- *Corresponding Author:
- Junling Shan
Department of Basic Medicine, Guangxi Medical University Nursing College, Nanning, Guangxi 530021, China
E-mail: shanjunling0907@gxmu.edu.cn
| Date of Received | 29 October 2022 |
| Date of Revision | 07 July 2023 |
| Date of Acceptance | 27 January 2024 |
| Indian J Pharm Sci 2024;86(1):110-116 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
To probe curcumin action on the proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells and its possible molecular mechanism. Human bone marrow mesenchymal stem cells were osteogenic induced for 14 d. Real-time quantitative reverse transcription-polymerase chain reaction and Western blotting analysis were utilized for the detection of microRNA-148a, alkaline phosphatase, osteocalcin and osteopontin levels in human bone marrow mesenchymal stem cells after curcumin incubation for 14 d. After curcumin treatment, the proliferation of human bone marrow mesenchymal stem cells was notably rose, and contents of alkaline phosphatase, osteocalcin and osteopontin in osteogenic induced-human bone marrow mesenchymal stem cells were elevated. Curcumin treatment elevated microRNA-148a expression. Overexpression of microRNA-148a could boost human bone marrow mesenchymal stem cells proliferation, and increased alkaline phosphatase, osteocalcin and osteopontin contents in human bone marrow mesenchymal stem cells during osteogenesis induction. Down-regulation of microRNA-148a reversed the promoting effect of curcumin on human bone marrow mesenchymal stem cells proliferation and the levels of alkaline phosphatase, osteocalcin and osteopontin in human bone marrow mesenchymal stem cells during osteogenesis induction (p<0.05). Curcumin promoted the proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells by up-regulating microRNA-148a.
Keywords
Curcumin, microRNA-148a, human bone marrow mesenchymal stem cells, proliferation, osteoporosis
Osteoporosis (OP) is characterized by a decrease in bone mass per unit volume and changes in bone microstructure, and is more likely to occur in elderly men and postmenopausal women[1]. Bone homeostasis is related to various factors[2,3]. Postmenopausal women face a high risk of OP due to estrogen deficiency and net bone loss, leading to faster bone turnover. Bone Marrow Mesenchymal Stem Cells (BMSCs) can differentiate into osteoblasts, cartilage, adipocytes and so on, and are key to new bone formation[4]. Targeting BMSCs may be a promising method for OP prevention.
Research has found that curcumin, a natural polyphenol compound isolated from Curcuma root, has anticancer, anti-inflammatory, antibacterial, antioxidant, and regulatory activity in bone metabolism[5,6]. The injection of curcumin in rats could improve bone microstructure and bone formation, and exerted protective effects to prevent OP[7]. Curcumin could alleviate the symptoms of postmenopausal OP[8,9]. Currently, increasing evidence has manifested the impact of epigenetic modifications on OP[10]. MicroRNAs (miRNAs) are one of epigenetic regulators and can modulate various cell biological processes by affecting mRNA expression[11]. Research has shown that miR-148a enhanced the differentiation of pluripotent stem cells into cardiomyocytes[12]. miR-148a expression was elevated in human BMSCs (hBMSCs) during myogenic differentiation, the decrease of miR-148a reduced myocardial cell specific markers Alpha- Myosin Heavy Chain (α-MHC), and miR-148a boosted hBMSC myocardial differentiation[13]. However, the role of miR-148a in OP and whether curcumin affected OP progression by miR-148a still need further exploration.
Here, this study mainly probed the action of curcumin on the proliferation and osteogenic differentiation of hBMSCs, and explored its regulatory effect on miR-148a.
Materials and Methods
Materials and reagents:
HBMSCs, Fetal Bovine Serum (FBS), trypsin and α-Minimum Essential Medium (MEM) (American Type Culture Collection, United States of America (USA)); curcumin, 3-(4,5-Dimethylthiazol- 2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) kit, dexamethasone, ascorbic acid, Beta (β)-glycerol phosphate and Dimethyl Sulfoxide (DMSO) (Beyotime, Beijing, China); Radioimmunoprecipitation Assay (RIPA) lysis buffer and Bicinchonic Acid (BCA) reagent kit (Shanghai Yanjing Biotechnology Co., Ltd); TRIzol, reverse transcription, and quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) reagents (Takara, Japan); Alkaline Phosphatase (ALP), Osteocalcin (OCN), Osteopontin (OPN) antibodies, and Horseradish Peroxidase (HRP)-labeled Immunoglobulin G (IgG) antibodies (Santa Cruz, USA); miR-148a mimic, inhibitor (anti-miR-148a) and the contrasts (miR-NC or anti-miR-NC) (Geenseed, Guangzhou, China). The primers of qRT-PCR.
