- *Corresponding Author:
- Anita Mahapatra
AVP Research Foundation, 136-137, Trichy Road, Ramanathapuram P.O, Coimbatore-641 045,India
E-mail: dranitads@gmail.com
| Date of Submission | 05 September 2014 |
| Date of Revision | 10 February 2015 |
| Date of Acceptance | 26 September 2015 |
| Indian J Pharm Sci, 2015;77(5):650-654 |
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Kaá¹£Ä?ya or decoction is an Ayurvedic dosage form, prescribed based on the stage of the disease according to the principles of Ayurveda. This dosage form is traditionally prepared fresh and consumed on the same day but for the sake of convenience; the process of preparation has been modified so that it can be stored with longer shelf life, easy availability and produced in large quantities. There is a need to understand the implications of this modification in terms of chemical changes. This work attempted to check the phytochemical profile of both freshly prepared decoction and commercially available decoction with reference to some analytical parameters like pH, total soluble solids, phenols, alkaloids, potassium and to assess the changes in the thin layer chromatography profiling of the decoction. The results showed that phenols and potassium are found to be two fold higher in freshly prepared decoction, compared to commercially available decoction diluted to dosage in practice (1:4 ratio). However, the total alkaloid content was found to be approximately ten fold higher in commercially available decoction. It was observed that the thin layer chromatography profile of decoctions was extracted into petroleum ether and chloroform was similar and consistent with different batches though the bands in commercially available decoction were slightly more intense compared to freshly prepared decoction. The total soluble solids in commercially available decoction were four times higher than freshly prepared decoction. The study reveals that there are differences in the phytochemical profiles of the freshly prepared decoction and commercially available decoction of the same formulation. However, the significance of these differences can be determined only by further clinical studies. On the other hand, the study lends support to the practice of diluting the commercially available decoction to make it equivalent to freshly prepared decoction.
Keywords
Ayurveda, ciravilva, consistency, decoction, secondary metabolites
Kaṣāya/Kvātha or decoction is a type of Ayurvedic dosage form, which is prepared with specified ingredients boiled in 8 times of water and reduced to ¼th of its original volume [1]. An Ayurvedic prescription mostly contains decoction as a key medicine and is being prescribed based on the strength of the patient and stage of the disease. Decoction is to be prepared fresh and consumed in the same day. This can be a cumbersome process for the patient as this involves chopping and cleaning the raw materials and boiling it for a few hours every day, not to speak of the challenge to ensure continuous availability of the raw materials. As a matter of convenience, for more than a century, the Ayurvedic industry has developed methods to prepare concentrates of the decoctions on an industrial scale that can be bottled and preserved for long periods of time. At the time of consumption, the patients have to dilute the specified dose with four times of water to make it equivalent to the freshly prepared decoction. Permitted or approved preservatives are added to the decoction to conserve, with a longer shelf life, and increase the availability and accessibility of the product. The modification in the original dosage form raises many questions that have not been adequately answered yet. What happens when the decoction is cooked for a longer period than recommended by the classical texts? Is there a significant change in the physicochemical profile of the final product and will it affect the efficacy of the product? This study is an attempt to find answers for these questions.
Cirivilvādi kaṣāya (CVK) formulation is indicated for hemorrhoids, fistula in ano, abdominal disturbance and as an appetizer. The ingredients of CVK formulation (comprising of Holoptelea integrifolia, Boerhavia diffusa, Plumbago zeylanica, Terminalia chebula, Piper longum and Zingiber officinale) [2] were procured in 3 different batches from Coimbatore local market and then checked for macroscopic and microscopic specifications to confirm their identity as per API guidelines. After proper identification and conformance to the standard specifications of quality, the ingredients were used in the preparation. CVK was freshly prepared in 3 different batches with the approved ingredients in a controlled temperature and humidity under hygienic conditions at AVPRF laboratory, which is administered within 24 h, whereas the commercial CVK are produced from the same ingredients, but concentrated and bottled with preservatives. Three different batches of CAD of CVK of GMP certified company were procured from Ayurvedic pharmacy for the study.
