- *Corresponding Author:
- Kavitha Sagar
Department of Studies in Botany, Vijayanagara Sri Krishnadevarya University, Ballari, Karnataka 583105, India
E-mail: drkamsgg@vskub.ac.in
| Date of Received | 21 June 2022 |
| Date of Revision | 21 July 2023 |
| Date of Acceptance | 11 October 2023 |
| Indian J Pharm Sci 2023;85(5):1478-1484 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Schouwia purpurea is a desert plant which is less explored for biological activities. The literature survey revealed that there are no reports on any pharmacological activities of Schouwia purpurea except one report on its antimicrobial activity and quantification of total phenols, flavonoids and tannins from the ethanolic leaf extract. Based upon our results of antioxidant property and pharmacognostic studies of Schouwia purpurea the present study was undertaken. The THP-1 cells were treated with various extracts or dimethyl sulfoxide concentrations. Cells in the positive control wells were treated with 1 % Triton X-100 solution, and negative control wells cells were incubated in culture media alone. Blank wells contained the corresponding extract concentrations or Triton X-100 solution or media without cells. The lactate dehydrogenase based in vitro toxicology assay kit was used to assay for cytotoxicity following the manufacturer instructions. The cytotoxicity study performed by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay suggests that the test compound, ethanolic leaf extract of Schouwia purpurea is moderately cytotoxic in nature against THP-1 cells. Based on the present study report it can be suggested for isolation, characterization and mechanisms of cytotoxicity by in vitro and in vivo studies of the extract of Schouwia purpurea is suggested in order to prove it as a potential anticancer, anti-inflammatory, immunomodulatory agent.
Keywords
In vitro, first report, 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay, anticancer, Schouwia purpurea
With an upsurge in the fields related to human disease development and prevention of diseases, there is an increasing concern especially regarding the impact of free radicals and cytotoxic compounds on human health. Hence, there is an increased demand of safe and natural herbal remedy throughout the world. The cytotoxicity studies are a useful initial step in determining the potential toxicity of a test substance, including plant extracts or biologically active compounds isolated from plants. Minimal to no toxicity is essential for the successful development of pharmaceutical or cosmetic preparation and in this regard, cellular toxicity studies play a crucial role. The concept of basal cytotoxicity, where lethal effects are noted on structure and functions common to human cells, is relevant when considering the relationship acute toxicity[1].
Cytotoxicity is one of the most important indicators for biological evaluation in vitro studies. In vitro, chemicals such as drugs and pesticides have different cytotoxicity mechanisms such as destruction of cell irreversible binding to receptors. In order to determine the cell death caused by these damages, there is need for cheap, reliable and reproducible short-term cytotoxicity and cell viability assays. Cytotoxicity assay is currently used in field of toxicology and pharmacology.
Disease that remains most challenging in today’s health care system as it has a complex, involving multiple mechanisms targets and drugs for effective disease management. On the other hand there are several current therapies with combination of drugs, of which however plant based therapy is cost effective, natural, with low side effects. There are herbal drugs which contain multiple components thereby saving considerable time and are inexpensive.
The most commonly engaged techniques for the detection cell viability and cytotoxicity are 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide assay (MTT assay), protein assay, neutral red and Lactate Dehydrogenase (LDH) leakage assay. The predictive value of in vitro cytotoxicity tests is based on the idea of ‘Basal’ cytotoxicity-that toxic chemical affect basic functions of cells, which are common in all cells and the toxicity can be measured by assessing cellular damage. The development of in vitro cytotoxicity assays has been driven by the need to rapidly evaluate the potential toxicity of large numbers of compounds. Cytotoxicity assays measures loss of some cellular or intercellular structure and functions, including lethal cytotoxicity. Thus leading us by indicating the potential of drug to cause cell and tissue injury.
The climatic condition of Ballari district, Karnataka is a semi-arid where most of the vegetation is composed of xerophytic plants are assessed which show numerous medicinal properties like anti-oxidant, anti-microbial, anti-cancer, anti-inflammatory, anti-diabetic etc., in plants like Ocimum sanctum, Cleome viscosa, Senna auriculata, Blumea lacera, Croton bonplandianum, Nerium oleander, Prosophis juliflora, Schouwia purpurea etc.