Cell culture and osteogenic differentiation:
hBMSCs were cultured in α-MEM containing 10 % FBS with 5 % Carbon dioxide (CO2) at 37°. For osteogenic differentiation, 100 μmmol/l-1 dexamethasone, 100 μg/ml ascorbic acid and 10 mm β-glycerol phosphate were added into α-MEM medium.
Experimental grouping:
hBMSCs, underwent osteogenic differentiation or not, were exposed to 1, 2, or 4 μg/ml curcumin for 48 h, namely 1, 2, or 4 μg/ml curcumin group. The untreated cells were used as the control group.
hBMSCs, underwent osteogenic differentiation or not, were transfected with miR-NC or miR-148a for 48 h, namely miR-NC or miR-148a group. hBMSCs with or without osteogenic differentiation were transfected with anti-miR-NC or anti-miR- 148a for 48 h, followed by treating with 4 μg/ml curcumin, namely 4 μg/ml+anti-miR-NC group or 4 μg/ml+anti-miR-148a group.
Cell transfection:
hBMSCs were inoculated into the 6-well plate (1×105/well), then as per the protocol of Lipofectamine 2000, cell transfection were conducted based on the experimental grouping using the Lipofectamine 2000.
MTT assay:
hBMSCs at 2.5×104 per well were inoculated overnight on a 96 well plate. After 24, 48, and 72 h of cultivation, adding 20 μl MTT (5 g/l) into per well, followed by incubating for 4 h and 150 μl DMSO for 2 h, then Optical Density (OD) value at 490 nm was assayed.
qRT-PCR:
Total Ribonuclic Acid (RNA) was extracted from the cells of each group and the purity of RNA were detected using NanoDrop 2000c spectrophotometer. Then complementary Deoxyribonucleic Acid (cDNAs) were produced by reverse transcript and qRT-PCR reaction were performed. The fold changes of miR-148a, ALP, OCN and OPN mRNA were calculated by 2-△△Ct method. U6 or Glyceraldehyde 3-Phosphate Dehydrogenase (GAPDH) was used as the internal reference. miR- 148a forward: 5’-ACACTCCAGCTGGGTCA GTGCACTACAGAA- 3’, reverse: 5'-TGGTGTCGTGGAGTCG-3'; U6 forward: 5'-CTCGCTTCGGCAGCACATA-3', reverse: 5'-CGAATTTGCGTGTCATCCT-3'; ALP forward: 5'-AGACCTTCATAGCGCACGTC-3', reverse: 5'-ACCTTTGGCTCTCGACCAG-3'; OCN forward: 5'-ACCGAGACACCATGAGAG-3', reverse: 5'-CTGGGTCTCTTCACTACCT-3’; OPN forward: 5'-AATATAAGCGCGAGGCCAAT-3', R5'-AATTCACGGCTCTGGGATTC-3; GAPDH forward: 5'-CACGTCTGCCACGATAACAC-3' and reverse: 5'-AGGCCTGTGATGGATTGTCT-3’.
Western blot:
The total protein of each group of cells was extracted and quantified by a BCA method.
After transferring to a Polyvinylidene Difluoride (PVDF) membrane, the antibody diluents including ALP (1:1000), OCN (1:1000), OPN (1:500) and GAPDH (1:3000) were incubated overnight at 4° with membranes, and then incubated at 37° for 1 h with the secondary antibodies. The protein bands were analyzed by Enhanced Chemiluminescence (ECL) incubation.
Statistical analysis:
The data were presented by x̄ ±s. Statistical Package for the Social Sciences (SPSS) 21.0 software was used to analyze the data. The comparison of the two groups or multiple groups was conducted using t-test or Analysis of Variance (ANOVA). p<0.05 suggested significant difference.
