- *Corresponding Author:
- Neena Bedi
Department of Pharmaceutical Sciences, Guru Nanak Dev University, Amritsar-143 005, India
E-mail: neena.pharma@gndu.ac.in
| Date of Submission | 06 June 2017 |
| Date of Revision | 14 April 2018 |
| Date of Acceptance | 15 November 2018 |
| Indian J Pharm Sci 2019;81(1):39-44 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The aim of the present investigation was to develop, validate and compare a spectrophotometric and a high performance liquid chromatography method for estimating canagliflozin in bulk and tablet dosage form. Spectrophotometry and high performance liquid chromatography were carried out using standard instrumental parameters, which were optimized. Both methods were validated in terms of linearity, accuracy, precision, robustness, ruggedness and stability according to the ICH guidelines. The optimized ratio of mobile phase in high performance liquid chromatography under low pressure gradient mode was 50:50 % v/v of acetonitrile:orthophosphoric acid (0.01 M), which provide a sharp peak with a short retention time of 4.732 minutes. In spectrophotometric analysis, methanol as a solvent gave adequate molar absorptivity at a λmax of 280 nm. Results indicated that both spectrophotometric and high performance liquid chromatography methods were linear, precise, accurate, rugged and robust with RSD values less than 2 % and percent recovery was within the standard limits (90-110 %). Both the methods were found to be statistically non-significant at 95 % confidence intervals (p<0.05) with respect to each other. The proposed methods were found to be highly effective and could be used for quantification of canagliflozin in bulk and tablet formulations for routine analysis.
Keywords
UV, HPLC, canagliflozin, ICH guidelines, quantification, validation
Gliflozins are the novel sodium glucose co-transporter (SGLT) type-II inhibitors, which prevent glucose absorption in proximal tubules of the kidneys leading to reduced plasma glucose levels and improved glycemic control [1]. These drugs have high target selectivity, low potential for causing hypoglycemia and have promising improvements in fast and post-prandial glucose levels (in contrast to other hypoglycemic drugs) [2]. There are several SGLT-II inhibitors from which canagliflozin (CFZ, Figure 1), the first in a new class of glucose lowering drugs, is already yielding promising data. CFZ inhibits SGLT-II protein in proximal convoluted tubules in the kidneys and provides an insulin-independent mechanism (kidney homeostasis) to lower blood glucose levels [3]. CFZ was approved by FDA in March 2013 and was marketed as Invokana® (100 mg, Janssen Pharmaceuticals) for twice a day dosage regimen.
High performance liquid chromatography (HPLC) analysis is widely implemented for quality control purposes due to high sensitivity, specificity and precise determination of analytes in various biological and analytical media [4]. On the other hand, spectrophotometric analysis is a simpler and inexpensive method of determining analytes in pharmaceutical formulations [5]. Liquid chromatographic methods have been widely employed for estimating drugs in various matrices. However, no spectrophotometric/HPLC method in combination has been reported for CFZ for analysis. An attempt was made to develop a UV and HPLC method for estimating CFZ in bulk and in tablets and both methods were validated as per ICH guidelines.
Materials and Methods
Spectrophotometric studies were performed on a double beam UV spectrophotometer (Blue Star- Au-2701) with spectral bandwidth of 2 nm and wavelength accuracy ±0.5 nm. The solvent used was methanol (100 % v/v) for preparing standard and serial dilutions of CFZ bulk form. The samples were placed in 1 cm quartz cells and absorbance was analysed using Systronics software. HPLC analysis was carried out using a reversed-phase column-based ultra-high performance liquid chromatographic method (Nexera X2, Shimadzu Asia Pacific Limited, Japan). The LC-30 AD system consisted of Shimadzu LC 20 AT pump containing Rheodyne 7725 injector with fixed loop at 20 μl having SIL-30 AC auto sampling configuration. The U-HPLC was equipped with C-18; 4.6 mm×150 mm; 5 μm analytical column (L-2013, Hitachi) and column oven was saturated with optimum temperature of 42°. The isocratic mode containing mobile phases (acetonitrile and orthophosphoric acid, 0.01 M) used at different concentrations were run at a constant flow rate of 0.9 ml/min to determine the optimized ratio for analysis. The prepared mobile phases were sonicated (Ultra Cleaner, Labpro International, India) and filtered with 0.22 μm filter membrane (Millipore, India) prior to analysis. The detection was done by the SPD-M 20A photodiode array detector. The data processing and acquisition was done on Lab Solutions System software (version 3.1.05.9).
