treatment (mm) | 50.132±3.215 | 50.152±3.01 | -0.032 | 0.61 |
| LVESD after treatment (mm) | 37.696±1.415 | 44.702±2.015 | -20.11 | 0.002 |
| LVEDD before treatment (mm) | 60.404±3.187 | 60.158±2.940 | 0.401 | 0.503 |
| LVEDD after treatment (mm) | 45.262±2.496 | 55.252±1.669 | -23.69 | 0.002 |
| LAD before treatment (mm) | 42.008±2.334 | 42.130±2.234 | -0.267 | 0.822 |
| LAD after treatment (mm) | 34.818±2.966 | 42.996±2.271 | -15.47 | 0.045 |
| LVMI before treatment | 190.14±13.06 | 187.98±11.64 | 0.873 | 0.182 |
| LVMI after treatment | 137.18±10.67 | 175.64±8.473 | -19.95 | 0.015 |
| Heart failure markers | ||||
| BNP before treatment (ng/l) | 652.02±138.9 | 633.26±135.1 | 0.684 | 0.709 |
| BNP after treatment (ng/l) | 262.72±69.60 | 358.76±51.83 | -7.825 | 0.034 |
| NT-PROBNP before treatment (ng/l) | 5078.4±129.3 | 5063.7±110.9 | 0.612 | 0.077 |
| NT-PROBNP after treatment (ng/l) | 3112.56±54.3 | 3780.80±70.2 | -53.17 | 0.011 |
| Clinical degree classification before treatment | ||||
| Killip classification | 1.527 | 0.676 | ||
| 1 | 11 (22 %) | 14 (28 %) | ||
| 2 | 14 (28 %) | 10 (20 %) | ||
| 3 | 13 (26 %) | 11 (22 %) | ||
| 4 | 12 (24 %) | 15 (30 %) | ||
Table 5: Comparison of cardiac function indexes between The two groups
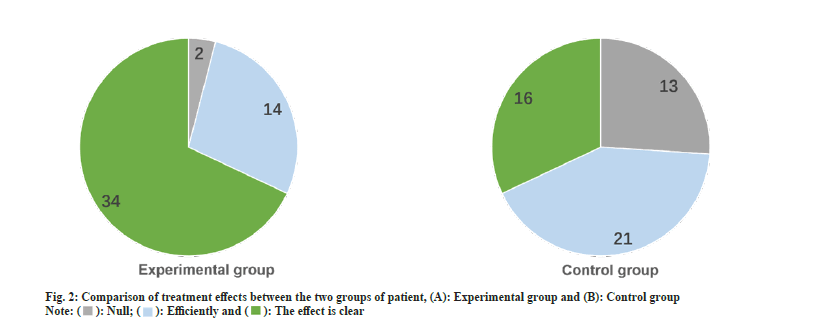
After treatment, there were 34 cases with obvious therapeutic effects in the experimental group and 16 cases with obvious therapeutic effects in the control group. The treatment response of the experimental arm was significantly better than the control arm, statistically significant different (p<0.05) as shown in fig. 2.
After the two groups were treated, the incidence of adverse reactions was 12 % in the experimental group and 38 % in the control group, the incidence of adverse reactions in the experimental group was lower than that of the control group patient (p<0.05) as shown in Table 6.
| Experimental group (n=50) | Control group (n=50) | χ2 | p | |
|---|---|---|---|---|
| Adverse reaction | 9.11 | 0.028 | ||
| Nausea | 3 (6 %) | 11 (22 %) | ||
| Vomiting | 2 (4 %) | 5 (10 %) | ||
| Bloating | 1 (2 %) | 3 (6 %) | ||
| Total | 6 (12 %) | 19 (38 %) |
Table 6: Incidence of adverse reactions in The two groups
AHF patient in the early stages of treatment can become unconscious and feel near death, resulting in reduced ventilator adaptability and has an impact on the safety of the patient's life. Therefore, the treatment of patient had AHF, it is necessary to quickly stabilize the dynamic level of the patient's blood cells and improve the respiratory situation and other symptoms[10]. In this study, in patients had AHF following the combination use of noninvasive ventilation had morphine therapy, the ventilation of patient was significantly improved, and the cardiac function indicators, including the levels of LVEF, LVESD, LVEDD, LAD and LVMI in echocardiography, heart failure marker BNP and NT-proBNP levels, showed significant changes, and the patient cardiac function recovered more quickly.
