- *Corresponding Author:
- H. N. Shivakumar
Department of Pharmaceutical Technology, K. L. E. S’s College of Pharmacy, Rajajinagar 2nd Block, Bangalore-560010,India
E-mail: shivakumarhn@yahoo.co.in
| Date of Submission | 18 October 2005 |
| Date of Revision | 01 April 2006 |
| Date of Acceptance | 17 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6): 781-787 |
Abstract
A pH sensitive multi-particulate system intended to approximate the chronobiology of angina pectoris is proposed for colonic targeting. The system comprising of Eudragit S-100 coated pellets was designed for chronotherapeutic delivery of diltiazem hydrochloride. The drug loaded core pellets were produced by aqueous extrusion spheronization technique using microcrystalline cellulose as a spheronizing aid and PVP K 30 as a binder. Different coat weights of Eudragit S-100 were applied to the drug loaded pellets in an automatic coating machine to produce the pH sensitive coated pellets. Scanning electron microscopy revealed that the core pellets were discrete, spherical, or oval with a slightly rough surface whereas the coated pellets were covered with a uniform and continuous acrylic film. Optical microscopic image analysis portrayed that the values of aspect ratio and pellet circularity were close to 1.0, indicating the core pellets were almost spherical in shape. The friability with glass spheres was below 1.0%, signifying the core pellets produced were sufficiently hard. In vitro dissolution studies of the coated pellets performed following pH progression method showed that the drug release from the coated pellets depended on the coat weights applied and pH of the dissolution media. Since, diltiazem hydrochloride is a drug, which exhibits a high solubility, it would be possible to minimize drug release from the coated pellets below pH 7.0, and effectively release the drug at colonic pH only with higher coat loads (15-20% weight gain).
Chronotherapeutics refers to a clinical practice of synchronizing drug delivery in a manner consistent with the body’s circadian rhythm including disease states to produce maximum health benefit and minimum harm [1]. It is now recognized that episodes of angina pectoris, asymptomatic ischemia, acute coronary syndromes, sudden death, ventricular ectopic activity, and stroke all exhibit an increased incidence in the early mornings (i.e., 6 AM to 12 noon) [2,3]. This temporal pattern is dependent both on the staging of the specific circadian rhythms and the morning occurrence of triggers of the conditions. The former include day-night pattern differences in sympathetic drive, blood coagulation, blood pressure, heart rate, coronary blood flow, and myocardial oxygen supply versus demand. The morning triggers include the change from supine to upright posture, increase in physical exertion, and rise of mental and emotional load due to work onset or engagement in other activities. A therapeutic system that would synchronize the drug delivery with the circadian variation in periods of increased risk is highly desirable for an antianginal regimen.[3] This can be achieved by a bed time administration of a drug delivery system which with a delayed start of drug release can provide adequate protection in the early mornings. In this context colon specific drug delivery systems has been utilized for chronotherapeutic drug administration [4,5] Block, Bangalore-560.
The site-specific delivery of drugs to the colon has implications in a number of therapeutic areas, which include topical treatment of colonic disorders such as Crohn’s disease, ulcerative colitis, constipation, colorectal cancer, spastic colon, and irritable bowel syndrome. A colonic delivery system would additionally be valuable when a delay in absorption is therapeutically desirable in treatment of diseases like angina, which is influenced by circadian rhythms [6].
Colon specific delivery can be achieved with a suitable mechanism that triggers off the drug release upon reaching the colon. Various approaches have been reported over the last few years to target the drug to the colon such as pH-sensitive release [7], time-dependent release [8-10], and microbially triggered release [11].
The major concern with the time dependent release systems is the potentially large variation in the gastric emptying time of the dosage forms in humans, [12] which may result in premature or incomplete drug release. Moreover a time dependent system can be developed and scaled up only if modification in the design and appropriate technology is developed and available [6]. Microbially degradable systems though promising suffer from the constraint of pre-mature release of the drug load in the upper segment of the GI tract and cannot function optimally with out a protective coat [13]. The other drawback with these systems is the use of non-approved biodegradable carriers.
Methods based on pH sensitive delivery still continue to be a simple and practical means for colon targeting [7]. Possible variation in the GI transit time risking incomplete carrier disintegration and a subsequent therapy failure is thought to be reduced with this approach [14]. Polymers such as the Eudragit® products with regulatory approval are preferred by the industrial developers for colonic targeting [13]. Several acrylic polymers particularly Eudragit S-100, [7] EudragitTMS [15] Eudragit® FS [16] and Eudragit® P-4135F [17] have been investigated for colonic delivery. Since the pH of the colon in normal subjects varies from 6.4 ± 0.6 to 7.5 ± 0.4 [18], these polymers have been designed to be soluble at pH values higher than 7, keeping in mind the pH prevalent in the large intestine.