For UV spectrophotometry, working standard (primary stock) of concentration 1000 μg/ml was prepared by adding 10 mg of CFZ in 10 ml of methanol. An appropriate dilution (secondary stock) was made to obtain a working standard of 100 μg/ml and was scanned in the range of 200-600 nm to ascertain its λmax. Gradual replicates were prepared from this stock solution to prepare 5-50 μg/ml linear range, filtered using 0.45 μm filter membrane and quantified spectrophometrically at observed λmax of the drug. For HPLC, working standard of 1000 μg/ml was prepared by dissolving 10 mg of CFZ in 10 ml HPLC grade acetonitrile.
Gradual injections were prepared in ranges from 2-40 μg/ml at room temperature and were quantified by HPLC at observed λmax of the drug. Quality control samples were run with each batch of working standards in order to validate the entire method.
The calibration curve was generated using different concentrations in linear progression, a 5-50 μg/ml range for UV and 2-40 μg/ml for HPLC. The linearity was determined by linear regression analysis by auto zeroing the intercept at the vertices of slope. The acceptance criteria involved was that the correlation coefficient (r2) should not be less that 0.990 according to least square method of analysis [5]. Accuracy is the percent amount of given analyte recovered from a known added amount. The methodology for both spectrophotometric and HPLC studies involves the preparation of concentration ranges at three different levels (80, 100 and 120 %) against a nominal set range of UV (30 μg/ml) and HPLC (20 μg/ml). After injection, percent recovery of each prepared concentration was determined. Samples were prepared for both methods in triplicate and assayed [6]. To ascertain the reproducibility of the proposed method, precision studies (intra and inter day) for spectrophotometric studies were carried out by preparing replicates of three different test concentrations (10, 20 and 30 μg/ml) at 100 % level and the drug amount was quantified for intraday and interday precision. For HPLC studies, four different drug concentrations (10, 20, 30 and 40 μg/ml) were analysed for intraday and interday precision [7]. Ruggedness defines the reproducibility of test results after giving variations in the laboratory test conditions like different analysts, different days and different reagents. For both spectrophotometric and HPLC studies, three replicates of different concentrations for two different analysts were prepared and analysed. The corresponding mean absorbance (UV) and peak area (HPLC) were noted and results were reported as % RSD with acceptable value of less than 2 [8].
Robustness involves reproducibility of test results after passing through different temperature conditions. For spectrophotometric studies, experiments were performed at room temperature (25°) and cold temperature of 18°. For HPLC studies, experiments were carried out by varying the flow rate, run time and detection wavelength [8]. The detection of lowest concentration of analyte in the sample defines lower limit of detection (LOD) and the upper concentration of sample that can be quantitatively determined defines upper limit of quantification (LOQ) and is calculated in accordance to the guidelines [9]. Samples evaluated for repeatability and reproducibility studies were preserved for 3 mo at accelerated temperature conditions (45°/ 75 % RH) and analysed for percent drug degradation against nominal concentration range [5]. Ten tablets (marketed product) were accurately weighed and uniformly crushed and passed through sieve no. 21 to obtain a fine powder. The powder equally proportionate to 100 mg was dissolved in 10 ml of acetonitrile and sonicated for 15 min. The solution obtained was filtered using 0.22 μm filter membrane (Millipore, India) and active drug was quantified in both UV and HPLC methods.
Statistical analysis
A statistical procedure was carried out to find statistical difference among these developed methods. The statistical tests, i.e. analysis of variance (ANOVA) and paired t-test were applied to statistically compare these two analytical methods at 95 % confidence interval level (p<0.05).
