- *Corresponding Author:
- T. M. Yunus Khan
Department of Mechanical Engineering, College of Engineering, King Khalid University, Abha 61421, Kingdom of Saudi Arabia
E-mail: yunus.tatagar@gmail.com
| This article was originally published in a special issue, “Trending Topics in Biomedical Research and Pharmaceutical Sciences” |
| Indian J Pharm Sci 2022:84(1) Spl Issue “235-245” |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The severe acute respiratory syndrome coronavirus 2 which is the source of pandemic coronavirus disease 2019 has engulfed almost whole world. This virus was first reported in Wuhan city (China) in December 2019. Since the discovery of the virus, till today the researchers and scientists have been working to develop new vaccines or therapeutic agents against severe acute respiratory syndrome coronavirus 2. However, thus far no vaccine has emerged that can be approved to treat or prevent coronavirus disease 2019. Due to lack of specific preventative and therapeutic options for the treatment of coronavirus disease 2019, the use of convalescent plasma therapy may be of great benefit in the current situation. Previous use of immune plasma has resulted in successful treatment of hemagglutinin type 1 and neuraminidase type 1 influenza virus, Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus 1 epidemics. In the current scenario raised by coronavirus disease 2019, the convalescent plasma therapy has been applied successfully among many patients across various regions. This article presents an up-to-date review of existing literature on recovery through convalescent plasma as a treatment of choice, safety and its efficacy, possibility and its challenges for the treatment of coronavirus disease 2019.
Keywords
Coronavirus disease 2019, convalescent plasma therapy, severe acute respiratory syndrome coronavirus 2
The Coronavirus Disease 2019 (COVID-19) pandemic infected more than 244 million people with 221 million recoveries and 4 953 017 deaths worldwide till 25th October 2021 [1]. The virus has spread throughout the globe and hence it has been declared a pandemic by the World Health Organization (WHO) [2,3]. The most affected countries being United States of America (USA), India and Brazil with a caseload of more than 46, 34 and 21 million infections respectively by 25th October 2021, with many other countries having substantial infections.
This pandemic has impacted all public and private sectors which have led to global socio-economic devastation [4]. The world has responded to this crisis with resilience and determination [5]. As of today, there is no prescribed vaccine to fight the coronavirus infection, though the research and efforts to develop a vaccine are going on war footage. It must be borne in mind that the development of any vaccine is a lengthy process that needs to be followed to ensure the safety and efficacy to treat the given disease. However, the current pandemic has spread in such a short span of time that the world is forced to depend on whatever alternate treatment methods are available until a safe vaccine is developed. Therefore, countries around the world have limited options to tackle this pandemic infection. Governments implemented lockdown, instructed to maintain strict social and physical distancing, cut trade and travels, employees were asked to stay at home or work from home, educational institutions were closed to break the chain of the infection. This method has been found effective in controlling the confirmed cases and death curves in counties such as China, France, United Kingdom (UK), Germany, Malaysia and Italy [6-8].
The social distancing within a region or among the regions is a method employed even centuries back to contain the spread of such diseases. For instance, Prophet Mohammad (peace be upon him) about 1400 y back instructed people that: ‘‘If you hear of an outbreak of plague in a land, do not enter it; but if the plague breaks out in a place while you are in it, do not leave that place” [9]. The lockdown and social distancing are suppressing strategies to contain the cycle of pandemic transmission [10]. These strategies are just precautionary measures to prevent COVID-19 infections but not a cure. However, the public lockdown strategies have negative socio-economic impacts; therefore, many countries are implementing lockdown exit strategies [11].
Presently there is no particular drug available to cure the COVID-19 infection. There are few studies that have reported contradictory and inconclusive results about Hydroxychloroquine (HCQ) and remdesivir drugs as measures to fight the COVID-19 [12-16]. Furthermore, the role of corticosteroid remains debatable in treating lung injuries caused by COVID-19, because of delayed clearance of viral load and related complications [17,18]. There are few potential therapies such as supportive intervention, antiviral therapy, immunomodulatory agents and Convalescent Plasma Transfusion/Therapy (CPT) or Convalescent Plasma Exchange (CPE) which has been reviewed by Li et al. [19]. CPT has emerged as a potential treatment for the coronavirus pandemic [20]. This paper reviews the recent literature on the use of plasma therapy to treat COVID-19 patients
Convalescent Plasma (CP)
Blood is made up of four important elements: Red blood cells, white blood cells and cell fragments called as platelets. The fourth component is a liquid portion called as plasma and constitutes nearly 50 % of blood volume. The main faction of plasma is circulation of proteins, nutrients and hormones in the body. But researchers are concerned about the presence of antibodies in plasma as a COVID-19 treatment tool. When a person with COVID-19 “convalesced” or recovers, antibodies to fight the disease are formed in the blood plasma to various Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) proteins within 2-3 w following infection. These antibodies can be detected by Enzyme-Linked Immunosorbent Assay (ELISA) or other quantitative assays. Blood is withdrawn from treated patients and plasma is isolated.
Furthermore, this isolated plasma is enriched and injected into the bloodstream of another infected person to treat the infection. It boosts the ability of severe ill patients to fight against the virus [21,22].