Results and Discussion
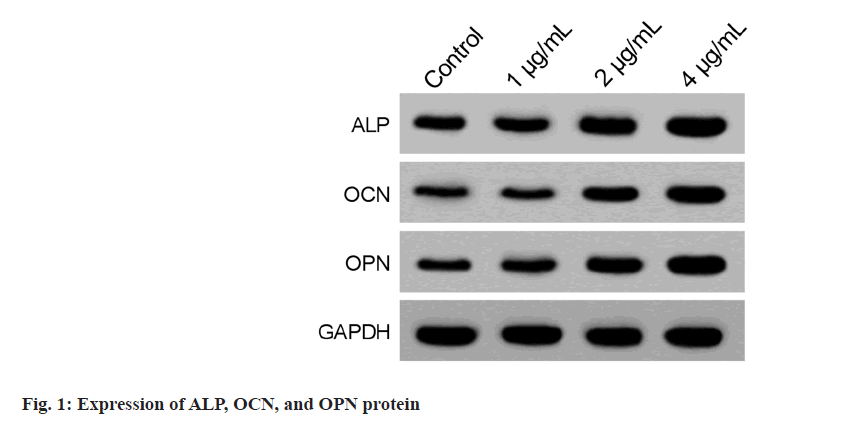
After culturing hBMSCs in a basic medium with 1, 2, and 4 μg/ml curcumin, it was found that OD values of hBMSCs in the 2 μg/ml and 4 μg/ ml curcumin group were markedly increased relative to the control group (Table 1), indicating the increased proliferation. After osteogenic differentiation for 14 d, we also found ALP, OCN, and OPN contents in hBMSCs of the 4 μg/ml curcumin group were notably increased relative to the control group (Table 2 and fig. 1).
| Group | OD values (490 nm) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Control | 0.39±0.03 | 0.61±0.05 | 0.95±0.08 |
| 1 μg/ml | 0.43±0.03 | 0.67±0.07 | 1.03±0.10 |
| 2 μg/ml | 0.49±0.05* | 0.75±0.06* | 1.19±0.09* |
| 4 μg/ml | 0.58±0.06* | 0.86±0.06* | 1.37±0.13* |
| F | 10.367 | 9.610 | 10.643 |
| p | 0.004 | 0.005 | 0.004 |
Note: In comparison to control group, *p<0.05
Table 1: Curcumin Promotes the Proliferation of Hbmscs (X̄±S, N=3)
| Group | mRNA | Protein | ||||
|---|---|---|---|---|---|---|
| ALP | OCN | OPN | ALP | OCN | OPN | |
| Control | 1.01±0.11 | 0.99±0.13 | 1.02±0.10 | 0.43±0.04 | 0.21±0.03 | 0.27±0.03 |
| 1 μg/ml | 1.32±0.17 | 1.28±0.14 | 1.23±0.15 | 0.52±0.05 | 0.27±0.03 | 0.34±0.04 |
| 2 μg/ml | 1.68±0.19* | 1.52±0.21* | 1.78±0.23* | 0.68±0.07* | 0.49±0.06* | 0.51±0.05* |
| 4 μg/ml | 2.26±0.28* | 1.89±0.26* | 2.52±0.31* | 0.85±0.08* | 0.63±0.08* | 0.72±0.07* |
| F | 22.232 | 11.752 | 29.674 | 26.649 | 38.644 | 48.727 |
| p | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: In comparison to control group, *p<0.05
Table 2: Curcumin Promotes Osteogenic Differentiation of Hbmscs (X̄±S, N=3)
After osteogenic differentiation for 14 d, hBMSCs were incubated with 1, 2, and 4 μg/ml curcumin, and then we observed that the expression of miR- 148a in hBMSCs at the 2 μg/ml and 4 μg/ml curcumin group were markedly increased relative to the control group (Table 3).