Results and Discussion
The development of a spectrophotometric method for routine analysis of drugs with precise determination reduces tedious sample preparation and is cost effective [9]. Following Beer Lambert’s law, CFZ with specific chromophore (Figure 1) allows detection at a specific wavelength [10]. The working standard scanned at wavelength of 200-600 nm presented with characteristic absorption spectra at λmax of 290 nm. The specified concentrations were prepared from working standard and the entire method was validated for its accuracy, precision, linearity, robustness, ruggedness as per ICH guidelines specified in the ICHQ2R1 [5].
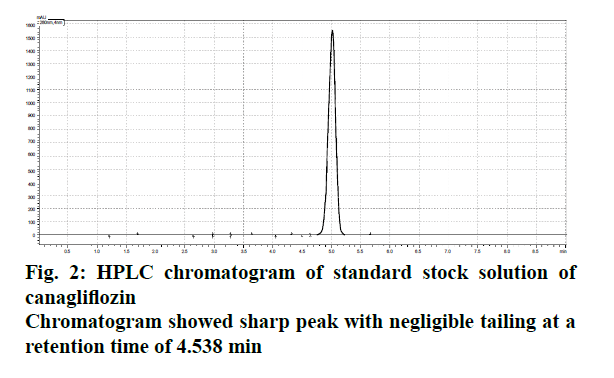
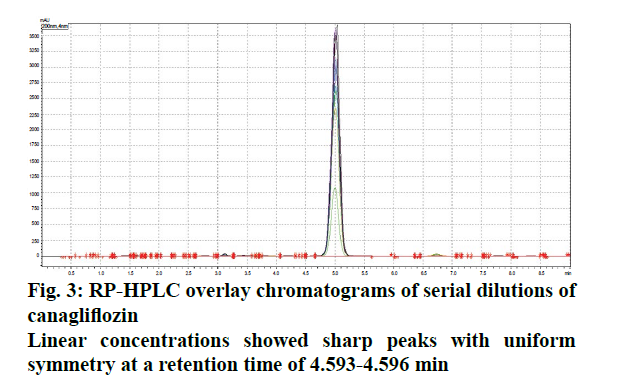
The liquid chromatographic method was developed and optimized in order to provide reproducibility and specificity. The selection criterion for mobile phase was based upon their polarity [8,9]. Since, CFZ is quite lipophilic and non-polar in nature[10]. (Log P>4.16), the mobile phase was modified with two different solvent systems (acetonitrile and ortho-phosphoric acid) and optimized ratio was evaluated on the basis of peak symmetry, peak purity and run time. The uniformity in flow rate is quite crucial as the longitudinal broadening is inversely proportional to the flow rate of mobile phase system [9,10]. Too high or low flow rate affects the Gaussian peak and may cause defects in the overall peak symmetry [11,12]. The optimized ratio of mobile phase was found to be 50:50 % v/v, which showed uniform peak symmetry at a flow rate of 0.9 ml/min (Figure 2). At this ratio, the retention time (RT) of eluted CFZ in standard stock solution was found to be 4.732 min with no interference that permits rapid determination of drug in analytical media. Figure 3 showed typical chromatograms obtained from serial dilutions of the standard stock solution of CFZ.
Linear correlation was observed in both spectrophotometric (concentration range: 5-50 μg/ml) and HPLC method (concentration range: 2-40 μg/ml) Beer’s law was well fitted in the developed linear concentrations in both analysis [13]. The regression coefficient and Eqn. were found to be 0.9955, Y = 0.0162x+0.0089 and 0.9971, Y = 11477x+32441, for UV and HPLC methods, respectively. Furthermore, detection limit depends upon instrument sensitivity as low detection limits give high sensitivity. The LOD/ LOQ in both analysis were found to be 0.00945, 2.8638 μg/ml (UV) and 0.00078, 0.0280 μg/ml (HPLC). The results concluded that developed method was linear according to least square method of analysis. The % RSD values for both spectrophotometric and HPLC analysis was observed to be less than 2, indicating uniform reproducibility and statistically significant in different replicates of test concentrations. A negligible variation in interday (repeatability) and intraday (reproducibility) studies among these developed analytical methods exhibited accurate precision for series of measurements (Table 1). Accuracy results displayed good reproducibility with % RSD values below 2. This was found to be accurate as percent recovery observed was high i.e. within the range of 99.305-100.375 % (spectrophotometric analysis) and 98.258-100.963 % (HPLC analysis, Table 2), suggesting that the proposed method showed good agreement between the standard and the observed values and demonstrate an adequate accuracy within the specified limits [14].