The treatment of AHF patient requires more research exploration in drug selection and device application. Arrigo et al. found that alejumab can improve cardiovascular function in AHF patient by regulating the function of endothelial cells[11]; however, another report pointed out that patient are prone to acute dyspnoea in the post-hospital period after treatment with alejumab, and increase the rate of re-admission of the patient with a concomitant risk of adverse reactions[12,13]. Kanai et al.[14] found that in AHF patient treated with medications, the prognostic outcome of patient treated with a single drug was better than that of patient treated with a combination of drugs, and the type of drug was associated with the mortality rate of patient and the incidence of adverse reactions, which suggests that a combination of drugs is not recommended for the treatment of patient with AHF. Therefore, in this study, only the opioid morphine was used, and the results showed that it could not only alleviate the symptoms of dyspnoea in patient with AHF, but also reduce the incidence of adverse effects and the rate of readmission, which proved that the application of morphine monotherapy in patient with AHF is safer.
Relevant studies have shown that in the application of non-invasive ventilator treatment, not only can improve the therapeutic effect of patient, but also can greatly reduce the occurrence of a series of adverse reactions[15]. Bello et al.[16] have shown that non-invasive ventilator-assisted ventilation can improve the therapeutic efficacy of patient with acute respiratory failure, and can also avoid complications and side effects such as endotracheal intubation. This coincides with the findings of this survey, in which patient with AHF were treated with noninvasive ventilator-assisted ventilation to improve their therapeutic efficacy and there were few adverse reactions. Based on the above conclusions, in the present study, the use of noninvasive ventilator combined with morphine in the treatment of patient with AHF was found to be effective in improving the levels of clinical indexes PaO2, RR, and HR with a low incidence of adverse reactions.
This is consistent with previous studies, for example, Cao et al.[17] found that compared with the traditional ventilator, the use of a combination of non-invasive ventilation and received morphine during treatment of patient had acute lung injury can effectively improve their prognosis and reduction in the incidence of side effects. In addition, Zhang et al.[18] found that by using noninvasive ventilatorassisted ventilation combined with morphine on the levels of PaO2, RR, PaCO2, HR, and other indexes in patient with pneumonia, it can more effectively improve the state of patient with severe hypoxia.
In summary, this study compared the levels of various clinical indicators and indicators of the function of the heart before a heart and after the treatment of the two groups of patients, found that the treatment of patient who have had noninvasive ventilation in combination had morphine have been effective in the improvement of various clinical indicators and cardiac function indicators, compared the therapeutic efficacy of the two groups of patients before and after the treatment, and then monitored the incidence of side effects in the two groups of patients, found that the treatment efficacy was better for patient on non-invasive ventilation in combination had morphine, and that the treatment efficacy had better for patient on noninvasive ventilation in combination had morphine. Patient on non-invasive ventilation combined had morphine were found to have better treatment outcomes, the incidence of adverse reactions was lower, and it was safer. However, it is undeniable that this study has certain limitations, because of the small sample size of the single-center study, the results were not statistical results and are inevitably subject to bias, and more clinical research centers are still needed for large sample verification.
AHF is a disease with a very high mortality and readmission rate. Patients treated with noninvasive ventilator-assisted ventilation combined with morphine not only improve the therapeutic efficacy and reduce the incidence of adverse effects, but also effectively improve their cardiac function indexes, including the levels of LVEF, LVESD, LVEDD, LAD, and LVMI in echocardiography, as well as the levels of BNP and NT-PROBNP in the markers of heart failure, which is safer and more reliable.
Author’s contributions:
Mei Li and Enyan Yang have contributed equally to this work.
Conflict of interests:
The authors declared no conflict of interests.
References
- Gupta AK, Tomasoni D, Sidhu K, Metra M, Ezekowitz JA. Evidence-based management of acute heart failure. Can J Cardiol 2021;37(4):621-31.