The three freshly prepared decoctions (FD), three commercially available decoction (CAD) and CAD diluted to dosage in practice (1:4 ratio) of the same CAD (a total of 9 samples) of CVK were checked for pH [3], total soluble solids [4], phenols [5], alkaloids [6], potassium [7] levels along with the TLC profile [8] that was carried out with petroleum ether extract (using petroleum ether:ethyl acetate:methanol (10:2:0.1) as mobile phase) and chloroform extracts (chloroform:toluene:ethanol (5:5:2) as mobile phase). All the parameters were analyzed on fresh decoctions and also in the dried form of the decoctions which were dried at 105° for 3 h to constant weight in an evaporating dish to avoid any inconsistency in the analytical results and were also checked for repeatability and reproducibility of the results.
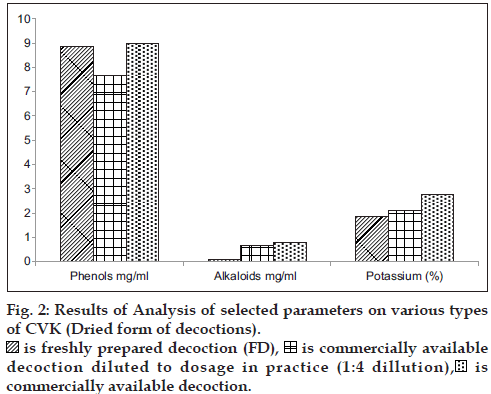
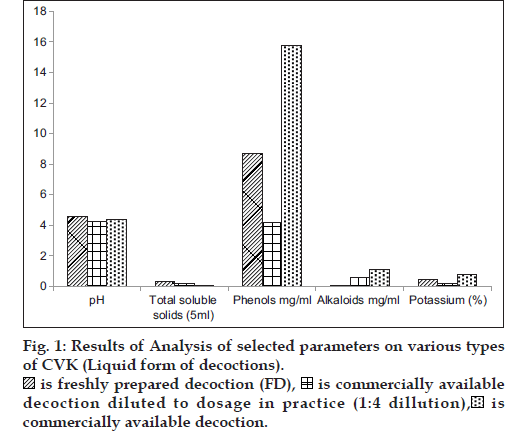
In general practice FD is administered as such and the CAD is diluted four times with water and hence a comparison in the variation of phytochemicals was carried in all the form of decoctions. The consolidated results of analytical parameters of the samples [3 FD+3CAD+3 CAD diluted to dosage in practice (1:4 ratio)] of CVK with mean and standard deviation values are tabulated in figs. 1 and 2.
TLC was performed for all the decoctions (3 FD+3CAD+3 CAD diluted to dosage in practice (1:4 ratio)] of CVK were repeated. The results of TLC obtained and fairly good reproducibility were seen when repeated for nine times, results are furnished below in Tables 1-4.