Schouwia purpurea (Brassicaceae) is widely distributed across the world in India, Iran Israel, Saudi Arabia and Egypt. In India Andhra Pradesh, Karnataka and Maharashtra. It is spread from Mauritania throughout the Sahel, Sahara, Northern Africa to Djibouti and Somalia in Arabia. It is considered as vegetable, food, fodder in Sahara and Sahel.
The literature survey revealed that there are no reports on any pharmacological activities of Schouwia purpurea except one report on its antimicrobial activity and quantification of total Phenols, flavonoids and tannins from the ethanolic leaf extract reported by Sagar et al.[2,3]. Based upon our results of antioxidant property and pharmacognostic studies of Schouwia purpurea the present study was undertaken.
The present study used to detect cell viability and cytotoxicity in response to plant extract. MTT assay is a colorimetric assay used for the determination of cell proliferation and cytotoxicity, based on reduction of yellow coloured water soluble tetrazolium dye MTT to formazan crystals. Mitochondrial LDH produced by live cells reduces MTT to insoluble formazan crystals, which upon dissolution into an appropriate solvent exhibits purple colour, the intensity of which is proportional to the number of viable cells and can be measured spectrophotometrically at 570 nm.
Materials and Methods
Chemicals and reagents:
Cell lines: THP-1-Human peripheral Blood Monocyte cell line (NCCS, Pune).
Cell culture medium: RPMI 1640 Media (#AL223A, Himedia), adjustable multichannel pipettes (Benchtop, USA) and a pipettor, fetal bovine serum (#10432, Himedia), MTT Reagents (#4060, Himedia) (5 mg/ml), Dimethyl Sulfoxide (DMSO) (#PHR1309, Sigma), doxorubicin used to treat different cancers that affect bladder, kidney, ovaries, nerve tissues. Dulbecco’s Phosphate- Buffered Saline (D-PBS) (#TL1006, Himedia) used for balanced salt solution, 96-Well plate (Corning USA for culturing the cells).
T25 flask ($12556009, Biolite-Thermo), 50 ml centrifuge tubes (#546043 TARSON), 1.5 ml centrifuge tubes (#TARSON), Pipettes (2-10 μl, 10-100 μl and 100-1000 μl) TARSON, 10 ml serological pipettes (TARSON), 10-1000 μl tips (TARSON).
Equipments:
Centrifuge (Remi: R-8°), Inverted Biological Microscope (Biolink), 37° incubator with humidified atmosphere of 5 % CO2 (Healthforce, China).
Assay controls:
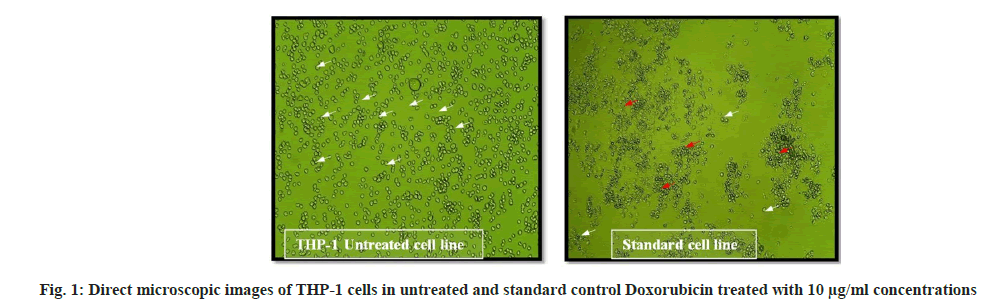
Medium control has medium without cells; negative control has medium with cells but without the extract of Schouwia purpurea and positive control has RPMI medium with cells and 10 μl of Doxorubicin (fig. 1).
Extracellular reducing components such as ascorbic acid, cholesterol, alpha-tocopherol, dithiothreitol present in the culture media may reduce the MTT to formazan. To account for this reduction, it was necessary to use the same RPMI medium in control to and also to the test wells.