Though a number of colonic drug delivery system have been proposed for chronotherapeutic administration, the systems reported were single unit dosage form [4,5]. Since the single unit forms are known to exhibit variable gastric emptying time and lower colon residence time, more emphasis was laid during the study to develop a multi-particulate system considering their advantages over single unit dosage forms. The multi-particulates are reported to exhibit higher colonic residence time [10], more predictable gastric emptying with gastric emptying being less dependent on the state of nutrition [19]. The aim of the present study was to investigate the feasibility of the combination of extrusion spheronization and pan coating to produce pH sensitive multi-particulates for colon targeting. Following a bedtime administration these coated multi-particulates are intended to maintain a low drug plasma concentration during night when the cardiovascular risk are reported to be the minimum and optimal therapeutic concentrations between 6 AM and 12 noon when the ischemic risk is found to be maximum.
Diltiazem hydrochloride is a benzothiazepine calcium channel antagonist used in treatment of angina pectoris [20].
The short biological half life (3-4 hour) and low dose (30-60 mg) of diltiazem coupled with good colonic absorption [21] makes it an ideal candidate for colon targeting.
Materials and Methods
Diltiazem hydrochloride was a gift sample from Anglo French Drug Company Ltd., Bangalore, Eudragit S-100 was generously donated by Rohm Pharma, Darmstadt, Germany. Microcrystalline cellulose (MCC), polyvinyl pyrollidone K 30 (PVP K 30) and triethyl citrate (TEC) were kindly donated by Zydus Health Care Ltd., Bangalore. The rest of the chemicals of analytical grade, supplied by S. D. Fine Chemicals, Mumbai included isopropyl alcohol, sodium hydroxide, potassium dihydrogen phosphate, disodium hydrogen phosphate.
Preparation of the drug-loaded pellets
The process of aqueous extrusion spheronization was employed to produce the drug loaded core pellets of diltiazem hydrochloride [15]. The dry mass consisting of diltiazem hydrochloride (20% w/w) and MCC (77% w/w) was blended in a rapid mixer granulator (Kevin RMG, model HSMG-10) for 10 min. The impeller speed was maintained at 80 rpm during the process of dry mixing. The uniform dry blend was granulated with aqueous solution of PVP K30, which was added at a rate of 135 g/ min over a period of 2 min. The impeller speed was set to 150 rpm during the process of addition. The wet mass was sheared at the chopper speed of 1000 rpm for 1 min followed by 3000 rpm for another 2 minutes. The wet mass was extruded immediately after granulation through a die of 1.0 mm diameter in gravity fed cylinder extruder (R. R. Enterprises, Thane, model 65) at the extruder speed of 70 rpm. The extrudate formed was immediately spheronized at a speed of 900 rpm for 5 minutes in a spheronizer (R. R. Enterprises, Thane, model 150) fitted with a radially cut stainless steel plate and dried in a oven at a temperature of 50° for 48 hours.
Acrylic film coating of the drug-loaded pellets
The acrylic film coating was performed in a automatic coating pan (Neocota model 15A, R & D model, Neomachine Mfg. Co., Kolkata) [22]. The coating dispersion was prepared by dispersing TEC in water to which IPA was added with stirring using a variable speed propeller stirrer (Remi Udyog, Mumbai). Eudragit S100 was added to the mixture and stirring continued to obtain homogeneous solution. Purified talc (#100/120) was dispersed in the coating solution and stirring was maintained at a low speed through out the coating process.
A #12/22 fraction of the pellets loaded with diltiazem hydrochloride was charged into the pan of automatic coating machine. Four batches of pellets with different coat weights ranging from 5 to 20% weight gain (w.g) were produced by intermittently applying the acrylic dispersion on the surface of the drug-loaded pellets. Samples of the coated pellets were withdrawn from the coating pan when theoretical weight gain of 5, 10, 15, and 20% was achieved and dried in the oven for 2 h at 50°. The detailed processing conditions for acrylic film coating are outlined in the Table 1.
| Core pellet composition (% w/w) | Coating dispersion composition | Processing condition for pan coating | |||
|---|---|---|---|---|---|
| (% w/w) | |||||
| DiltiazemHCl | 20 | Eudragit S-100 | 6.0 | Inlet air temp. | 40–50° |
| M.C.C | 77 | Triethyl citrate | 0.6 | Exhaust air temp. | 30–40° |
| PVP k-30 | 3 | Talc | 3.0 | Bed temp. | 40° |
| Purified Water | 5.0 | Spray rate | 5 g/min | ||
| IPA | Q.S | Spray nozzle diameter | 1 mm | ||
| Spray pressure | 4 Kg/cm2 | ||||
| Pan speed | 20 rpm | ||||
The composition of the core pellet and coating dispersion along with the processing conditions for acrylic film coating of diltiazem hydrochloride loaded pellets
Table 1: Core Pellet And Coating Dispersion Composition Along With The Processing Conditions For Acrylic Film Coating
Scanning electron microscopy (SEM)
Morphology and surface topography of the core and the coated pellets were studied by scanning electron microscopy [23] (SEM-JEOL, JSM-840A, Japan). The samples were mounted on the SEM sample stab, using a double-sided sticking tape and coated with gold (200A°) under reduced pressure (0.001 torr) for 5 min using an Ion sputtering device (JEOL, JFC-1100 E, Japan). The gold-coated samples were observed under the SEM and photomicrographs of suitable magnifications obtained.