| FD | CAD diluted todosage in practice(1:4 dilution) | CAD | |||
|---|---|---|---|---|---|
| Colour ofthe spot | Rf value | Colour ofthe spot | Rf value | Colour of the spot | Rf value |
| Pink | 0.08 | Pink | 0.17 | Pink | 0.13 |
| Green | 0.20 | Yellow | 0.57 | Purple | 0.20 |
| Pink | 0.55 | Violet | 0.93 | Blue | 0.28 |
| Yellow | 0.61 | Blue | 0.37 | ||
| Violet | 0.95 | Pink | 0.42 | ||
| Purple | 0.56 | ||||
| Yellow | 0.66 | ||||
| Violet | 0.95 | ||||
Results are derived from triplicate analysis. CVK: Cirivilvādi kaṣāya, FD: freshly prepared decoctions, CAD: commercially available decoction, TLC: thin layer chromatography
Table 1: Tlc Spots Of Cvk Test Solution (Petroleum Ether Extract) After Derivatization With Anisaldehyde Sulphuric Acid
| FD | CAD diluted to dosage in practice (1:4 dilution) | CAD | |||
|---|---|---|---|---|---|
| Colour of the spot | Rf value | Colour of the spot | Rf value; | Colour of the spot | Rf value |
| Pale pink | 0.60 | Pink | 0.67 | Pale pink | 0.61 |
| Pink | 0.75 | Pink | 0.73 | Pink | 0.65 |
| Pale pink | 0.81 | Light pink | 0.67 | ||
| Pink | 0.88 | Pink | 0.71 | ||
Results are derived from triplicate analysis. CVK: Cirivilvādi kaṣāya, FD: freshly prepared decoctions, CAD: COMMERCIALLY available decoction, UV: ultra violet, TLC: thin layer chromatography
Table 2: Tlc Spots Of Cvk Test Solution (Chloroform Extraction) Visualized Under Uv 254 Nm
| FD | CAD diluted to dosage in practice (1:4 dilution) | CAD | |||
|---|---|---|---|---|---|
| Colour of the spot | Rf value | Colour of the spot | Rf value | Colour of the spot | Rf value |
| Fluorescent yellow | 0.28 | Pale fluorescent yellow | 0.8 | Fluorescent blue | 0.65 |
| Fluorescent blue | 0.60 | Fluorescent blue | 0.67 | Fluorescent blue | 0.67 |
| Fluorescent blue | 0.75 | Fluorescent blue | 0.73 | Fluorescent blue | 0.71 |
| Fluorescent pink | 0.81 | Fluorescent blue | 0.76 | ||
| Fluorescent blue | 0.88 | ||||
Results are derived from triplicate analysis. CVK: Cirivilvādi kaṣāya, FD: freshly prepared decoctions, CAD: commercially available decoction, UV: ultra violet, TLC: thin layer chromatography
Table 3: Tlc Spots Of Cvk Test Solution (Chloroform Extraction) Visualized Under Uv 366 Nm
| FD | CAD diluted todosage in practice(1:4 dilution) | CAD | |||
|---|---|---|---|---|---|
| Colour ofthe spot | Rf value | Colour ofthe spot | Rf value | Colour ofthe spot | Rf value |
| Purple | 0.60 | Purple | 0.5 | Purple | 0.67 |
| Green | 0.75 | Green | 0.67 | Green | 0.65 |
| Light violet | 0.68 | Violet | 0.76 | Violet | 0.71 |
Results are derived from triplicate analysis. CVK: Cirivilvādi kaṣāya, FD: freshly prepared decoctions, CAD: commercially available decoction, TLC: thin layer chromatography
Table 4: Tlc Spots Of Cvk Test Solution (Chloroform Extraction) After Derivatization With Anisaldehyde Sulphuric Acid
A comparative analysis of the changes in the levels of major phytochemicals in freshly prepared decoctions (FD), three commercially available decoction (CAD) and CAD diluted to dosage in practice (1:4 ratio) was carried out to assess the deviations in the dose administration of CAD from the FD. The analysis was done on fresh and dry weight basis of the prepared decoction to minimize analytical errors and the compiled results are the average of six analysis.
The major metabolites and marker compounds of the plants that was used in the preparation of decoction was taken into consideration to analyse the marked variations in FD and diluted CAD. Comparative changes in TLC of the secondary metabolic that was carried out, since it is poly herbal formulation. The alkaloid content (Piper longum and Plumbago zeylanica); potassium (Boerhavia diffusa); phenolic compounds (Terminalia chebula) and the soluble solids were taken as critical parameters to understand the phytochemical variations in the FD and CAD, as the major objective of the study was to justify the dose and use of diluted CAD instead of FD.
The formulation was extracted in different solvent systems to evolve a finger print profile for the polyherbal formulation. The solvent that was suitable for eluting the greater part of compounds was found to be petroleum ether and chloroform compared to methanol, water, hexane and ethanol. The developed plates were visualized under visible (derivatised), 366 nm and 254 nm for the chloroform extract, whereas the petroleum ether extract showed well resolved bands only after derivatisation indicating that the compounds that eluted with petroleum ether was different from the compounds eluted with chloroform and could be the phenolic group of compounds due to its reaction with anisaldehyde sulphuric acid. The TLC profile of the decoction was standardized with petroleum ether and chloroform extraction based on the consistency and resolution of the bands. The TLC profile of the decoction was observed for any marked variations with the type of the formulation i.e., CAD, CAD diluted to dosage in practice (1:4 ratio) and FD.