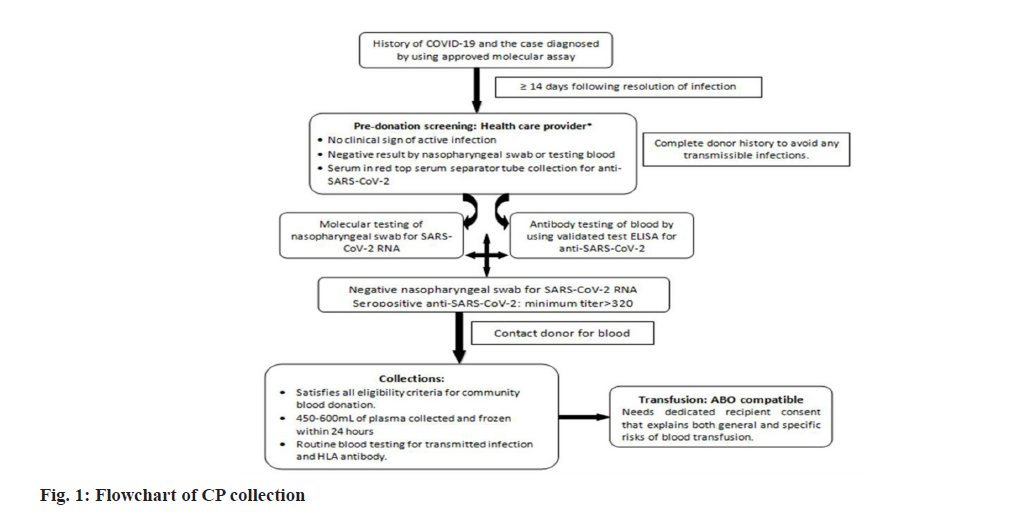
Currently there is a lot of interest towards the use of CP and a high number of ongoing trials are documented in terms of research and review publications [23]. In past epidemics also the antibody-mediated tools have been proved effective in treating the viral infections [24,25]. The blood plasma treatment has been used by many countries including China but its efficacy levels have not been tested through documented clinical studies. On 23rd August 2020, the United States Food and Drug Administration (FDA) declared Emergency Use Authorization (EUA) of CP in critically ill hospitalized patients with COVID-19. A typical workflow of the CP collection is demonstrated in fig. 1.
A completely recovered patient can donate plasma using plasmapheresis technique, otherwise the whole blood is collected from the donor and plasma is isolated for transfusion. The isolated liquid plasma can be preserved for 40 d between temperature of 1° and 6°, whereas frozen plasma can be stored up to a span of 12 mo at −18°, if stored within 24 h of blood collection. According to the suggestions the titers of SARS-CoV-2 Neutralizing Antibodies (NAbs) should be higher than 1:320, but lower thresholds could also be efficient in the treatment. In emergency situations the stored plasma can be transfused to the patients without antibody testing, later the archived samples can be evaluated to confirm levels of NAbs. People with elevated levels of antibodies can donate plasma fortnightly being their titers remain sufficient. Serum or plasma samples (before and after) transfusion should be collected from the recipient to perform the hemovigilance.
CP-A Possible Treatment
In the past century, CP treatment has been used as armament against the 1918 Spanish flu, polio, measles, rabies and hepatitis B with different degrees of achievements [26,27]. CP has been used as an alternative to treat critical patients in a few countries. The CP was even used during the outbreak of SARS as illustrated in Table 1 [28,29]. Results from case series during the previous Middle East Respiratory Syndrome (MERS) and SARS coronavirus infections recommended that CP is safe and may grant beneficial clinical outcomes and rapid viral clearance if administered in the early stage of the disease.
| Country/Reference | Sample size | Male/Female | Plasma volume | Virus clearance | Comment |
|---|---|---|---|---|---|
| Taiwan [28] | 3 | 1/2 | 500 ml | 1 d | All patients recovered |
| Hong Kong [29] | 80 | 37/43 | 279.3±127.1 ml | - | 33 patients has good outcome (Discharged after 22 d of onset of symptoms) Patients given CP before d 14 had a better outcome than those given plasma after d 14 |
Table 1: CP in Case of SARS
Keith et al. [30] obtained remarkable results in terms of mortality benefit with Therapeutic Plasma Exchange (TPE) (47.8 % mortality vs. 81.3 % mortality, p=0.05). They concluded that TPE is a promising tool to fight the coronavirus but at the same time, they proposed randomized trials for further investigations [30]. A pilot randomized study is going on to evaluate the safety of TPE in adult patients requiring Intensive Care Unit (ICU). A summary of randomization, trial status and full protocol is available in the letter [31]. The infections in India are rising exponentially and the death rate is increasing gradually. In India also the immune plasma or CP has emerged as one of the hopeful treatments [32]. People in Delhi were finding it difficult to get the plasma and the situation was getting worse. Hence, recently the state government of Delhi has opened plasma banks to have a systematic approach to treat the corona patients [33]. People with a history of chronic diseases are at greatest risk of COVID-19 infection. Pawar et al. [34] have proved that CP is a potential treatment for diabetic and liver dysfunction patients infected with coronavirus without any adverse effects. They too stressed the need for randomized clinical trials in their conclusion to save valuable lives [34]. There is a case report available for two males (56 y old and 46 y old) infected with severe 2019 novel coronavirus. Initially, they were treated with supportive care and antiviral therapy but despite this treatment, their health deteriorated. Their health improved after CP infusion clinically and radiologically and finally, they got discharged after successful treatment. The report concluded that CP might be an effective therapy with no adverse effects [35].