Collection of plant materials and preparation of extracts:
The plant Schouwia purpurea was collected from Ballari and identified through Floras, followed by preparation of herbarium by the procedure proposed by Jain et al.[4]. The herbarium was deposited at and authenticated by Curator, Mahatma Gandhi Botanical Garden and Herbarium, GKVK, University of Agricultural Sciences, Bengaluru (UASB 5417). The Leaves of this plant were collected during March 2022 from its natural habitat which is located 2 km from Vijayanagara Sri Krishnadevaraya University Campus, Ballari.
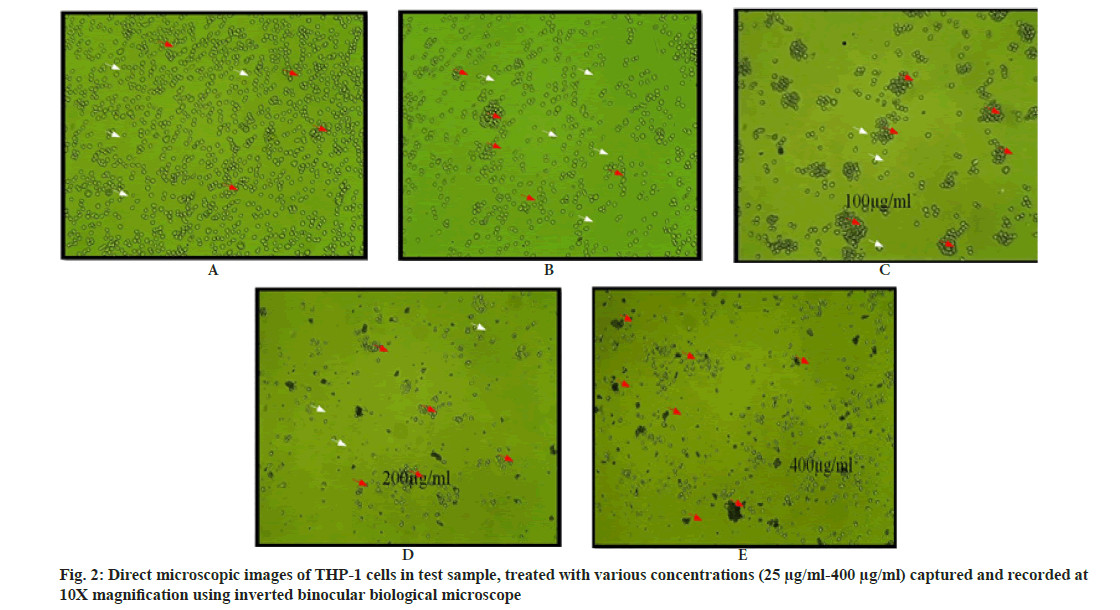
The leaves were separated, washed carefully under the tap water, rinsed with distilled water, air dried (100°) for 1 h and shade dried at room temperature (25-30°). The dried plants parts were ground by using mortar and pestle to make a powder. The powder thus obtained was sieved and packed in a sealed plastic covers or air tight bottles and stored in room temperature. The extract of the sample were prepared by soaking 50 g of powder sample in 300 ml of ethanol in 500 ml of conical flask and air tight them with aluminium foil and kept at dark place for 24 h on a rotary shaker at 100 rpm overnight and filtered with Whatman No. 1 paper and concentrated to dryness at 40°, lyophilized and stored in tight screw tube for further use at 4° degree. Different concentrations Schouwia purpurea leaves extract i.e. 25, 50, 100, 200 and 400 μg/ml in 100 % ethanol was prepared for the study (fig. 2).
Cell line culturing and maintenance:
The effect of methanol extract of the plants on the viability of cells was determined using MTT assay. The reduction of tetrazolium salts is now widely accepted as a reliable way to examine cell proliferation. The yellow tetrazolium MTT (3-(4, 5-dimethylthizolyl-2)-2, 5-diphenyl tetrazolium bromide) is reduced by metabollically active cells, in part by the action of dehydrogenase enzymes to generate reducing equivalents such as nicotinamide adenine dinucleotide hydrogen and nicotinamide adenine dinucleotide phosphate hydrogen. The resulting intracellular purple Formazan can be solubilized and quantified by spectrophotometric means. The assay measures the cell proliferation rate and conversely, whether metabolic events lead to apoptosis or necrosis with reduction in cell viability MTT cell proliferation assay.