| Group | miR-148a |
|---|---|
| Control | 1.01±0.13 |
| 1 μg/ml | 1.35±0.19 |
| 2 μg/ml | 1.83±0.23* |
| 4 μg/ml | 2.72±0.32* |
| F | 31.740 |
| p | 0.000 |
Note: In comparison to control group, *p<0.05
Table 3: Curcumin Promotes Mir-148a Expression in Hbmscs (X̄±S, N=3)
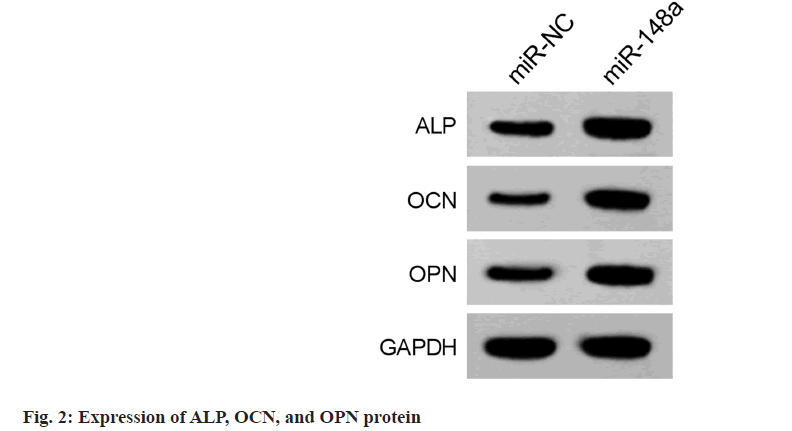
Transfection of miR-NC and miR-148a into hBMSCs suggested miR-148a levels in hBMSCs in the miR-148a group was significantly higher than those in the miR-NC group (Table 4), indicating successful transfection. Moreover, miR-148a overexpression increased the OD values of hBMSCs in the miR-148a group (Table 4). After osteogenic differentiation for 14 d, we also found that levels of ALP, OCN, and OPN in hBMSCs of miR-148a group were elevated (fig. 2 and Table 5).
| Group | miR-148a | OD values (490 nm) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| miR-NC | 1.01±0.09 | 0.35±0.04 | 0.57±0.05 | 0.89±0.07 |
| miR-148a | 2.83±0.36* | 0.54±0.06* | 0.81±0.08* | 1.33±0.13* |
| t | 8.495 | 4.564 | 4.406 | 5.162 |
| p | 0.001 | 0.010 | 0.012 | 0.007 |
Note: In comparison to miR-NC group, *p<0.05
Table 4: Mir-148a Promotes the Proliferation of Hbmscs (X̄±S, N=3)
| Group | mRNA | Protein | ||||
|---|---|---|---|---|---|---|
| ALP | OCN | OPN | ALP | OCN | OPN | |
| miR-NC | 1.02±0.12 | 1.01±0.10 | 1.01±0.08 | 0.39±0.05 | 0.23±0.02 | 0.31±0.03 |
| miR-148a | 2.17±0.34* | 1.82±0.21* | 2.35±0.28* | 0.82±0.07* | 0.59±0.07* | 0.75±0.08* |
| F | 5.524 | 6.032 | 7.970 | 8.658 | 8.565 | 8.92 |
| p | 0.005 | 0.004 | 0.001 | 0.001 | 0.001 | 0.001 |
Note: In comparison to miR-NC group, *p<0.05
Table 5: Mir-148a Promotes Osteogenic Differentiation of Hbmscs (X̄±S, N=3)
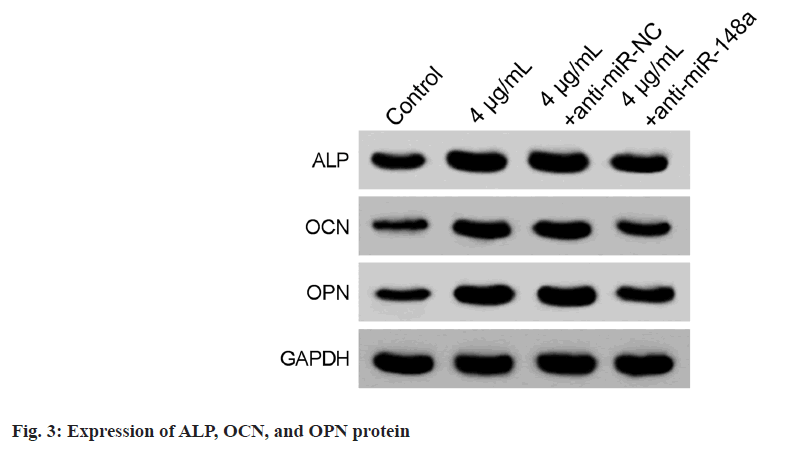
Comparison with the 4 μg/ml+anti-miR-NC group, miR-148a levels in hBMSCs of the 4 μg/ ml+anti-miR-148a group were declined, indicating successful transfection (Table 6). Besides, the OD values of hBMSCs in the 4 μg/ml+anti-miR-148a group were markedly decreased (Table 6). After the osteogenic differentiation, the levels of ALP, OCN, and OPN were notably reduced in hBMSCs of 4 μg/ml+anti-miR-148a group were downregulated (fig. 3 and Table 7).