The safety of the CP transfusion has been validated by Rojas et al. [36]. While discussing the possible mechanisms of action of CP and their impact, they concluded that CP is safe, effective and beneficial in the hope of reducing morbidity and mortality [36]. The first report of the use of CP therapy for COVID-19 in Korea was published in April 2020. It reports about two patients who were suffering from severe pneumonia with Acute Respiratory Distress Syndrome (ARDS). Though there were some limitations, but it was concluded that CP showed favorable outcomes without causing any negative effects [37]. The case series on hospitalized patients suggested that the chances of survival of critically ill patients will improve if they were administered with CP in early stage of the disease [38]. The early use of CP is being generally recommended which is further observed by Zeng et al. that during a study of 6 patients, 5 patients who received CP eventually died even though they were cleared of SARS-CoV-2 before the death [39]. In this case, the CP was infused at median 21.5 d whereas the one patient who survived received CP on 11th d. Kesici et al. [40] recommended that for severely ill patients within 1st w of the symptom onset they should be considered for CP transfusion [40]. The time of clin41ical improvement was prolonged for the infected persons when they received CP later than 7 w after onset [41]. However, the questions regarding the exact timing of administering plasma during disease period, suitable antibody types and dosing remain unanswered. But in all the studies it was found that the CP administered in different patients in different stages had benefitted the patients in terms of clinical improvement in some studies to reducing the progression to severe forms, reducing mortality, more ventilator free days and better ICU outcomes in other studies. The observational studies related to CP are shown in Table 2 [42-56].
| Country/Reference | Sample size | Male/Female | Plasma volume | Virus clearance | Comment |
|---|---|---|---|---|---|
| Korea [35] | 2 patients P1: 71/M P2: 67/F |
1/1 | 500 ml in two doses at 12 h interval | SARS-CoV-2 was negative after d 16 of CP | No adverse reaction during the plasma transfusion Leukocytosis and lymphopenia were immediately recovered after CP infusion |
| China [37] | 6 patients Age=61.5 (median) | 5/1 | 300 ml, median | 1 patient was cleared of SARS-CoV-2 on the day of transfusion, 4 patients, on the 2nd d of transfusion and 1 on the 3rd d after transfusion | 5 out of 6 patients eventually died even though they were cleared of SARS-CoV-2 before death The patient who survived was given CP on 11th d where as those died were infused of CP on median day of 21.5 |
| Wuhan, China [42] | 10 patients P1: 46/M P2: 34/F P3: 42/M P4: 55/F P5: 57/M P6: 78/F P7: 56/M P8: 67/M P9: 49/F P10: 50/M |
6/4 | 200 ml | The clinical symptoms significantly improved within 3 d | No severe adverse effects were observed The viral load was undetectable after transfusion in seven patients who had previous viremia |
| Shenzhen, China [43] | 5 patients P1: 70/M P2: 60/M P3: 50/ F P4: 30/F P5: 60/M |
3/2 | 2 consecutive transfusions of 200 to 250 ml | Average 2 d | Out of 5 critical patients 3 recovered and 2 were in stable condition at the time of reporting Cycle threshold (Ct) value improved within 1 d after transfusion Four patients who had been receiving mechanical ventilation and Extracorporeal Membrane Oxygenation (ECMO) no longer required respiratory support by 9 d after plasma transfusion |
| China [44] | 4 patients P1: 69/F P2: 55/M P3: 73/M P4: 31/F |
2/2 | P1 d 18: 200 ml d 28: 400 ml d 29: 300 ml Discharged on d 43 P2 d 12: 200 ml Discharged on d 19 P3 d 15: 400 ml d 23: 400 ml d 27: 400 ml d 30: 400 ml d 32: 200 ml d 34: 200 ml d 38: 200 ml d 41: 200ml Discharged on d 51 P4 d 19: 300 ml Discharged on d 46 |
It took 3 to 22 d for negative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) test after transfusion in 3 patients | The third and fourth cases produced anti-SARS-CoV-2 IgG approximately 14 d after CP transfusion No serious adverse reactions observed, (associated with the transfusion of CP) |
| China [45] | 6 patients P1: 69/M P2: 75/ F P3: 56/ M P4:, 63/ F P5: 28/F P6: 57/M |
3/3 | 200 ml of each cycle P1=3 cycles P2=2 cycles P3=3 cycles P4=1 cycle P5=1 cycle P6=1 cycle |
P1: Throat swab test was negative after 10 d of CP P2 and P3: Throat swab test was negative after 8 d of CP P4: Throat swab test was negative after 1 d of CP P5: Throat swab test was negative after 2 d of CP P6: Patient discharged after 4 d of CP days of CP |
No adverse reactions were observed in the six patients during plasma transfusion and in the following 3 d All 6 patients cured |
| Iran [46] | 115 patients | 67/48 | - | The patients with CP were discharged on an average 6.25 d as against 12.88 d of those without CP | Two groups of patients with CP (115 patients) and control group (74 patients) were studied The death rate among those who did not receive CP was 24.3 %, whereas it was 14. 8 % amongst those received CP Patients were discharged within 5 d of admission with CP was 28.1 % whereas it was merely 8.9 % among without CP |
| India [47] | 10 patients | - | 200 ml | Disappearance of viremia in 7 d | Clinical symptoms rapidly improved in 3 d |
| India [48] | 452 patients A total of 52 institutions participated in a trial study by Indian Council of Medical Research (ICMR) |