Cell Preparation and cell treatment:
The THP-1 monocyte culture was diuted to 2×105 cell/ml in a 50 ml vial and pretreated with 25 μl of 100 μg/ml phorbol myristate acetate to get final concentration of 50 ng/ml in 50 ml vial. Then the THP-1 cells were trypsinized and aspirated into a 50 ml centrifuge tube. Cell pellet was obtained by centrifugation at 300 ×g. Approximately 20 000 cells per well were plate seeded into a 96-well micro-titre plate in triplicates without plant extract and incubated at 37° and 5 % CO2 atmosphere 24 h. After 24 h, the spent medium was aspirated from wells. The various extract concentrations i.e. 25, 50, 100, 200 and 400 μg/ml which were prepared in 100 % ethanol was filtered with 0.22 μm Millex-GP syringe filters and 200 μl of different concentrations of each Schouwia purpurea extracts were added to the respective wells. 200 μl of medium containing 10 % MTT reagent was then added to each well to get a final concentration of 0.5 mg/ml and the plate was incubated at 37° and 5 % CO2 atmosphere for 3 h. The RPMI culture medium was removed completely without disturbing the Formazan crystals formed and 100 μl of DMSO was added to solubilize the crystals. The absorbance was measured at 570 nm and 630 nm using a micro plate reader. The percentage growth inhibition was calculated by subtracting the absorbance of test from the blank divided by 100. The IC50 value was determined by using linear regression equation i.e., Y=Mx+c, here Y=50 M and C values were derived from the viability graph[5]. Leaf extract is taken as sample and THP-1 is the cell line.
Cytotoxicity assay:
THP-1 cells were treated with 25, 50, 100, 200 and 400 μg/ml concentration of extract and or DMSO concentrations. Cells in the positive control wells were treated with 1 % Triton X-100 solution and negative control wells cells were incubated in culture media alone. Blank wells contained the corresponding extract concentrations or Triton X-100 solution or media without cells. The LDH based in vitro toxicology assay kit was used to assay for cytotoxicity following the manufacturer instructions. The assay measures membrane integrity as a function of the amount of cytoplasmic LDH released into the medium. LDH reduces NAD into NADH, which is utilized in the reduction of a tetrazolium dye to colored Formazan. The amount of formazan which is proportional to the amount of LDH release from dead cells was measured colorimetrically at 450 nm. Absorbance for background correction was determined at 620 nm. The percentage of cell viability was calculated as follows:
Percentage cell viability=100-percentage cell cytotoxicity
Percentage cell cytotoxicity=100×(experimental well absorbance-negative control well absorbance)/ (positive control well absorbance-negative control well absorbance)
All calculations were performed after background absorbance correction and blank absorbance subtraction.
Results and Discussion
The Ethanolic leaves extract of Schouwia purpurea is evaluated to analyze the cytotoxicity effect on THP-1 cell lines. The concentrations of the test compound used to treat the cells are given in Table 1.
| S. No | Test Compound | Cell Line | Concentration treated to cells |
|---|---|---|---|
| 1 | Untreated | THP-1 | No treatment |
| 2 | Doxorubicin | THP-1 | 10 µg/ml |
| 3 | Blank | - | Only media without cells |
| 4 | Leaf extract | THP-1 | 25, 50, 100, 200 and 400 µg/ml |
Table 1: Concentrations of Test and Standard Treated to Cells
The cytotoxicity study performed by MTT assay suggests that the test compound, ethanolic leaf extract of Schouwia purpurea is moderately cytotoxic in nature against THP-1 cells. The percentage cell viability was calculated using the formula:
Percentage cell viability=Mean absorbance of a sample-Blank/Mean absorbance of untreated- Blank×100
Where, b=blank and c=control. The results reported are the mean values of two different experiments performed in triplicates.
The cell death is increased on dose dependent manner of increased concentrations of test sample and cell density is decreased due to reduced cell numbers. Clustered morphology of cells also observed in higher concentrations of test sample due to toxic potency of ethanolic extracts of Schouwia purpurea on THP-1 cells (Table 2).
| Sample | IC50 (µg/ml) |
|---|---|
| Leaves extract | 292.55 |
Table 2: Ic50 Value of the Test Compound Tested Against Thp-1 Cell Lines
The cell viability can be measured by MTT assay which is a sensitive, reliable and quantitative colorimetric assay which is based on the capacity of the cellular mitochondrial dehydrogenase enzyme in living cells to reduce the yellow watersoluble substrate MTT into a dark blue/purple formazan product which is insoluble in water and is measured by spectrophotometer. There is direct relation between the amount of formazan produced and the cell number in range of cells lines which are proportional to each other.