| Group | miR-148a | OD values (490 nm) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| Control | 1.01±0.08 | 0.36±0.04 | 0.58±0.05 | 0.87±0.09 |
| 4 μg/ml | 2.81±0.31* | 0.59±0.06* | 0.83±0.08* | 1.40±0.16* |
| 4 μg/ml+anti-miR-NC | 2.75±0.36 | 0.62±0.07 | 0.80±0.07 | 1.45±0.19 |
| 4 μg/ml+anti-miR-148a | 1.56±0.18# | 0.45±0.05# | 0.69±0.05# | 1.08±0.11# |
| F | 36.115 | 14.127 | 9.546 | 11.028 |
| p | 0.000 | 0.001 | 0.005 | 0.003 |
Note: In comparison to control group, *p<0.05 and relative to 4 μg/ml+anti-miR-NC group, #p<0.05
Table 6: Mir-148a Silencing Reverses Curcumin-Evoked Proliferation in Hbmscs (X̄±S, N=3)
| Group | mRNA | Protein | ||||
|---|---|---|---|---|---|---|
| ALP | OCN | OPN | ALP | OCN | OPN | |
| Control | 1.02±0.10 | 1.00±0.08 | 1.01±0.12 | 0.41±0.04 | 0.22±0.02 | 0.29±0.03 |
| 4 μg/ml | 2.23±0.26* | 1.91±0.21* | 2.39±0.32* | 0.83±0.08* | 0.61±0.07* | 0.71±0.08* |
| 4 μg/ml+anti-miR-NC | 2.31±0.22 | 1.85±0.25 | 2.47±0.28 | 0.80±0.07 | 0.64±0.05 | 0.69±0.06 |
| 4 μg/ml+anti-miR-148a | 1.46±0.21# | 1.36±0.19# | 1.65±0.17# | 0.57±0.06# | 0.36±0.04# | 0.42±0.05# |
| F | 27.299 | 14.932 | 25.310 | 28.818 | 52.117 | 38.112 |
| p | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
Note: In comparison to control group, *p<0.05 and relative to 4 μg/ml+anti-miR-NC group, #p<0.05
Table 7: Mir-148a Silencing Reverses Curcumin-Evoked Osteogenic Differentiation in Hbmscs (X̄±S, N=3)
OP is the most common skeletal disease among elderly patients of all races and genders worldwide, especially in developed and aging societies[14]. The prevalence rate of the elderly over 60 y old in China is 36 %, and OP is an asymptomatic disease, there are no obvious symptoms in the early stage, which is called "silent disease", therefore both diagnosis and treatment are insufficient. Owing to impaired bone strength, decreased bone mass, and deteriorating microstructure, the fractures are prone to occur in OP patients[15,16]. Mesenchymal Stromal Cells (MSCs) is an important cell bank involved in tissue regeneration. Under the action of special signals caused by tissue injury, MSC migrate to the damaged site, accumulate and proliferate locally, and differentiate along different pathways according to different injury signals. MSC is easy to isolate and expand, has strong in vitro multiplication ability, and can maintain its multidirectional differentiation ability even if amplified 100 million times. In addition, transplantation of MSCs can change the hematopoietic microenvironment, rebuild the immune system, and promote the recovery of hematopoietic function. Therefore, MSC is a practical seed cell for tissue repair, and BMSCs play a significant role in bone repair processes[17,18].