- | - | In two groups of 24 patients, 85 % patients with CP recovered as against 60 % in non-CP group CP implementation at right time resulted in decrease in hospital stay time, viral load, oxygen requirements and time till a negative test result |
General summery of 36 participating institutions is reported of which 24 institutions felt that CP was effective It was felt that timing of the CP infusion is crucial It is recommended that patients should receive CP in 5-10 d of diagnosis In another institution, administered CP, 85 % of 25 patients given plasma, 85 % tested negative after 3 d of CP transfusion with 11 d hospital time as against 16 for non CP patients |
| China [49] | 10 patients | 6/4 | 200 ml | The symptoms in all patients, like cough, fever, breathing problems and chest pain, got relieved in 1 d to 3 d after CP transfusion Upon treating with CP, two patients were shifted from mechanical ventilation to high-flow nasal cannula and in one patient high-flow nasal cannula was stopped. Also, one patient was shifted to intermittent oxygenation from the previous treatment with conventional nasal cannula oxygenation and continuous oxygenation All patients exhibited various degrees of absorption of pulmonary lesions |
After CP no major adverse reactions were recorded Patients had evanescent facial red spot Lymphocytopenia got better after transfusion In 5 patients neutralizing antibody titres increased and in 4 patients it remained the same level after transfusion 3 patients got discharged and 7 patients got better and ready for discharge in CP group |
| Italy [50] | 46 patients | 28/18 | 1 unit=250-300 ml 24 patients got one unit of plasma, 21 got two units and 1 patient received three units |
After 7 d of CP infusion the Arterial Oxygen Partial Pressure (PaO2)/ Fractional Inspired Oxygen (FiO2) escalated by 112 units (95 % Confidence Interval (CI): 82-142) in survivors and the bilateral multilobe infiltrates on the chest X-ray healed in 23 % of patients (95 % CI: 5 %-42 %). Ferritin, C-Reactive Protein (CRP) and lactate dehydrogenase levels all reduced, by 36 %, 90 % and 20 %, respectively | 42 patients tolerated the plasma infusion, but 4 patients had serious unfavourable events 3 patients died within 7 d (on d 1, 4 and 6). 2 patients had comorbities and the third had a low PaO2/FiO2 ratio of 67 40 enrolled patients survived |
| USA [51] | 25 patients | 11/14 | 1 unit=300 ml 24 patients got 1 unit 1 patient got 2 units The 2nd unit is given after 6 d |
On d 7 after CP, 9 patients (36 %) got better from baseline, 13 (52 %) had same condition and 3 worsened. 7 of the nine improved patients (28 %) got discharged By d 14 after transfusion, 19 patients (76 %) got better from baseline, four patients more were discharged, eight patients got better from baseline, three patients remained same, three got worsened and one patient died by a condition not due to plasma transfusion |
No adverse events attributed to plasma transfusion occurred within 24 h after transfusion. One patient developed a morbilliform rash 1 d after transfusion that lasted for several days The median value for CRP reduced from 14.66 mg/dl at d 0 to 2.9 and 0.45 mg/dl at d 7 and 14 after CP, respectively Levels of ferritin that were increasing till d 3, got reduced by d 7. No significant increase in liver enzymes |
| Argentina [52] | Total 160 patients 80 patients receivedCP 80 patients received placebo |
60/100 | 250 ml, CP or placebo administered within 72 h at the onset of symptoms, over a period of 1.5 to 2.0 h | 13 (16 %) patients progressed to severe respiratory disease in CP group and in 25 (31 %) in the placebo group (relative risk, 0.52; 95 % CI, 0.29 to 0.94; p=0.03) The median time for the development of severe respiratory disease in the CP patients group (15 d; Interquartile Range (IQR), 15 to 15) was longer than that for the placebo group (15 d; IQR, 9 to 15) (p=0.03) |
A combined secondary end-point event (life-threatening respiratory disease, critical systemic illness and death, or any of these outcomes) happened in 7 patients (9 %) that got CP and 12 patients (15 %) that got placebo After 24 h the distribution of anti-SARS-CoV-2 serum S IgG titres was higher concentrations in patients in CP group (median log anti-SARS-CoV-2 S IgG titer, 5.7 IQR, 4.9 to 6.3) than the patients in the placebo group |
| Indonesia [53] | 10 patients | 5/5 | 3 doses at 2 d intervals (approximately 3 ml/kg of recipient body weight) 10-20 ml for the first 15 min, which was slowly increased and completed in 4 h |
All patients got relieved form the symptoms, such as fever, cough and chest pain, within 1 to 3 d following the first CP transfusion The median Ct value of 33.2 (range: 21.5-43.1) before transfusion, progressed to 35.5 (range: 15.4-43.3), 35.1 (range: 21.0-45.2), 37.1 (range: 23.8-43.1) and 40.2 (range: 28.8-49.8) at w 1, 2, 3 and 4 after the CP transfusion, respectively |
At w 3, 2 among the 4 patients with comorbidities deteriorated and passed away At 28 d, 4 patients got discharged from the hospital, 2 treated without supplemental oxygenation and 1 treated with nasal oxygenation. 1 patient required mechanical ventilation although had improved clinical and laboratory parameters before The Neutrophil-to-Lymphocyte Ratio (NLR), varied between w 1 and 3 after CP treatment, but got reduced at w 4 in surviving patients CRP significantly got better in most patients compared to earlier levels A reduction in D-dimer, was also seen in most patients after transfusion, except in 1 patient |
