- *Corresponding Author:
- K. K. Aravindakshan
Department of Chemistry, University of Calicut, Malappuram-673 635, India
E-mail: aravindkuttamath@yahoo.com
| Date of Submission | 19 December 2013 |
| Date of Revision | 16 October 2014 |
| Date of Acceptance | 11 November 2015 |
| Indian J Pharm Sci, 2015;77(6):655-660 |
This is an open access article distributed under the terms of the Creative Commons Attribution?NonCommercial?ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non?commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Cytotoxic activities of acetoacetanilide N(4)-methyl(phenyl)thiosemicarbazone (L2H) and its seven different metal complexes were studied. Of these, IC50value of the copper complex was found to be 46 μg/ml. Antitumour studies of this copper complex was carried out using Daltons Lymphoma Ascites cell-induced solid tumour model and Ehrlich's Ascites Carcinoma cell-induced ascites tumour model. Administration of the copper complex at different concentrations (10, 5 and 1 mg/kg b. wt) inhibited the solid tumour development in mice and increased the mean survival rate and the life span of Ascites tumour bearing mice in a concentration dependent manner.
Keywords
Daltons lymphoma ascites, Ehrlich ascites carcinoma, cytotoxicity, ligand, acetoacetanilide N(4)- methyl(phenyl)thiosemicarbazone
Coordination compounds have immense applications in different fields such as metallurgy, industry, biology and medicine. Although metals are used in the medical field, the actual role and potential of metal based drugs were fully understood only after the discovery of cisplatin, i.e., cis- [dichlorodiammine] platinum (II) [1]. Cisplatin and carboplatin still play prominent role in cancer chemotherapy [2,3].
Since the discovery of the antitubercular activity of thiosemicarbazones [4], a great deal of work has been done on the pharmacology of this type of compounds. Literature survey revealed that the transition metal complexes of thiosemicarbazones show antitumour property [5]. Thiosemicarbazones exercise their biological activity in mamalian cells by inhibiting ribonucleotide reductase, a necessary enzyme in the synthesis of DNA precursors [6]. Studies on iron and copper complexes have shown that they can be more active in the inhibition of DNA synthesis than the free thiosemicarbazone [7]. Previous studies showed that the copper(II) complex of 2-formylpyridine thiosemicarbazone is more powerful antitumour agent than the free ligand [8]. The antitumour activities of Co(II), Ni(II), Cu(II) and Zn(II) complexes of thiosemicarbazone derived from 3-acetylumbelliferone was studied [9]. It was confirmed that the Co(II) and Cu(II) complexes produced more inhibitory effect compared to the other complexes.
Since the cost of platinum complexes is very high, complexes of cheaper metals like copper and iron were tried in cancer chemotherapy. According to Petering [10], Cu(II) ion alone will not have any antitumour activity, but will act as an inhibitor of tumour growth in the chelated form. Even though, the actual pathway by which the copper chelate inhibits the cancer growth is not known, it is believed that this will be on the basis of structure activity correlation as in the case of cisplatin [11] (i.e. DNA intercalation). Earlier studies based on the inhibition of DNA biosynthesis showed that copper complexes were more active as antitumour agents than those of Fe(III), probably due to the reversible reduction processes at accessible electrode potential of copper compounds [12]. Secondly, 4-coordinate planar copper complexes will get attached to nitrogen base of DNA, thus blocking the base replication leading to the inhibition of tumour growth than 6-coordinate complexes of Fe(III) [13].
Among the important mechanistic conclusions emanated from the structural studies, a prominent one is the finding that the ligand systems with NNS, ONO or ONS donor atoms have ample carcinostatic potency. Recently, it has been shown that substitution on the 4th nitrogen of thiosemicarbazone can enhance its biological activity [14]. Our literature survey revealed that semicarbazone, thiosemicarbazone and related compounds of 1,3-diketoderivatives have been seldom investigated for their biological activities [15,16]. Hence, it is considered to be worthwhile and interesting to evaluate the antitumour activity of acetoacetanilide N-(4)methyl(phenyl) thiosemicarbazone (AcTSC) and its metal complexes.
Materials and Methods
All chemicals used were of AnalaR quality and purchased from Merck. Commercial solvents used for the synthesis were purified by standard methods [17]. Hydrated metal salts purchased were used as such for the preparation of the complexes.