Curcumin is the main component of curcumin compounds extracted from the roots and stems of Curcuma, and multiple studies have found that curcumin can improve OP. For instance, curcumin could improve bone biomechanical properties and preserved bone microstructure through Transforming Growth Factor-Beta (TGF-β)/ Mothers against Decapentaplegic Homolog (SMAD) 2/3 pathway protects OP in type 2 diabetes rats, and protected type 2 diabetes rats against OP[19]. The dysfunction of osteoblasts caused by oxidative stress could be improved by curcumin through Glycogen Synthase Kinase-3β (GSK- 3β)-Nuclear Factor Erythroid 2-related factor 2 (Nrf2) pathway[20]. The bone microstructure of glucocorticoid-evoked secondary OP mice could be ameliorated by curcumin through activating miR-365 via Matrix Metallopeptidase 9 (MMP- 9)[21]. Curcumin improved bone metabolism and trabecular microstructure in ovariectomized OP model rats, and inhibit bone resorption[22]. Curcumin ameliorated bone microstructure and prevented bone loss by downregulating Enhancer of Zeste Homolog-2 (EZH2) expression[23]. Curcumin blocked Nuclear Factor Kappa B (NF-κB) activation to promote osteogenic differentiation in BMSCs[24]. ALP is a key marker in early differentiation of osteoblasts, OCN functions in bone remodeling and mineralization, OPN has been shown to be implicated in bone accelerated hBMSC proliferation, and induced osteogenic differentiation of hBMSCs by elevating ALP, OCN, and OPN levels.
Research has shown that miR-148a can promote cell differentiation. In an in vitro rabbit induced differentiation model, miR-148a-3p enhanced precursor adipocyte differentiation by downregulating PTEN[25]. miR-148a promoted MSC differentiation into cardiomyocytes by targeting DNA Methyltransferase 1 (DNMT1) [26]. In our work, we found curcumin increased miR-148a expression in hBMSCs. Functionally, miR-148a I overexpression promoted hBMSCs proliferation, and elevated ALP, OCN, and OPN levels in cells. Furthermore, the downregulation of miR-148a reversed the effects of curcumin on hBMSCs.
In all, curcumin could promote the proliferation and osteogenic differentiation of hBMSCs, which might be linked with the upregulation of miR- 148a, providing a theoretical basis for curcumin treatment of OP.
Conflict of interests:
The authors declared no conflict of interests.
References
- Ruiz-Esteves KN, Teysir J, Schatoff D, Elaine WY, Burnett-Bowie SA. Disparities in osteoporosis care among postmenopausal women in the United States. Maturitas 2022;156:25-9.
[Crossref] [Google Scholar] [PubMed]
- Suzuki T, Harada A, Shimada H, Hosoi T, Kawata Y, Inoue T, et al. Assessment of eldecalcitol and alendronate effect on postural balance control in aged women with osteoporosis. J Bone Miner Metab 2020;38(6):859-67.
[Crossref] [Google Scholar] [PubMed]
- Arjmand B, Sarvari M, Alavi-Moghadam S, Payab M, Goodarzi P, Gilany K, et al. Prospect of stem cell therapy and regenerative medicine in osteoporosis. Front Endocrinol 2020;11:430.
- Yang YR, Li CW, Wang JH, Huang XS, Yuan YF, Hu J, et al. Ubiquitylomes analysis of the whole blood in postmenopausal osteoporosis patients and healthy postmenopausal women. Orthop Surg 2019;11(6):1187-200.
[Crossref] [Google Scholar] [PubMed]
- Wei Y, Ma H, Zhou H, Yin H, Yang J, Song Y, et al. miR-424-5p shuttled by bone marrow stem cells-derived exosomes attenuates osteogenesis via regulating WIF1-mediated Wnt/β-catenin axis. Aging 2021;13(13):17190.
[Crossref] [Google Scholar] [PubMed]
- Giordano A, Tommonaro G. Curcumin and cancer. Nutrients 2019;11(10):2376.
- Kim Y, Clifton P. Curcumin, cardiometabolic health and dementia. Int J Environ Res Public Health 2018;15(10):2093.
[Crossref] [Google Scholar] [PubMed]
- Jiang Q, Lei YH, Krishnadath DC, Zhu BY, Zhou XW. Curcumin regulates EZH2/Wnt/β-catenin pathway in the mandible and femur of ovariectomized osteoporosis rats. Kaohsiung J Med Sci 2021;37(6):513-9.
[Crossref] [Google Scholar] [PubMed]
- Khanizadeh F, Rahmani A, Asadollahi K, Ahmadi MR. Combination therapy of curcumin and alendronate modulates bone turnover markers and enhances bone mineral density in postmenopausal women with osteoporosis. Arch Endocrinol Metab 2018;62:438-45.
[Crossref] [Google Scholar] [PubMed]
- Bukhari SN, Hussain F, Thu HE, Hussain Z. Synergistic effects of combined therapy of curcumin and Fructus Ligustri Lucidi for treatment of osteoporosis: Cellular and molecular evidence of enhanced bone formation. J Integr Med 2019;17(1):38-45.