| USA [54] | 151 patients | 200 ml | By the d 14, the in-hospital mortality in early CP participants was 15 %, compared to 23 % in their matched unexposed cohort By the d 30, early CP participants had in-hospital mortality of 38 % compared to 49 % in their matched unexposed cohort. Broadly, early CP participants were nearly 50 % less likely to die in the hospital in comparison to patients in the matched unexposed cohort On the other hand, no differences in mortality were seen in late CP participants in comparison to their matched unexposed cohort (Heart Rate (HR) 0.98, [95 % CI 0.53 to 1.83]; p=0.95). Among the late cohort, the estimates of 14 d and 30 d in-hospital mortality were 28 % vs. 29% and 42% vs. 47%, comparing CP participants vs. matched unexposed patients, respectively |
No sudden transfusion-related unfavourable events were seen after CP administration in any of the cohorts and no transfusion reactions seen to the blood bank The early CP cohort patients were more days “alive and ventilator free” by 30 d post-index date compared with their matched unexposed cohort (mean 3.3 d, [95 % CI 0.2 to 6.3]; p=0.04). There were no differences comparing CP participants and matched unexposed patients in the late cohort (mean-3.5 d, [95 % CI-8.6 to 1.7]; p=0.18) |
|
| USA, Brazil [55] | Total 223 patients 150-CP 73-control plasma |
200-250 ml infused over 2 h | After 28 d mortality was significantly lower in patients randomized to CP versus control plasma (19/150 [12.6 %] versus 18/73 [24.6 %], Odds ratio (OR) 0.44, 95 % CI 0.22-0.91, p=0.034) | Serious adverse events occurred in 39 (27 %) patients who received CP and 26 (36 %) patients who received control plasma The median titer of anti-SARS-CoV-2 neutralizing antibody in infused CP units was 1:160 (IQR 1:80-1:320) |
|
| Austria [56] | Total 120 patients 48-CP 72-no CP |
12/26 | 400 ml CP d 1, 200 ml d 2 | CP participants had a better 3 mo survival than the others | The 30 d ICU overall survival was 69 % in CP group and 54 % in non-CP group |
Table 2: Observational Studies of CP in Case Of COVID-19 Patients
Efficacy and Safety
It is a fact that there is no approved specific drug or vaccine for the treatment of coronavirus patients. This problem of non-availability of specific treatment is multiplied when the person infected by COVID-19 has history of chronic disease or severely ill. Therefore, it is an urgent need to look for alternate methods for treating the COVID-19 patients especially severely ill patients. CP may be the potential treatment for rescuing from the deadly disease. Nevertheless, its risks and benefits are still unclear as of now; a pilot study was conducted with the inclusion of three hospitals with 10 patients. The aim of the study was to explore the viability of CP treatment. The study showed the treatment is fine tolerated besides improved clinical outcomes [49].
The present coronavirus pandemic situation is of great uncertainty and utmost care must be taken not to harm the patient that is the fundamental principle of “first do no harm” must be practiced. Employment of TPE with plasma received from the donor might be both efficient and harmless [57]. There is one case report which has used the combination of CP and HCQ for the first time. The results suggested that the combined therapy is non-optimal and specific treatment must be explored to treat the infection [58]. Some reports have cautioned about employing the CP therapy without reviewing and analyzing the quality of existing data because “We are not sure” [59]. A study was conducted at the infectious disease department, Shenzhen hospital China on five critically ill patients with COVID-19 infection. It was found that the CP therapy is helpful in fighting the virus but the conclusion precludes a definitive statement regarding the efficacy of this treatment because this method needs evaluation in randomized clinical trials [42]. To date many patients around the world have received CP therapy, specifically in the US around 72 000 patients have received it. According to the initial safety data published for 5000 patients receiving CP in the US, it was found that the incidence of severe unfavorable events was less than 1 % [60]. Another study in US found CP to be a safe option for treating patients with severe COVID-19 disease [51]. A randomized clinical study in Argentina found that the early administration (within 72 h after the onset of mild symptoms) of high titer CP (>1:1000 Immunoglobulin G (IgG)) in mild COVID-19 infected old age patients reduced the progression to severe forms [52] though clinical improvement has also been observed in patients with moderate and severe infections in Indonesia after CP treatment, but the results could not be solely attributed to CP treatment [53]. But like wise in a study on moderate to severe COVID-19 patients in US it was that the early administration of CP (within 6 d of hospitalization) improved the outcomes along with more 30 d alive and ventilator free days [54]. A randomized clinical study in US and Brazil on hospitalized patients with severe COVID-19 had significantly less mortality after the CP treatment in comparison to the patients that had received control plasma [55]. Similarly in a study on severely ill and immunocompromised COVID-19 patients admitted to ICU with acute respiratory failure, CP treatment improved the ICU outcomes [56].