Preparation of the ligand (acetoacetanilide N(4)- methyl(phenyl)thiosemi-carbazone)
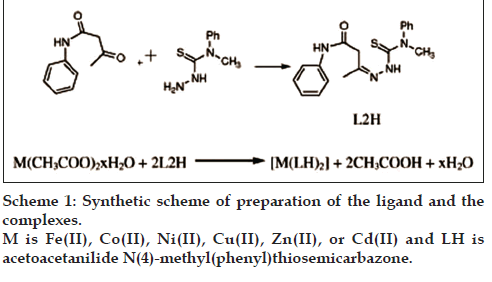
Acetoacetanilide (0.005 mol) in 30 ml methanol was added to N(4)-methyl(phenyl)thiosemicarbazide (0.005 mol) in 30 ml methanol and refluxed on a water bath for 3 h. The mixture was cooled to room temperature. The solid product obtained was filtered, dried and kept over fused calcium chloride (Yield: 70%, M.P=158°) [18].
Preparation of the thiosemicarbazone complexes
A methanol solutions of metal acetate (0.025 mol in 20 ml) was added to a methanol solution of AcTSC (0.05 mole in 40 ml) and the mixture was refluxed for 4 h. Then it was evaporated and cooled. The solid complex that formed was filtered off, washed several times with methanol and water. It was dried in a desiccator. The product was kept over fused calcium chloride. The complexes of Fe(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) were prepared using a reaction mixture containing metal acetate and ligand in 1:2 molar ratio. Yield and melting point of complexes were noted (Yield: 70–80%, m.p.=210–220°) [18] (Scheme 1).
Characterization of the ligand and complexes
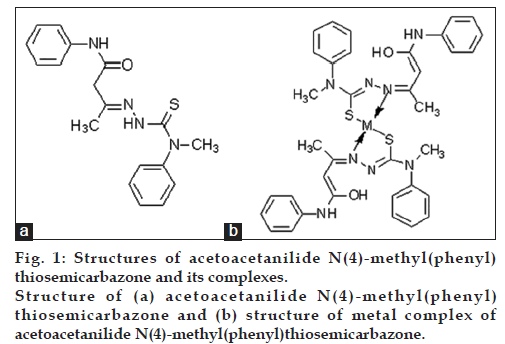
The ligand (fig. 1a) and the complexes (fig. 1b) were characterized by elemental analysis, magnetic moment measurements, IR, UV/Vis spectrum and 1H NMR spectral studies [18].
The preparation of the drug
Fifty milligrams of the compound was dissolved in 1 ml of dimethyl sulphoxide (DMSO). For in vitro studies, the drug was dissolved in DMSO and for in vivo studies 50 mg of drug was first dissolved in 1 ml DMSO and further it was diluted using distilled water to desired concentration.
Evaluation of anticancer potential
Dalton’s Lymphoma Ascites (DLA) and Ehrlich Ascites Carcinoma (EAC) cell lines were initially procured from Adayar Cancer Institute, Chennai and propagated as transplantable tumors in the peritoneal cavity of BALB/C mice. L929 (mouse lung fibro blast) cell line was obtained from National Centre for Cell Sciences, Pune.
Swiss albino female mice (20-25 g) were obtained from the Small Animal Breeding Station (SABS), Mannuthy, Thrissur, Kerala. They were kept under standard conditions of temperature and humidity in animal house of Amala Cancer Research Centre. The animals were provided with standard mouse chow (Sai Durga Feeds and Foods, Bangalore, India) and water ad libitum. All the animal experiments in this study were carried out with the prior approval of the Institutional Animal Ethics Committee (IAEC) and were conducted strictly according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Animal Welfare Division, Government of India.
Mouse lung fibroblast (L929 cells) were cultured in DMEM medium supplemented with FBS (10% v/v), streptomycin (100 μg/ml) and penicillin (100 U/ml) and kept at 37° in an incubator with 5% CO2. Dalton’s Lymphona Ascites (DLA) and Ehrlich’s Ascites Carcinoma (EAC) cells maintained in the intraperitoneal cavity of mouse, were used for the study.