| Interday precision | ||||||||||||||
| Method | Concentration (µg/ml) | Day 1 | Day 2 | Day 3 | ||||||||||
| UV Method | (Absorbance±SD) | % RSD | (Absorbance±SD) | % RSD | (Absorbance±SD) | % RSD | ||||||||
| 10 | 0.283±0.043 | 1.519 | 0.284±0.053 | 1.8661 | 0.291±0.067 | 2.3024 | ||||||||
| 20 | 0.445±0.077 | 1.730 | 0.440±0.056 | 1.2727 | 0.444±0.056 | 1.2612 | ||||||||
| 30 | 0.635±0.072 | 1.133 | 0.631±0.088 | 1.3946 | 0.645±0.098 | 1.5193 | ||||||||
| HPLC Method | Concentration (µg/ml) | (Peak area±SD) | % RSD | (Peak area±SD) | % RSD | (Peak area±SD) | % RSD | |||||||
| 10 | 151343±3.139 | 1.353 | 150382±3.037 | 1.359 | 151998±3.238 | 1.554 | ||||||||
| 20 | 262748±2.659 | 1.339 | 264732±2.954 | 1.363 | 265343±2.833 | 1.657 | ||||||||
| 30 | 361232±3.092 | 1.432 | 369422±3.069 | 1.366 | 360132±3.735 | 1.556 | ||||||||
| 40 | 493210±3.929 | 1.229 | 492323±3.132 | 1.461 | 495848±3.234 | 1.461 | ||||||||
| Intraday precision | ||||||||||||||
| Method | Concentration (µg/ml) | Morning | Afternoon | Evening | ||||||||||
| UV Method | (Absorbance±SD) | % RSD | (Absorbance±SD) | % RSD | (Absorbance±SD) | % RSD | ||||||||
| 10 | 0.287±0.050 | 1.7421 | 0.289±0.055 | 1.903 | 0.279±0.057 | 2.031 | ||||||||
| 20 | 0.429±0.085 | 1.9831 | 0.438±0.081 | 1.849 | 0.430±0.086 | 2.056 | ||||||||
| 30 | 0.639±0.091 | 1.4241 | 0.638±0.0121 | 1.896 | 0.640±0.0131 | 2.046 | ||||||||
| HPLC Method | Concentration (µg/ml) | (Peak area±SD) | % RSD | (Peak area±SD) | % RSD | (Peak area±SD) | % RSD | |||||||
| 10 | 154357±3.629 | 1.349 | 156237±3.036 | 1.559 | 160562±2.355 | 1.607 | ||||||||
| 20 | 262129±3.456 | 1.553 | 277293±3.178 | 1.341 | 260530±3.822 | 1.425 | ||||||||
| 30 | 365683±2.287 | 1.628 | 362348±3.199 | 1.233 | 365273±3.899 | 1.355 | ||||||||
| 40 | 495480±3.988 | 1.531 | 490342±2.389 | 1.330 | 494438±2.689 | 1.333 | ||||||||
Inter and intraday precision good reproducibility and repeatability with % RSD <2 %
Table 1: Interday and Intraday Precision of UV and HPLC Methods
| Method | Nominal concentration (µg/ml) | Level of addition (%) | Concentration prepared (µg/ml) | Amount recovered | % RSD | % Recovery |
|---|---|---|---|---|---|---|
| U V Method |
30 | 80 | 24 | 24.09±0.022 | 1.3067 | 100.37 |
| 30 | 100 | 30 | 29.88±0.071 | 1.4251 | 99.613 | |
| 30 | 120 | 36 | 35.75±0.055 | 1.3475 | 99.305 | |
| HPLC Method | 20 | 80 | 22 | 22.212±0.391 | 1.7603 | 100.96 |
| 20 | 100 | 24 | 23.582±0.282 | 1.1958 | 98.258 | |
| 20 | 120 | 26 | 26.986±0.323 | 10771 | 103.79 |
Accuracy showed acceptable recovery of 90-110 %, as per ICH guidelines
Table 2: Accuracy of Spectrophotometric and HPLC Methods