Possibilities and Challenges
A review of the literature summarizes that in the absence of any specific treatment for the corona pandemic, transfusion of CP has emerged as an effective and safe strategy for treating critically ill patients. In order to obtain complete benefit of this potential treatment option, there are several critical problems and challenges that need to be illuminated. Generally, CP has been employed for increasing the survival rate of the patients but at the same time, there are some critical issues that need to be addressed by the research fraternity [61,62].
The therapeutic efficacy of CP in COVID-19 patients is evaluated by the level of circulating SARS-CoV-2 Neutralizing Antibody Titer (NAT). An experimental study on SARS established that particularly IgG begins to rise about 3rd w after onset and reached to the maximum at 12th w [63]. Moreover, another research on influenza recommended that CP with a NAT level of more than 1:160 decreased the mortality rate [64]. There are certain limitations of procuring CP such as age, weight, health status, patient consent, the required amount; the ratio of convalesced patients The therapeutic efficacy of CP in COVID-19 patients is evaluated by the level of circulating SARS-CoV-2 Neutralizing Antibody Titer (NAT). An experimental study on SARS established that particularly IgG begins to rise about 3rd w after onset and reached to the maximum at 12th w [63]. Moreover, another research on influenza recommended that CP with a NAT level of more than 1:160 decreased the mortality rate [64]. There are certain limitations of procuring CP such as age, weight, health status, patient consent, the required amount; the ratio of convalesced patients to those who need it causes the shortage of CP. As a result, the source of CP restricts its extensive use, particularly in nations that are in the acceleration phase of COVID-19.
The following are the few challenges that need rigorous scientific strategies in order to avoid the harms and benefit the patients and the overall safety and efficacy of CP therapy. Development of an immune response may cause cytokine storm [41,65]; CP strategy may lessen serum cytokine response [66]; risk of hyper-immune attacks [67]; high costs, troublesome logistics, lack of randomized controlled trials, lack of information on virus variability and mutations, short duration of the effect due to passive immunization [24]; risks of paradoxical worsening [67,68].
“A mistake repeated consciously is a decision”. Sunny Dzik has reminded the importance of “study first before wide-scale implementation” failure to do so may harm the patient as well as the entire health care system in general and the overall worldwide health care response to the pandemic. He has reviewed the four areas of potential concerns in his research [69].
Conclusion
The current articles provide an updated overview of CPT to treat the patients suffering from COVID-19. It is found that the CP care can provide effective and timely alternative till vaccines and the treatment protocols are fully established. CP therapy may be a curative treatment of choice for early COVID-19 patients who do not require mechanical ventilation and may reduce the mortality in moderately/severely affected patients. However, it requires more clinical studies with larger sample size covering different parameters to further establish its usefulness.
Author’s contributions:
We declare that this work was done by the authors named in this article and all liabilities pertaining to claims relating to the content of this article will be borne by the authors.
Acknowledgements:
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia for funding this work through the General Research Group Project under grant number (GRP/134/1442).
Conflict of interests:
The author reports no conflicts of interest in this work.
References
- COVID-19 Map. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Coronavirus Resource Center 2020.
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382(8):727-33.
[Crossref] [Google Scholar] [PubMed]
- Sohrabi C, Alsafi Z, O'neill N, Khan M, Kerwan A, Al-Jabir A, et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg 2020;76:71-6.
[Crossref] [Google Scholar] [PubMed]
- Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int J Surg 2020;78:185-93.
[Crossref] [Google Scholar] [PubMed]
- Legido-Quigley H, Asgari N, Teo YY, Leung GM, Oshitani H, Fukuda K, et al. Are high-performing health systems resilient against the COVID-19 epidemic? Lancet 2020;395:848-50.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Jiang B, Yuan J, Tao Y. The impact of social distancing and epicenter lockdown on the COVID-19 epidemic in mainland China: A data-driven SEIQR model study. MedRxiv 2020.
- Assessment RR. Coronavirus disease 2019 (COVID-19) pandemic: Increased transmission in the EU/EEA and the UK-seventh update. European Centre for Disease Prevention and Control; 2020.
- Othman ADPB. Countries continue with restrictions. Thestar; 2020.
- Bukhari SA. Sahih al-Bukhari 5728-Medicine. Book 76, Hadith 43, USC-MSA web 2020:7.
- Rahman MA, Zaman N, Asyhari AT, Al-Turjman F, Bhuiyan MZ, Zolkipli MF. Data-driven dynamic clustering framework for mitigating the adverse economic impact of COVID-19 lockdown practices. Sustain Cities Soc 2020;62:102372.