Trypan blue exclusion method
The test compounds were studied for short-term in vitro cytotoxicity using Dalton’s lymphoma ascites cells (DLA). The tumour cells aspirated from the peritoneal cavity of tumour bearing mice were washed thrice with PBS or normal saline. Cell viability was determined by Trypan blue exclusion method [18]. Viable cell suspension (1×106 cells in 0.1 ml) was added to tubes containing various concentrations of the test compounds and the volume was made up to 1 ml using phosphate buffered saline (PBS). Control tube contained only cell suspension. These assay mixtures were incubated for 3 h at 37°. Further cell suspension was mixed with 0.1 ml of 1% Trypan blue and kept for 2–3 min and loaded on a haemocytometer. Dead cells take up the blue colour of Trypan blue while live cells do not take up the dye. The stained and unstained cells were counted separately.
Methyl tetrazolium assay
For long-term cytotoxicity, L929 cells were used. The cells were seeded in to 96 well flat bottom titre plate (5000 cells/well) containing 200 μl MEM (minimum essential medium) with 10% FCS (fetal calf serum) and incubated for 24 h at 37° in 5% CO2 atmosphere for the attachment of cells. After incubation, various concentrations of the test compound were added to the wells in triplicates and the incubation was continued for 48 h. Twenty microlitres of MTT (5 mg/mL in PBS) was added to each well before 4 h of the completion of incubation. After the incubation period, the plates were centrifuged, supernatant liquid was removed and 100 μl of DMSO was added to each well. The plate was then incubated at room temperature for 15 min and the optical density (OD) was measured at 540 nm. The percentage of dead cells was determined using the formula: % of dead cells=(I–OD of drug treated/OD of control)×100.
Toxicity studies of metal complexes
Twenty four Swiss albino mice were divided into 4 groups (6 animals/group). Group 1: 1 mg/kg, treated, Group 2: 5 mg/kg, treated, group 3: 10 mg/kg, treated and group 4: 25 mg/kg, treated. The drug was administrated once daily (i.p.) and continued for 10 weeks. The animals were observed for their mortality.
Effect of copper complex of acetoacetanilide N(4)- methyl(phenyl)thiosemicarbazone on the survival rate of ascites tumour bearing animals
Animals (female, 6–8 weeks old) weighing 28–30 g were divided into 4 groups of 6 animals each. Viable EAC cells 106 in 0.1 ml of phosphate buffered saline (PBS) were injected in to the peritoneal cavity. Group 1: control, group 2: 1 mg/kg, treated, group 3: 5 mg/kg, treated, group 4: 10 mg/kg, treated, and group 5: standard drug (cyclophosphamide), treated.
Drug and cyclophosphamide were given by intraperitoneal injection from the first day of tumour induction. The death pattern of animals due to tumour burden was noted and the percentage increase in life span was calculated as, %ILS=(T-C/C)×100, where T and C are mean survival of treated and control mice, respectively.
Effect of copper complex of acetoacetanilide N(4)- methyl(phenyl)thiosemicarbazone on solid tumour development
Swiss albino mice (female, 5–6 w old) weighing 20–25 g were divided into 4 groups comprising of 6 animals in each group for the above studies. Tumour was induced by injecting DLA cells (0.1 ml of 106 cells per mouse) in to the right hind leg of mice. Group 1 was kept as control Groups 2, 3 and 4 were treated with copper complex of AcTSC. Group 5 was treated with cyclophosphamide. The tumour development on animals of each group was determined by measuring the diameter of tumour growth in two perpendicular planes using a digital vernier caliper starting from 7th day of tumour induction up to 34th day. The tumour volume was calculated using the formula, V=4/3πr1 2r2, where r1 is the minor diameter and r2 is the major diameter [19].
Statistical analysis
The values were expressed as mean±standard deviation (SD). The mean values were statistically analyzed using one-way analysis of variance (ANOVA) using graph pad Instat 3 software (Graph Pad Software, San Diego, California, USA) followed by appropriate post-hoc test (Dunnett multiple comparison test). P<0.05 was considered statistically significant and are indicated by “*”.