No major difference in % RSD was observed between analysts, instruments and environmental conditions in both spectrophotometric and HPLC analysis (Table 3), suggesting that the developed methods (UV and HPLC) are rugged in nature. Experimental findings from spectrophotometric analysis revealed that there was no effect of % RSD on different temperature conditions. Furthermore; in HPLC analysis, no significant difference in % RSD was observed by slightly changing the flow rate, run time and detection (Table 4). The minuscule drug degradation was detected very precisely by both UV and HPLC methods as percent amount recovered was within the acceptable range (90-110 %), indicating that samples were stable for over 3 mo period of time (Table 5). Previous reports also indicated that CFZ was most stable under different stress conditions like oxidation, thermal hydrolysis and photolytic exposure with negligible degradation [15].
Method |
Analyst 1 | Analyst 2 | ||||
| Concentration (µg/ml) | Absorbance±SD | % RSD | Concentration (µg/ml) | Absorbance±SD | % RSD | |
| UV Method | 10 | 0.292±0.035 | 1.1986 | 10 | 0.288±0.049 | 1.4013 |
| 20 | 0.447±0.056 | 1.2527 | 20 | 0.456±0.055 | 1.2061 | |
| 30 | 0.638±0.071 | 1.1598 | 30 | 0.651±0.087 | 1.3364 | |
| Analyst 1 | Analyst 2 | |||||
| HPLC Method | Concentration (µg/ml) | Peak area±SD | % RSD | Concentration (µg/ml) | Peak area±SD | % RSD |
| 20 | 262394±2.377 | 1.3776 | 20 | 265682±3.282 | 1.9846 | |
| 40 | 493456±3.372 | 1.2617 | 40 | 491985±2.899 | 1.7725 | |
Ruggedness performed by different analysts and considered method as rugged with % RSD <2 %
Table 3: Ruggedness of UV and HPLC Methods
| UV METHOD | ||||||||||
| Room temperature (25°) | Temperature (18°) | |||||||||
| Conc. (µg/ml) |
Absorbance±SD | % RSD | Concentration (µg/ml) | Absorbance±SD | % RSD | |||||
| 10 | 0.295±0.022 | 1.7457 | 10 | 0.292±0.060 | 2.047782 | |||||
| 20 | 0.442±0.045 | 1.6181 | 20 | 0.459±0.091 | 1.982571 | |||||
| 30 | 0.644±0.039 | 1.6055 | 30 | 0.677±0.088 | 1.899852 | |||||
| HPLC METHOD | ||||||||||
| Flow rate (±0.1 ml/min) | Run time (±2 min) | Detection wavelength (±2 nm) | ||||||||
| Conc. (µg/ml) | Peak area±SD | % RSD | Peak area±SD | %RSD | Peak area±SD | % RSD | ||||
| 20 | 273834±2.17 | 1.4192 | 274363±2.271 | 1.3022 | 277232±3.292 | 1.5821 | ||||
| 40 | 493725±2.99 | 1.7516 | 495362±3.917 | 1.5315 | 497312±2.343 | 1.7614 | ||||
Table 4: Robustness of UV and HPLC Methods
Robustness performed by varying instrumental conditions and considered method as robust with % RSD <2 %
| Method | Concentration prepared (µg/ml) | Zero day | First month | Second month | Third month | |||
|---|---|---|---|---|---|---|---|---|
| Concentration (µg/ml) | Concentration (µg/ml) | Drug degradation (%) | Concentration (µg/ml) | Drug degradation (%) | Concentration (µg/ml) | Drug degradation (%) | ||
| UV Method | 10 | 9.87±1.75 | 9.15±2.38 | 0.376±0.094 | 8.73±1.38 | 0.614±0.055 | 8.37±1.35 | 0.738±0.053 |
| 20 | 19.83±2.06 | 19.27±1.87 | 0.722±0.061 | 19.02±2.11 | 0.951±0.045 | 18.23±1.84 | 1.098±0.065 | |
| 30 | 29.78±2.35 | 29.12±1.36 | 0.812±0.056 | 28..26±2.46 | 0.957±0.042 | 27.47±2.56 | 0.997±0.076 | |
| HPLC Method | 20 | 19.87±2.65 | 19.34±1.91 | 0.173±0.044 | 19.51±2.94 | 0.343±0.031 | 17.44±2.66 | 0.673±0.093 |
| 30 | 29.92±2.86 | 29.72±2.22 | 0.255±0.036 | 28.12±3.49 | 0.564±0.067 | 27.54±2.18 | 0.855±0.085 | |
| 40 | 39.65±3.04 | 39.11±2.04 | 0.847±0.046 | 38.06±3.345 | 0.952±0.073 | 37.73±3.05 | 1.033±0.083 | |