[Crossref] [Google Scholar] [PubMed]
- Dawoud D. Emerging from the other end: Key measures for a successful COVID-19 lockdown exit strategy and the potential contribution of pharmacists. Res Social Adm Pharm 2021;17(1):1950-3.
- Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020;395(10236):1569-78.
- Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis 2020;20(10):1118-9.
[Crossref] [Google Scholar] [PubMed]
- Raoult D, Hsueh PR, Stefani S, Rolain JM. COVID-19 therapeutic and prevention. Int J Antimicrob Agents 2020;55(4):105937.
[Crossref] [Google Scholar] [PubMed]
- Khuroo MS, Khuroo M, Khuroo MS, Sofi AA, Khuroo NS. COVID-19 vaccines: A race against time in the middle of death and devastation! J Clin Exp Hepatol 2020;10(6):610-21.
- Anderson J, Schauer J, Bryant S, Graves CR. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: A case report. Case Rep Womens Health 2020;27:e00221.
[Crossref] [Google Scholar] [PubMed]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395(10223):473-5.
[Crossref] [Google Scholar] [PubMed]
- Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395(10225):683-4.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Liu S, Zhang S, Ju Q, Zhang S, Yang Y, et al. Current treatment approaches for COVID-19 and the clinical value of transfusion-related technologies. Transfus Apher Sci 2020;59(5):102839.
[Crossref] [Google Scholar] [PubMed]
- Brown BL, McCullough J. Treatment for emerging viruses: Convalescent plasma and COVID-19. Transfus Apher Sci 2020;59(3):102790.
[Crossref] [Google Scholar] [PubMed]
- Malani AN, Sherbeck JP, Malani PN. Convalescent plasma and COVID-19. JAMA 2020;324(5):524.
[Crossref] [Google Scholar] [PubMed]
- Zeng F, Chen X, Deng G. Convalescent plasma for patients with COVID-19. Proc Natl Acad Sci USA 2020;117(23):12528.
[Crossref] [Google Scholar] [PubMed]
- Franchini M. Why should we use convalescent plasma for COVID-19? Eur J Intern Med 2020;77:150-1.
[Crossref] [Google Scholar] [PubMed]
- Tamburello A, Marando M. Immunoglobulins or convalescent plasma to tackle COVID-19: Buying time to save lives-Current situation and perspectives. Swiss Med Wkly 2020;150(1718):20264.
[Crossref] [Google Scholar] [PubMed]
- Zhu M, Hu K, Zhu Z. Use of convalescent plasma in COVID-19 patients in China. Transfus Clin Biol 2020;27(3):168-9.
[Crossref] [Google Scholar] [PubMed]
- Casadevall A, Pirofski LA. The convalescent sera option for containing COVID-19. J Clin Invest 2020;130(4):1545-8.
[Crossref] [Google Scholar] [PubMed]
- Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020;130(6):2757-65.
[Crossref] [Google Scholar] [PubMed]
- Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother 2005;56(5):919-22.
[Crossref] [Google Scholar] [PubMed]
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis 2005;24(1):44-6.
[Crossref] [Google Scholar] [PubMed]
- Keith P, Day M, Perkins L, Moyer L, Hewitt K, Wells A. A novel treatment approach to the novel coronavirus: An argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care 2020;24(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Faqihi F, Alharthy A, Alodat M, Asad D, Aletreby W, Kutsogiannis DJ, et al. A pilot study of therapeutic plasma exchange for serious SARS CoV-2 disease (COVID-19): A structured summary of a randomized controlled trial study protocol. Trials 2020;21:1-3.
- da Silva JA. Convalescent plasma: A possible treatment of COVID-19 in India. Med J Armed Forces India 2020;76(2):236-7.
[Crossref] [Google Scholar] [PubMed]
- Butani A, Saxena A. As plasma bank opens, Kejriwal says will end ‘chaos’ in looking for donors. The Indian Express 2020.
- Pawar AY, Hiray AP, Sonawane DD, Bhambar RS, Derle DV, Ahire YS. Convalescent plasma: A possible treatment protocol for COVID-19 patients suffering from diabetes or underlying liver diseases. Diabetes Metab Syndr 2020;14(4):665-9.
[Crossref] [Google Scholar] [PubMed]
- Abdullah HM, Hama-Ali HH, Ahmed SN, Ali KM, Karadakhy KA, Mahmood SO, et al. Severe refractory COVID-19 patients responding to convalescent plasma: A case series. Ann Med Surg 2020;56:125-7.
[Crossref] [Google Scholar] [PubMed]
- Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid-19: Possible mechanisms of action. Autoimmun Rev 2020;19(7):102554.
[Crossref] [Google Scholar] [PubMed]
- Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci 2020;35(14):e149.
[Crossref] [Google Scholar] [PubMed]
- Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with COVID-19: Case series. Blood 2020;136(6):759-62.
[Crossref] [Google Scholar] [PubMed]
- Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis 2020;222(1):38-43.
[Crossref] [Google Scholar] [PubMed]
- Kesici S, Yavuz S, Bayrakci B. Get rid of the bad first: Therapeutic plasma exchange with convalescent plasma for severe COVID-19. Proc Natl Acad Sci USA 2020;117(23):12526-7.