Results
Short-term in vitro cytotoxic analysis
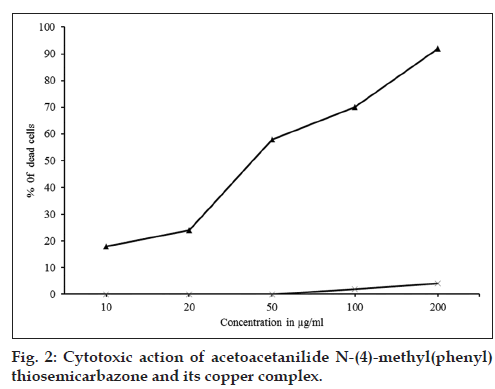
The ligand, AcTSC and its Fe(II), Co(II), Ni(II), Cu(II), Zn(II) and Cd(II) complexes showed marked cytotoxic activity for DLA cell line (Table 1). The copper complex showed maximum activity (fig. 2) and the concentration required for 50% death (IC50) was found to be 46 μg/ml. The free ligand showed only very low cytotoxic activity.
| Concentration (µg/ml) | Percentage cytotoxicity | ||||||
|---|---|---|---|---|---|---|---|
| Complexes | Ligand | ||||||
| Fe | Co | Ni | Cu | Zn | Cd | AcTSC | |
| 10 | 0 | 0 | 3 | 18 | 7 | 2 | 0 |
| 20 | 2 | 0 | 7 | 24 | 14 | 9 | 0 |
| 50 | 6 | 5 | 11 | 58 | 20 | 12 | 0 |
| 100 | 10 | 8 | 20 | 70 | 35 | 36 | 2 |
| 200 | 20 | 18 | 33 | 92 | 58 | 42 | 4 |
AcTSC: Acetoacetanilide N-(4)?methyl(phenyl)thiosemicarbazone
Table 1: In Vitro Cytotoxicity Of Actsc And Its Different Complexes
Long-term in vitro cytotoxic analysis (methyl tetrazolium assay)
The results of long-term in vitro cytotoxicity of the copper complex of AcTSC showed that it is nontoxic up to 0.5 μg/ml towards L929 cells (Table 2).
| Concentration (µg/ml) | Cytotoxicity (%) |
|---|---|
| Control | 0 |
| 0.05 | 0.37±0.03 |
| 0.1 | 0.51±0.03 |
| 0.25 | 0.61±0.03 |
| 0.5 | 1.05±0.03 |
AcTSC: Acetoacetanilide N-(4)-methyl(phenyl)thiosemicarbazone, MTT: methyl tetrazolium
Table 2: Mtt Assay Of Copper Complex Of Actsc
Toxicity studies
The results of toxicity studies of copper complex on 24 Swiss albino mice, 4 groups, at four concentrations (25, 10, 5 and 1 mg/kg) showed that 25 mg/kg was toxic to the animals. Therefore, this concentration was avoided and 10, 5 and 1 mg/kg were only selected for in vivo studies, as they were nontoxic to the animals.
Effect of copper complex on ascites tumour development
The animals of the tumour control group survived for a period of 15.8±0.65 d. Those treated with cyclophosphamide survived for 26.63±1.8 d. The copper complex at 10, 5 and 1 mg/kg increased the survival rate of animals by 21±2.1 d, 19.6±1.4 d, and 18.6±1.2 d, respectively (Table 3). Thus the copper complex was found to be effective in increasing the average life span of the animals by 32.9, 24.05 and 17.7%, respectively, at 10, 5 and 1 mg/kg doses (Table 4).
| Treatment (mg/kg) | Mean survival rate |
|---|---|
| Control | 15.8±0.65 |
| 10 | 21±2.1 |
| 5 | 19.6±1.4 |
| 1.5 | 18.6±1.2 |
| Standard?cyclophosphamide (10) | 26.63±1.8 |
AcTSC: Acetoacetanilide N-(4)-methyl(phenyl)thiosemicarbazone
Table 3: Effect Of Copper Complex Of Actsc On The Mean Survival Rate Of Ascites Tumor Bearing Mice
| Treatment (mg/kg) | Increase in life span (%) |
|---|---|
| Control | ? |
| 10 | 32.9 |
| 5 | 24.05 |
| 1 | 17.7 |
| Standard?cyclophosphamide (10) | 68.4 |
AcTSC: Acetoacetanilide N-(4)-methyl(phenyl)thiosemicarbazone
Table 4: Effect Of Copper Complex Of Actsc On The Life Span Of Ascites Tumour Bearing Mice
Effect of copper complex on solid tumour development
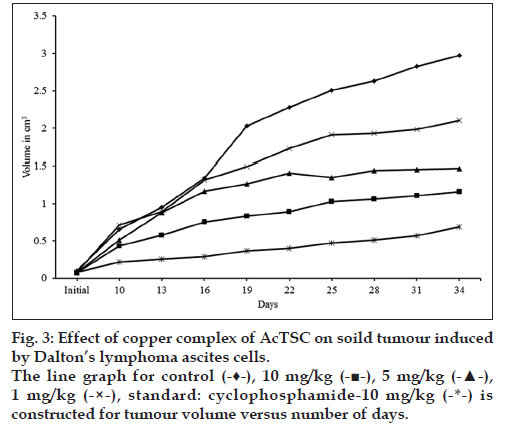
In the control animals, the volume of tumour was increased to 2.973 cm3 on 34th d, while in the animals treated with the copper complex, there was a significant reduction of tumour volume. At 10 mg/kg, the volume was 1.1605 cm3, while at lower concentrations (5 and 1 mg/kg) the tumour volumes were 1.4692 and 2.1011 cm3, respectively. Treatment with cyclophosphamide, a standard antitumour drug reduced the tumour volume to 0.583 cm3 (Table 5 and fig. 3).