Miniscule drug degradation at accelerated stability conditions showed statistically non-significant (p<0.05) with respect to each other
Table 5: Short term Stability Data of Canagliflozin
Furthermore, no new drug peak emerged in analysis of bulk drug after storage at high temperature and humidity, which confirms the stability indicating property of the proposed method. The analysis of standard drug in marketed tablets showed acceptable content in both UV and HPLC analysis (92.362 and 96.484 %, respectively) with a % RSD of less than 2 (Table 6). Thus, both UV and HPLC methods justified good agreement with the analysis of labelled claim for the tablets and were endorsed for rapid determination of CFZ in routine analysis [16]. Furthermore, the p-value for marketed product was greater than that from standard degree of freedom, implying that there is negligible difference in drug assay in both UV and HPLC methods, thus both methods were considered as statistically insignificant. Table 7 enlists the summary of all the parameters that were analysed by both analytical methods. The developed UV and HPLC methods were found to be linear, precise and accurate. The cost effective, simple and low cost reagents in spectrophotometric method allow routine use in pharmaceutical research. The overall results from both spectrophotometric and HPLC methods demonstrate rapid determination of CFZ and is endorsed for routine analysis for quality control purpose.
| Analysis Method | Name of the formulation | Labelled claim | Amount found (mg) | % RSD | Paired t-test | Significant (2 tailed) |
|---|---|---|---|---|---|---|
| UV | Marketed product | 100 mg | 92.362±1.232 | 1.3338 | 1.3212 | 1.1232 |
| HPLC | Marketed product | 100 mg | 96.484±1.198 | 1.2417 |
At 95 % confidence intervals, the mean results comparison of UV and HPLC in pharmaceutical formulation is not significant with respect to each other
Table 6: Drug Assay and Statistical Comparison of UV and HPLC Methods
| Parameters | Result (UV Analysis) | Result (HPLC Analysis) |
|---|---|---|
| Absorption maxima | 290 nm | 280 nm |
| Beer’s law range | 5-50 μg/ml | 2-40 μg/ml |
| Correlation coefficient | 0.9955 | 0.9971 |
| Standard regression equation | Y = 0.0162 X+0.0089 | Y =11477 X+32441 |
| Slope | 0.0162 | 11477 |
| LOD (µg/ml) | 0.00945 | 0.00078 |
| LOQ (µg/ml) | 2.86389 | 0.00280 |
| Accuracy ( Average % recovery) | 99.46-100.31 % | 91.70-102.03 % |
| Precision (Average % RSD) | Intraday (1.8764) Interday (1.5535) |
Intraday (1.4377) Interday (1.4285) |
| Robustness (Average % RSD) | 1.81655 | 1.55812 |
| Ruggedness (Average % RSD) | 1.39618 | 1.59917 |
| Stability study (% Drug degradation) |
0.376-1.098 | 0.173-1.033 |
Table 7: Summary of the Validation Parameters of UV and HPLC Analysis
Acknowledgements
The authors are grateful to Zydus Cadila Limited, Ahmedabad for providing gift sample of canagliflozin for this research work. Emerging Life Sciences Facility in Guru Nanak Dev University for carrying out HPLC studies is highly acknowledged.