[Crossref] [Google Scholar] [PubMed]
- Kurmani S, Squire I. Acute heart failure: Definition, classification and epidemiology. Curr Heart Failure Rep 2017;14:385-92.
[Crossref] [Google Scholar] [PubMed]
- Mcdonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-726.
- Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, et al. Acute heart failure. Nat Rev Dis Primers 2020;6(1):16.
- L?Her E, Duquesne F, Girou E, de Rosiere XD, Conte PL, Renault S, et al. Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients. Intensive Care Med 2004;30:882-8.
[Crossref] [Google Scholar] [PubMed]
- Masip J. Noninvasive ventilation in acute heart failure. Curr Heart Fail Rep 2019;16(4):89-97.
[Crossref] [Google Scholar] [PubMed]
- Gil V, Dominguez-Rodriguez A, Masip J, Peacock WF, Miro O. Morphine use in the treatment of acute cardiogenic pulmonary edema and its effects on patient outcome: A systematic review. Curr Heart Fail Rep 2019;16(4):81-8.
[Crossref] [Google Scholar] [PubMed]
- Kawaguchi J, Hamatani Y, Hirayama A, Nishimura K, Nakai E, Nakamura E, et al. Experience of morphine therapy for refractory dyspnea as palliative care in advanced heart failure patients. J Cardiol 2020;75(6):682-8.
[Crossref] [Google Scholar] [PubMed]
- Hamatani Y, Iguchi M, Moriuchi K, Anchi Y, Inuzuka Y, Nishikawa R, et al. Effectiveness and safety of morphine administration for refractory dyspnoea among hospitalised patients with advanced heart failure: The morphine-HF study. BMJ Support Palliat Care 2023;2023:004247.
[Crossref] [Google Scholar] [PubMed]
- Janssens U. Acute heart failure. Med Klin Intensiv Med Not Med 2012;107:397-423.
- Voors AA, Kremer D, Geven C, Ter Maaten JM, Struck J, Bergmann A, et al. Adrenomedullin in heart failure: Pathophysiology and therapeutic application. Eur J Heart Fail 2019;21(2):163-71.
[Crossref] [Google Scholar] [PubMed]
- Henney RP, Vasko JS, Brawley RK, Oldham HN, Morrow AG. The effects of morphine on the resistance and capacitance vessels of the peripheral circulation. Am Heart J 1966;72(2):242-50.
[Crossref] [Google Scholar] [PubMed]
- Vasko JS, Henney RP, Brawley RK, Oldham HN, Morrow AG. Effects of morphine on ventricular function and myocardial contractile force. Am J Physiol 1966;210(2):329-34.
[Crossref] [Google Scholar] [PubMed]
- Kanai M, Minamisawa M, Motoki H, Seko Y, Kimura K, Okano T, et al. Prognostic impact of hyperpoly pharmacy due to non cardiovascular medications in patients after acute decompensated heart failure-insights from the clue of risk stratification in the elderly patients with heart failure (CURE-HF) registry. Circ J 2023.
[Crossref] [Google Scholar] [PubMed]
- Garguilo M, Lejaille M, Vaugier I, Orlikowski D, Terzi N, Lofaso F, et al. Noninvasive mechanical ventilation improves breathing-swallowing interaction of ventilator dependent neuromuscular patients: A prospective crossover study. PLoS One 2016;11(3):e0148673.
[Crossref] [Google Scholar] [PubMed]
- Bello G, de Pascale G, Antonelli M. Noninvasive ventilation. Clin Chest Med 2016;37(4):711-21.
[Crossref] [Google Scholar] [PubMed]
- Cao J, Zhang J, Feng J, Wu Q, Chen BY. A study of noninvasive positive-pressure mechanical ventilation in the treatment of acute lung injury with a complex critical care ventilator. J Int Med Res 2014;42(3):788-98.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Wang Y, Shen J, Zou D, Huang J, Han X, et al. Application of non-invasive ventilator in treatment of severe COVID-19 patients. Clin Lab 2022;68(1).
[Crossref] [Google Scholar] [PubMed]