| Treatment (mg/kg) | Observation (number of days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 10 | 13 | 16 | 19 | 22 | 25 | 28 | 31 | 34 | |
| Mean volume | 0.099 | 0.659 | 0.835 | 1.344 | 2.035 | 2.283 | 2.514 | 2.636 | 2.83 | 2.973 |
| 10 | 0.075 | 0.436 | 0.576 | 0.756 | 0.834 | 0.889 | 1.032 | 1.059 | 1.11 | 1.161 |
| 5 | 0.08 | 0.511 | 0.877 | 1.168 | 1.263 | 1.408 | 1.351 | 1.440 | 1.46 | 1.469 |
| 1 | 0.083 | 0.714 | 0.885 | 1.314 | 1.495 | 1.777 | 1.912 | 1.929 | 1.98 | 2.101 |
| Standard?cyclophosphamide (10) | 0.080 | 0.220 | 0.260 | 0.290 | 0.370 | 0.400 | 0.470 | 0.510 | 1.57 | 0.690 |
Values are in cm3. AcTSC: Acetoacetanilide N-(4)-methyl(phenyl)thiosemicarbazone
Table 5: Effect Of Copper Complex Of Actsc On The Reduction Of Tumour Volume
Discussion
In 2008, approximately 12.7 million cancer cases were diagnosed and 7.6 million people died of cancer. The most common, being the cancer that affecting stomach, lung, liver and breast. Though many diseases (such as heart failure) may have worse effect on the society, it is believed that cancer is the most dangerous and deadly disease affecting human being. Therefore, cancer is regarded as a disease that must be “fought” to end. Here lies the importance of cancer research, which includes the intense scientific efforts to understand the disease processes and to discover the possible therapies.
Bearing in mind that the N-N-S or O-N-O or O-N-S donor system is a common feature for all compounds with carcinostatic potency, we proceeded the antitumour studies of acetoacetanilide N(4)-methyl(phenyl)thiosemicarbazone and its metal complexes and we got promising results. In vitro cytotoxicity studies on AcTSC and its different complexes showed cytotoxicity against DLA cell lines. The copper complex showed maximum cytotoxicity with an IC50 value of 46 μg/ml.
Ehrlich ascetic tumor is a rapidly growing carcinoma with very aggressive behavior [20]. It is able to grow in almost all strains of mice. The Ehrlich ascetic tumor implantation induces a local inflammatory reaction with increasing vascular permeability, which results in an intense edema formation, cellular migration and a progressive acetic fluid formation [21]. The ascetic fluid is essential for tumor growth since it constitutes a direct nutritional source for tumor cells [22]. The copper complex of AcTSC was found to be effective against DLA-induced solid tumour and EAC-induced ascites tumour. The 10 mg/kg body weight was more effective than the other two concentrations (5 and 1 mg/kg b.wt) in both the cases. The present study of in vitro cytotoxic and antitumour properties of the copper complex of acetoacetanilide N(4)- methyl(phenyl)thiosemicarbazone (AcTSC) suggests its potential use as an anticancer agent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode.Nature 1965;205:698-9.
- Wheate NJ, Walker S, Craig GE, Oun R. The status of platinumanticancer drugs in the clinic and in clinical trials. Dalton Trans2010;39:8113-27.
- Kelland L. The resurgence of platinum-based cancer chemotherapy. NatRev Cancer 2007;7:573-84.