[Crossref] [Google Scholar] [PubMed]
- Mao Q, Pang Y, Lu Y, Liang X. miRNA-148a promotes the differentiation of human induced pluripotent stem cells into cardiomyocyte-like cells. Chin J Tissue Eng Res 2020;24(19):2978.
- Jiang CK, Gong F. The regulatory role and mechanism of miR-148a in 5-aza induced myocardial like differentiation of mesenchymal stem cells. Chin J Appl Physiol 2017;33(6):514-43.
- Huang JL, Shi F, Zhang FM. Curcumin promotes in vitro proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. J Nanjing Med Univ 2020;40(12):1868-73.
- Sikora M, Śmieszek A, Marycz K. Bone marrow stromal cells (BMSCs CD45+/CD44+/CD73+/CD90+) isolated from osteoporotic mice SAM/P6 as a novel model for osteoporosis investigation. J Cell Mol Med 2021;25(14):6634-51.
[Crossref] [Google Scholar] [PubMed]
- Yan G, Huang Y, Cao H, Wu J, Jiang N, Cao X. Association of breastfeeding and postmenopausal osteoporosis in Chinese women: A community-based retrospective study. BMC Women's Health 2019;19(1):110.
[Crossref] [Google Scholar] [PubMed]
- Zhao H, Li X, Zhang D, Chen H, Chao Y, Wu K, et al. Integrative bone metabolomics—lipidomics strategy for pathological mechanism of postmenopausal osteoporosis mouse model. Sci Rep 2018;8(1):16456.
- Zhang W, Dong R, Diao S, Du J, Fan Z, Wang F. Differential long noncoding RNA/mRNA expression profiling and functional network analysis during osteogenic differentiation of human bone marrow mesenchymal stem cells. Stem Cell Res Ther 2017;8(1):30.
[Crossref] [Google Scholar] [PubMed]
- Liu Y, Yang G, Ji H, Xiang T, Luo E, Zhou S. Synergetic effect of topological cue and periodic mechanical tension-stress on osteogenic differentiation of rat bone mesenchymal stem cells. Colloids Surf B Biointerfaces 2017;154:1-9.
[Crossref] [Google Scholar] [PubMed]
- Liang Y, Zhu B, Li S, Zhai Y, Yang Y, Bai Z, et al. Curcumin protects bone biomechanical properties and microarchitecture in type 2 diabetic rats with osteoporosis via the TGFβ/Smad2/3 pathway. Exp Ther Med 2020;20(3):2200-8.
[Crossref] [Google Scholar] [PubMed]
- Li X, Chen Y, Mao Y, Dai P, Sun X, Zhang X, et al. Curcumin protects osteoblasts from oxidative stress-induced dysfunction via GSK3β-Nrf2 signaling pathway. Front Bioeng Biotechnol 2020;8:625.
[Crossref] [Google Scholar] [PubMed]
- Li G, Bu J, Zhu Y, Xiao X, Liang Z, Zhang R. Curcumin improves bone microarchitecture in glucocorticoid-induced secondary osteoporosis mice through the activation of microRNA-365 via regulating MMP-9. Int J Clin Exp Pathol 2015;8(12):15684-95.
[Google Scholar] [PubMed]
- Zhang QG, Zhang QH, Zhang K, Yao M, Tian Y, Mao X, et al. Effects of curcumin on bone metabolism balance in ovariectomized osteoporosis model rats based on OPG/RANKL signaling pathway. China Pharm 2020;31(17):2119-24.
- Jiang Qi, Zhu BY, De W. The effect of curcumin on EZH2 gene expression in the mandible and femur of ovariectomized osteoporosis rats. Shanghai Stomatol 2019;28(3):241-5.
- Xie YJ, Deng Z, Fan DS. The role and mechanism of curcumin in promoting osteogenic differentiation of bone marrow mesenchymal stem cells in high glucose environments. Shanghai J Tradit Chin Med 2020;54(2):85-90.
- He HB. miR-148a-3p targets to promote the differentiation of rabbit precursor adipocytes. Sichuan Agric Univ 2018;26:52-9.
- Zhang WC, Gong JL, Yang F. miR-148a regulates the differentiation of rat mesenchymal stem cells into cardiomyocytes by targeting DNMT1. J Wuhan Univ 2019;40(1):11-6.