It can be inferred from the values of phytochemical parameters that, pH and total soluble solids did not differ in the decoction that was diluted from CAD in comparison to FD, the phenol content in FD was two-fold higher than the diluted CAD, the levels of potassium were also observed to be two fold higher in the FD, compared to the diluted CAD, whereas the alkaloid content was found to be significantly higher in diluted CAD. The presence of major bands (Pink, Yellow and Violet) was consistent in all decoctions, but less intense in the diluted CAD, which is indicative that considerable changes in the constituents did not occur, during the concentration stage of decoction preparation that is carried out for convenience of treatment and wide accessibility and availability of the drugs. Chemical profiling by TLC did not reveal variations in the Rf, and color of the bands except for the presence of an additional green colored band with an Rf of 0.20 (extracted in petroleum ether) and fluorescent pink (chloroform extracted and visualized under 366 nm) in the FD. The FD showed higher intensity of the separated bands on TLC, which reflects the respective concentration of the compounds. All the samples subjected to chloroform extraction (Purple, Green and Violet) showed similar major and minor bands when visualized under 366 nm and 254 nm, though the intensity of the bands was high in fresh compared to bottled and bottled diluted sample. Similar observations were noted with petroleum ether extracts that was visualized under 366 nm, 254 nm and visible light (derivatised).
The diluted versions of CAD fairly matched with the profile of the FD. This suggests the appropriateness of the current practice to dilute the CAD by adding four times water before consumption. However, the alkaloid contents were found to be higher in the CAD even after dilution in comparison with the FD.
Phenols and potassium were found to be higher in FD, which could be due to the relative extractability of the phenolic compounds in a neutral aqueous phase. The alkaloid content and total soluble solids were significantly high in CAD, as it is extracted under high temperature and pressure and concentrated, for longer durations compared to the preparation of FD. The presence of additional bands and higher/lower concentration of compounds needs to be further evaluated at every step of manufacture and in large number of samples to validate the analytical findings. This preliminary study points out trends in the similarity and dissimilarity of FD and CAD and warrants the necessity of further studies to understand its implications in clinical efficacy of the formulations. Studies are required to be conducted on other formulations to find out if the findings vary depending on the ingredients in various formulations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Vagbhata. AshtangaHridaya. In: Paradakar HS, editor. Jvaracikitsitam. 9th ed. Varanasi: ChaukhambhaSurbhartiPrakashan; 2002. p. 773.
- Anonymous. CikitsasarasarvasvamAthavaSahasrayogam. In: Krishnan KV, Gopalapillay. editors. Gulikayogangal. 19th ed. Mullaykkal, Alapuzha: Vidyarambham Publishers; 1990. p. 50.
- Physical tests and determinations. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 6. New Delhi: Govt. of India; 2009. p. 291.
- Physical tests and determinations. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 6. New Delhi: Govt. of India; 2009. p. 298.
- Chemical tests and assays. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 6. New Delhi: Govt. of India; 2009. p. 336.
- Determination of quantitative data of vegetable drugs. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 5. New Delhi: Govt. of India; 2006. p. 221.
- Chemical tests and Assays. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 6. New Delhi: Govt. of India; 2009. p. 347.
- Determination of quantitative data of vegetable drugs. In: The Ayurvedic Pharmacopoeia of India, Part-1. 1st ed., Vol. 5. New Delhi: Govt. of India; 2006. p. 218.
 is freshly prepared decoction (FD),
is freshly prepared decoction (FD), is commercially available
decoction diluted to dosage in practice (1:4 dillution),
is commercially available
decoction diluted to dosage in practice (1:4 dillution), is
commercially available decoction.
is
commercially available decoction.