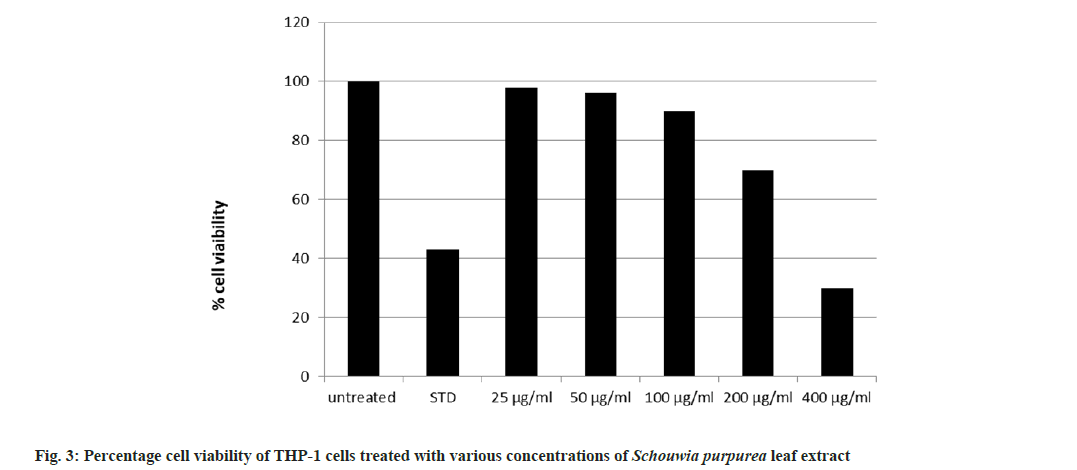
The cytotoxicity activity of the extracts of Schouwia purpurea on cell lines THP-1 Human peripheral blood monocyte cell line was investigated in vitro MTT assay. Five different concentrations (25, 50, 100, 200 and 400 μg/ml) of leaf extract were used to study the cytotoxicity potential of the plant on cell lines. The IC50 of ethanolic extract is 292.55 μg/ml which shows that this concentration of Schouwia purpurea reduced 50 % of viable cell THP 1 cell number. Hence it is observed that the ethanolic leaf extract of Schouwia purpurea was moderately cytotoxic on THP-1 cell lines. The result showed that the cytotoxicity of the plant extract was dose dependent. At the highest concentration i.e. 400 μg/ml of cell viability reduced to 30 % when compared to 200 μg/ml 70 %, at 100 μg/ml 85 %, at 50 μg/ml, 95 % and at least concentration 25 μg/ ml, 95 % cell viability was observed. Incidently, the reports are in consideration with the studies of Miceli et al.[6], where they reported cytotoxicity of Brassica sp. (Brassicaceae) leaf extracts against Human Colorectal adenoarcinomma and breast cancer (MCF-7) cell lines. The leaf extracts showed cytotoxic efficacy against Caco- 2 cells, with the flowering top extract being the most effective (about 90 % activity at the highest concentration tested). Similar studies were done by Siddiqui et al.[7] where they demonstrated dose dependant cytotoxic activity of the methanol extract of Catharanthus sp. against HCT-116 colorectal carcinoma cell line, where n-hexane, chloroform fraction showed the highest activity with chloroform fraction also showing the highest activity.
Similar reports were demonstrated by Antoney et al.[8] showed that the methanolic extract of Embelia ribes. Nemati et al.[9] also reported that ethanolic extract of Coronilla sp. has significant cytotoxicity effect on HeLa cell line in concentration range between 10 mg/ml by using MTT assay. The highest cytotoxicity of this extract against Hela cell was found in 5 mg/ml concentration with 94.18 % of cell growth inhibition IC50 value of the highest cytotoxicity of this extract against HeLa cell was found in 5 mg/ml concentration with 94.18 % of cell growth inhibition IC50 value of Coronilla sp. cytotoxicity rate was increased along with the concentrations of leaf extracts. Sultan et al.[10] observed similar concentration dependant activity of of methanolic extract of Artemisia sp. using MCF-7,HT-29 and HeLa cells, all of the extracts showed cytotoxic activity in all three cell lines to varying extract.