Conflict of Interest
None.
Financial Support and Sponsorship
Nil.
References
- Kalra S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A review of their basic and clinical pharmacology. Diabetes Ther 2014;5:355-66.
- Nair S, Wilding JP. Sodium Glucose Co-transporter 2 inhibitors as a new treatment of diabetes mellitus. J Clin Endocrinol Metab 2010;95(1):34-42.
- Nisly SA, Kolanczyk, DM, Walton AM. Canagliflozin, a new sodium-glucose co-transporter 2 inhibitor in the treatment of diabetes. Am J Health Syst Pharm 2013;70:311-9.
- Latha ST, Thangadurai AS, Jambulingam M, Sereya K, Kamalakannan D, Kumar A. Development and validation of RP-HPLC method for the estimation of erlotinib in pharmaceutical formulation. Arab J Chem 2017;10:S1138-S44.
- International Conference on Harmonization (ICH). Validation of Analytical Procedures: Text and Methodology Q2 (R1), Geneva, Switzerland, 2005. Available from: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf.
- Sarkar M, Khandavilli S, Panchagnula R. Development and validation of RP-HPLC and ultraviolet spectrophotometric method of analysis for the quantitative estimation of antiretroviral drugs in pharmaceutical dosage forms. J Chromatogr B Analyt Technol Biomed Life Sci 2006;830:349-54.
- Parmar VK, Desai SB, Vaja T. RP-HPLC and UV Spectrophotometric Methods for Estimation of Pirfenidone in Pharmaceutical Formulations. Indian J Pharm Sci 2014;76(3):34-9.
- Joshi H, Patel A, Captain A. Spectrophotometric and Reversed-Phase High-Performance Liquid Chromatographic Method for the Determination of Doxophylline in Pharmaceutical Formulations. J Young Pharm 2014;2(3):289-96.
- Mahboubifar M, Sobhani Z, Dehghanzadeh G, Javidnia AA. Comparison between UV spectrophotometer and high pressure liquid chromatography method for the analysis of sodium benzoate and potassium sorbate in food products. Food Anal Methods 2011;4:150-4.
- Committee for Medicinal Product for Human use. Assessment Report February 2014. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Public_assessment_report/human/002656/WC500166672.pdf.
- Mendez AS, Steppe M, Sachapoval EE. Validation of HPLC and UV spectrophotometric methods for the determination of meropenem in pharmaceutical dosage form. J Pharm Biomed Anal 2003;33:947-54.
- Maleque M, Hasan MR, Hossen F, Safi S. Development and validation of a simple UV spectrophotometric method for the determination of levofloxacin both in bulk and marketed dosage formulations. J Pharm Anal 2012;2(6):454-7.
- Sistla R, Tata VS, Kashyap PV, Chandrasekar D, Diwan PV. Development and validation of reversed phase HPLC method for the determination of exetimibe in pharmaceutical dosage forms. J Pharm Biomed Anal 2003;39:517-22.
- Rimavi FA. Development and validation of a simple reversed phase HPLC-UV method for determination of oleuropein in olive leaves. J Food Drug Anal 2014;13:1-5.
- Kaur I, Wakode S, Singh HP, Manachanda S. Development and validation of a stability indicating reverse phase HPLC-PDA method for determination of canagliflozin in bulk and pharmaceutical dosage form. Pharm Methods 2016;7(1):54-62.
- Kommana R, Rebecca SD. Development and validation of HPLC and UV spectrophotometric method for determination of pioglitazone hydrochloride in bulk and its formulations. Pharm Lett 2013;5(1):269-78.