- Domagk G, Behnish R, Mietzsch F, Schmidt H. New compound activeagainst tuberculosis bacilli in vitro. Naturwissenschaften 1946;33:315.
- West DX, Carison CS, Liberta AE, Albert JN, Daniel CR.Transition metal ion complexes of thiosemicarbazones derived from2-acetylpyridine. Part 8. The chemical and antifungal properties ofnickel(II) complexes of 2-acetylpyridine 4N-diethyl- and 4N-dipropylthiosemicarbazones and their copper(II) complexes. Transit Met Chem1990;15:341-4.
- French FA, Blanz EJ Jr, DoAmaral JR, French DA. Carcinostaticactivity of thiosemicarbazones of formylheteroaromatic compounds.VI 1-Formylisoquinoline derivatives bearing additional ring substituents,with notes on mechanism of action. J Med Chem 1970;13:1117-24.
- Saryan LA, Mailer K, Krishnamurti C, Antholine W, Petering DH.Interaction of 2-formylpyridine thiosemicarbazonato copper (II) withEhrlich ascites tumor cells. BiochemPharmacol 1981;30:1595-604.
- West DX, Lockwood MA, Owens MD, Liberta AE. Copper (II)complexes of formylpyrazineN(4)-substituted thiosemicarbazones.Transit Met Chem 1997;22:366-71.
- Wang M, Wang LF, Li YZ. Antitumour activity of transitionmetal complexes with the thiosemicarbazone derived from3-acetylumbelliferone. Transit Met Chem 2001;26:307-10.
- Petering HG, Buskirk HH, Crim JA. The effect of dietary mineralsupplements of the rat on the antitumor activity of 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazone). Cancer Res 1967;27:1115-21.
- Kuncheria J, Jayasree S, Aravindakshan KK, Kuttan G. Antitumouractivity of some pyrazolone-copper complexes. Indian J Pharm Sci1994;56:37-40.
- Padhye S, Chikate R, Kumar A, Shallom JM, Chitnis MP. Novel,quinone-thiosemicarbazone hybrid (QTSCHY) non-platinum antitumoragents: Inhibition of DNA biosynthesis in P388 Iymphocytic cellsby coordinatively unsaturated copper (II) and iron (III) complexes ofnaphthoquinonethiosemicarbazones. Biometals 1992;5:67-71.
- Antholine WE, Knight JM, Petering DK. Some properties of copperand zinc complexes of 2-formylpyridine thiosemicarbazone. InorgChem1977;16:569-74.
- Deepa KP, Aravindakshan KK, Suhara F. Synthesis, characterizationand antifungal studies of transition metal complexes of N-methylandN-ethyl acetoacetanilideisonicotinylhydrazones. Asian J Chem2001;13:1549-59.
- Jayasree S, Aravindakshan KK. Structural and antitumour studies ofmetal complexes with thiosemicarbazones of β-diketoesters. Polyhedron1993;12:1187-92.
- Deepa KP, Aravindakshan KK, Suhara F. Synthesis, characterizationand antifungal studies of metal complexes of Benzoyl-andSalicyloylhydrazone of N-Methyl acetoacetanilide. Asian J Chem2001;13:1513-23.
- Weissberger A, Proskauer ES, Riddick JA, Toops EE. Organic Solvents.New York: Interscience; 1956. p. 7.
- Jeevana R. Metal Complexes of Thiosemicarbozons. Ph.D. Thesis.University of Calicut, 2012.
- Moldeus P, Hogberg J, Orrenius S. Isolation and use of liver cells.In: Fleischer S, Packer L, editors. Methods in Enzymology. Vol. 52.New York: Academic Press; 1978. p. 60-71.
- Ma Y, Mizuno T, Ito H. Antitumor activity of some polysaccharidesisolated from a Chinese mushroom, “huangmo”, the fruiting body ofHohenbueheliaserotina. AgricBiolChem 1991;55:2701-10.
- Segura JA, Barbro IG, Marquez J. Ehrlich ascites tumour unbalancessplenice cell population and reduces responsiveness of T Cellsto Staphylococcus aureusenteroxin B stimulation. ImmunolLett2000;74:111-5.
- Fecchio D, Sirois P, Russo M, Jancar S. Studies on inflammatoryresponse induced by Ehrlich tumor in mice peritoneal cavity.Inflammation 1990;14:125-32.