There are other reports where MTT assay has been used to know cytotoxic effect of Calotropis procera, Moringa oleifera, Millettia pinnata on A549 non-small-cell lung cancer cells where they found significant cytotoxicity effect. the cytotoxicity of the chloroform (37.45±1.04) and ethyl acetate extracts (34.20±0.81) of Millettia pinnata against A549 cells was found relatively higher with respect to another extract. In contrast, a study with the L132 normal epithelial lung cell line revealed less toxicity from the chloroform extract (0.33±0.19) compared to the ethyl acetate extract (6.65±0.59)[11].
Potestà et al.[12] showed that particularly boiled Moringa oleifera extract showed a specific antiproliferative activity on cancer cells, but not on the Peripheral Blood Mononuclear Cell (PBMC) [12]. In addition, the results of Mfotie Njoya et al.[13] demonstrated that the methanol extract from leaves of Sarcocephalus pobeguinii was selectively cytotoxic to cancer cell lines compared to the normal Vero cells, with the Selectivity Index (SI) ranging from 3.15 to 18.28 on the four cancer cells lines (MCF-7, HeLa, Caco-2, and A549), suggesting the potential and antiproliferative effect of this extract[14]. Heliotropium bacciferum chloroform extract showed a concentrationdependent inhibitory effect on the growth of the treated cancer cell lines with half maximal inhibitory concentration (IC50) values of 95 μg/ml on HCT116 and 62 μg/ml on DLD1[15]. Curcuma aqueous extract has been shown to induce apoptosis in human colon cancer LS-174-T cells. Njoya et al.[13] revealed the role of curcumin in inducing apoptosis in rabbit osteoclasts as well as inhibiting bone resorption. Curcumin’s proapoptotic effect in leukaemic Jurkat cells, COLO 205 cells, human lung carcinoma A549 cells, murine myelomonocytic leukemia WEHI-3 cells, human nasopharyngeal carcinoma cells, and NPC-TW 076 has previously been documented[16]. Capsaicin regulate different molecular targets in breast cancer like, caspase-3, Reactive Oxygen Species (ROS), Rac1, and HER- 2 etc. Capsaicin produced apoptosis in breast cancer (H-Ras, MCF1 cells) by inducing ROS and Rac1 signaling pathways. These ROS and Rac1 pathways are specifically induced by proteins like, p38, c-Jun N-terminal protein kinase-1[17].
The present investigation is first study on cytotoxic study of Schouwia purpurea. It was observed that Schouwia purpurea exhibited a selective dose dependent inhibition of THP-1 at various concentrations (fig. 3). The medicinal uses and therapeutic properties of Schouwia purpurea are not known. Sagar et al.[2] have reported that phenols were present in more quantity when compared to flavonoids and tannins in ethanolic leaves extract of Schouwia purpurea. Since Phenols offer protection against oxidative damages by donating hydrogen or electron to free radicals and thus, in this process, they aid in stabilizing cell membrane networks and inhibiting the formation and expression of inflammatory cytokines like Tumor Necrosis Factor alpha (TNF-α), Transforming Growth Factor beta (TGF-β) and varieties of Interleukins (IL-6, IL-2, IL-8). The IC50 value suggests that Schouwia purpurea extract is moderately cytotoxic on THP- 1 cell lines.
Based on the present study report it can be suggested for isolation, characterization and mechanisms of cytotoxicity by in vitro and in vivo studies of the extract of S. purpuea is suggested in order to prove it as a potential anticancer, antiinflammatory, immunomodulatory agent.
Conflict of interests:
The authors declared no conflict of interests.
References
- McGaw LJ, Elgorashi EE, Eloff JN. Cytotoxicity of African medicinal plants against normal animal and human cells. In: Toxicological survey of African medicinal plants 2014: pp. 181-233).