[Crossref] [Google Scholar] [PubMed]
- Xia X, Li K, Wu L, Wang Z, Zhu M, Huang B, et al. Improved clinical symptoms and mortality on severe/critical COVID-19 patients utilizing convalescent plasma transfusion. Blood 2020;136:755-59.
[Crossref] [Google Scholar] [PubMed]
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA 2020;323(16):1582-9.
[Crossref] [Google Scholar] [PubMed]
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: A pilot study. MedRxiv 2020.
- Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020;158(1):e9-13.
[Crossref] [Google Scholar] [PubMed]
- Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID‐19 patients in Wuhan, China. J Med Virol 2020;92(10):1890-901.
[Crossref] [Google Scholar] [PubMed]
- Abolghasemi H, Eshghi P, Cheraghali AM, Fooladi AA, Moghaddam FB, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: Results of a multicenter clinical study. Transfus Apher Sci 2020;59(5):102875.
[Crossref] [Google Scholar] [PubMed]
- DCGI clears clinical trial of plasma therapy in COVID-19 patients. The Hindu Business Line 2020.
- Plasma therapy effective for moderately ill patients, say most doctors of ICMR trial. The Indian Express 2020.
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA 2020;117(17):9490-6.
[Crossref] [Google Scholar] [PubMed]
- Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S, et al. Mortality reduction in 46 patients with severe COVID-19 treated with hyperimmune plasma. A proof-of-concept, single-arm, multicenter trial. Haematologica 2020;105(12):2834-40.
[Crossref] [Google Scholar] [PubMed]
- Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol 2020;190(8):1680-90.
[Crossref] [Google Scholar] [PubMed]
- Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N Engl J Med 2021;384(7):610-8.
[Crossref] [Google Scholar] [PubMed]
- Rejeki MS, Sarnadi N, Wihastuti R, Fazharyasti V, Samin WY, Yudhaputri FA, et al. Convalescent plasma therapy in patients with moderate-to-severe COVID-19: A study from Indonesia for clinical research in low-and middle-income countries. EClinicalMedicine 2021:100931.
[Crossref] [Google Scholar] [PubMed]
- Briggs N, Gormally MV, Li F, Browning SL, Treggiari MM, Morrison A, et al. Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19. PLoS One 2021;16(7):e0254453.
[Crossref] [Google Scholar] [PubMed]
- O'Donnell MR, Grinsztejn B, Cummings MJ, Justman JE, Lamb MR, Eckhardt CM, et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J Clin Invest 2021.
[Crossref] [Google Scholar] [PubMed]
- Hatzl S, Posch F, Sareban N, Stradner M, Rosskopf K, Reisinger AC, et al. Convalescent plasma therapy and mortality in COVID-19 patients admitted to the ICU: A prospective observational study. Ann Intensive Care 2021;11(1):1-1.
[Crossref] [Google Scholar] [PubMed]
- Stahl K, Bode C, David S. First do no harm-beware the risk of therapeutic plasma exchange in severe COVID-19. Crit Care 2020;24(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Xu TM, Lin B, Chen C, Liu LG, Xue Y. Non-optimal effectiveness of convalescent plasma transfusion and hydroxychloroquine in treating COVID-19: A case report. Virol J 2020;17(1):1-3.
[Crossref] [Google Scholar] [PubMed]
- Honore PM, Mugisha A, Kugener L, Redant S, Attou R, Gallerani A, et al. Therapeutic plasma exchange as a routine therapy in septic shock and as an experimental treatment for COVID-19: We are not sure. Crit Care 2020;24(1):1-2.
[Crossref] [Google Scholar] [PubMed]
- Zhao Q, He Y. Challenges of convalescent plasma therapy on COVID-19. J Clin Virol 2020;127:104358.
[Crossref] [Google Scholar] [PubMed]
- Langhi DM, Santis GC, Bordin JO. COVID-19 convalescent plasma transfusion. Hematol Transfus Cell Ther 2020;42:113-5.
[Crossref] [Google Scholar] [PubMed]
- Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: A prospective study. Lancet 2003;361(9371):1767-72.
[Crossref] [Google Scholar] [PubMed]
- Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011;52(4):447-56.
[Crossref] [Google Scholar] [PubMed]
- Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest 2020;130(9):4791-7.
[Crossref] [Google Scholar] [PubMed]
- Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfus 2016;14(2):152-7.
[Crossref] [Google Scholar] [PubMed]
- Yuan FF, Tanner J, Chan PK, Biffin S, Dyer WB, Geczy AF, et al. Influence of FcγRIIA and MBL polymorphisms on severe acute respiratory syndrome. Tissue Antigens 2005;66(4):291-6.
[Crossref] [Google Scholar] [PubMed]
- Li G, Chen X, Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med 2003;349(5):508-9.
[Crossref] [Google Scholar] [PubMed]
- Fleming AB, Raabe V. Current studies of convalescent plasma therapy for COVID-19 may underestimate risk of antibody-dependent enhancement. J Clin Virol 2020;127:104388.
[Crossref] [Google Scholar] [PubMed]
- Dzik S. COVID-19 convalescent plasma: Now is the time for better science. Transfus Med Rev 2020;34(3):141-4.
[Crossref] [Google Scholar] [PubMed]