- Sagar K, Shivashankar PN, Banu Y. Antimicrobial efficacy of few less known semi-arid plants of Ballari, Karnataka, India. Int J Curr Microbiol Appl Sci 2021;10(4):552-8.
- Sagar K, Shivashankar PN, Amarappa S. Phytochemical studies and quantification of total phenols, tannins and flavonoids in less known semi-arid plants of Ballari, Karnataka. Int J Pharm Sci Res 2022;13(5):1000-06.
- Jain SK, Rao RR. A Handbook of field and herbarium methods. Today and Tomorrow's Publishers. New Delhi. 1980.
- Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 1988;48(3):589-601.
[Google Scholar] [PubMed]
- Miceli N, Cavò E, Ragusa M, Cacciola F, Mondello L, Dugo L, et al. Brassica incana Ten.(Brassicaceae): Phenolic constituents, antioxidant and cytotoxic properties of the leaf and flowering top extracts. Molecules 2020;25(6):1461.
[Crossref] [Google Scholar] [PubMed]
- Siddiqui MJ, Ismail Z, Aisha AF, Abdul Majid AM. Cytotoxic activity of Catharanthus roseus (Apocynaceae) crude extracts and pure compounds against human colorectal carcinoma cell line. Int J Pharmacol 2010;6(1):43-7.
- Antoney J, Britto AJ, Abida P, Stephan TL. in vitro cytotoxicity studies on methanolic leaf extract of Embelia ribes burm f-an important traditional medicinal plant of Kerala. Adv Cytol Pathol 2016;1(1):6-8.
- Nemati F, Dehpouri AA, Eslami B, Mahdavi V, Mirzanejad S. Cytotoxic properties of some medicinal plant extracts from Mazandaran, Iran. Iran Red Crescent Med J 2013;15(11):e8871.
[Crossref] [Google Scholar] [PubMed]
- Sultan MH, Zuwaiel AA, Moni SS, Alshahrani S, Alqahtani SS, Madkhali O, et al. Bioactive principles and potentiality of hot methanolic extract of the leaves from Artemisia absinthium L in vitro cytotoxicity against human MCF-7 breast cancer cells, antibacterial study and wound healing activity. Curr Pharm Biotechnol 2020;21(15):1711-21.
[Crossref] [Google Scholar] [PubMed]
- Kumar G, Gupta R, Sharan S, Roy P, Pandey DM. Anticancer activity of plant leaves extract collected from a tribal region of India. Biotech 2019;9(11):399-405.
[Crossref] [Google Scholar] [PubMed]
- Potestà M, Minutolo A, Gismondi A, Canuti L, Kenzo M, Roglia V, et al. Cytotoxic and apoptotic effects of different extracts of Moringa oleifera Lam on lymphoid and monocytoid cells. Exp Ther Med 2019;18(1):5-17.
[Crossref] [Google Scholar] [PubMed]
- Mfotie Njoya E, Munvera AM, Mkounga P, Nkengfack AE, McGaw LJ. Phytochemical analysis with free radical scavenging, nitric oxide inhibition and antiproliferative activity of Sarcocephalus pobeguinii extracts. BMC Complement Altern Med 2017;17:1-9.
[Crossref] [Google Scholar] [PubMed]
- Aïssaoui H, Mencherini T, Esposito T, de Tommasi N, Gazzerro P, Benayache S, et al. Heliotropium bacciferum Forssk.(Boraginaceae) extracts: Chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat Prod Res 2019;33(12):1813-8.
[Crossref] [Google Scholar] [PubMed]
- Ozaki K, Kawata Y, Amano S, Hanazawa S. Stimulatory effect of curcumin on osteoclast apoptosis. Biochem Pharmacol 2000;59(12):1577-81.
[Crossref] [Google Scholar] [PubMed]
- Su CC, Lin JG, Li TM, Chung JG, Yang JS, Ip SW, et al. Curcumin-induced apoptosis of human colon cancer colo 205 cells through the production of ROS, Ca2+ and the activation of caspase-3. Anticancer Res 2006;26(6B):4379-89.
[Google Scholar] [PubMed]
- Chang HC, Chen ST, Chien SY, Kuo SJ, Tsai HT, Chen DR. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum Exp Toxicol 2011;30(10):1657-65.
[Crossref] [Google Scholar] [PubMed]