Pellet shape
The shapes of core pellets were investigated by optical microscopic image analysis. The image analyzer consisted of a optical microscope (magnification 4×) linked to a computer and a digital camera (Labomed, India). The digitalized images were analyzed by image analyzing software (Digipro version 2, Labomed, India). The maximum (dmax) and minimum (dmin) Feret diameter, circumference and area were recorded for 100 pellets. The two parameters namely the aspect ratio [15] and the pellet circularity [23] were computed using the formulae, Aspect ration = dmax/dmin and Pellet circularity = 4πA/P, where A and P stands for the projected area and the perimeter of the pellet as seen through the microscope.
Particle size analysis
The core pellets were subjected to sieve analysis using a set of standard sieves (1700, 1400, 1000, 710, and 600 mm) in a vibratory sieve shaker for a period of 10 min [24]. The weight distribution data were fitted into log-normal distribution and the geometric mean diameter was computed from the log probability plots [25].
Friability
Friability of the core pellets were determined by subjecting 10 g of the core pellets of the #12/22 mesh fraction with 200 glass beads to abrasion in a automated USP friabilator (Electrolab EF-2, India) for 4 min at 25 rotation/min [26]. The abraded samples were sieved using sieve #22 mesh for 2min. The pellets retained on the sieve were weighed and % friability was calculated from the difference in the weight of pellets before and after friability.
Flow properties
The Carr Compressibility Index and Hausner ratio of the coated pellets were computed on the basis of tapped bulk density and poured bulk densities27. Tapped bulk density (ρt) was determined by taking 20 g of the pellets in 50 ml measuring cylinder and tapping it to a constant volume in a bulk density apparatus (Cambell Electronics, India). Poured bulk density (ρp) was determined by three- tap method using the same apparatus. Carr compressibility index = 100 (ρt-ρp)/ρp and Hausner ratio= ρt/ρb
Drug content estimation
The drug content of different batches of the coated pellets was estimated in triplicate. The coated pellets were allowed to equilibrate in phosphate buffer of pH 7.5 for 24 h. The solution was filtered (0.22 μm, Millipore, India) and assayed spectrophotometrically at 236 nm (Jasco V 530 UV visible spectrophotometer, Japan).
In vitro dissolution studies
Dissolution studies of the coated pellets were performed in triplicate employing USP XIII dissolution rate test apparatus-1 (Electrolab, TDT-06T, India) following pH progression method28 simulating the gastrointestinal tract conditions. Weighed quantities of the coated pellets were loaded into the basket of the dissolution apparatus, the pH changes were performed starting with 900 ml of 0.1 N hydrochloric acid for 2 h, mixed phosphate buffer of pH 5.5 for 1 hour, phosphate buffer of pH 6.8 for 2 hour followed by mixed phosphate buffer of pH 7.5 till the end of the test. The temperature of the dissolution fluid was maintained at 37±0.5° with a stirring speed of 100 rpm. The samples withdrawn every hour were filtered (0.22 μm, Millipore) and assayed spectrophotometrically at 236 nm. The raw dissolution data was analyzed to calculate the amount of drug released and percentage cumulative drug released at different time intervals.
Fourier transform infrared spectroscopy
The IR spectrum of the coated pellets was compared with that of diltiazem hydrochloride to confirm the chemical integrity of the drug in the formulations developed [29]. The samples were powdered and intimately mixed with dry powdered potassium bromide. The powdered mixture was taken in a diffuse reflectance sampler and the IR spectra recorded by scanning in the wavelength region of 2.5- 25 μm in a FTIR spectrophotometer (Jasco 460 Plus, Japan).
Results and Discussion
Microcrystalline cellulose was a key ingredient for effective aqueous spheronization. The formulation was designed to have enough of MCC for successful spheronization of pellets. Since pellets prepared without PVP K 30 were found to demonstrate high values of percentage friability (>15%), PVP K30 was used as a binder to impart sufficient mechanical strength to the core pellets. The friability with glass spheres was below 1% (0.71 ± 0.15), indicating that the pellets produced were sufficiently hard to withstand further processing including the acrylic film coating process in automatic coating machine.
The water content in the wet mass was critical to obtain a good yield and to produce spherical pellets. The diversity of the formulation components made it difficult to predict the exact amount of water for successful completion of spheronization. The amount of water required was empirically determined based on the visual inspection of the pellets produced. It was noticed that pellets produced with lesser water content were elongated (oval or dumb bell-shaped). When acceptable pellets were not obtained, the water content was adjusted and the batch repeated. The water to be added was finally optimized to 67.5% w/ w of the dry mass during granulation. The weight distribution data of the core pellets indicated that majority of the pellets fall in the size range of #12/22 mesh fraction. The yield of #12/22 mesh fraction was found to be good (93%) which was considered as an overall indicator of the process viability. Those which were retained on #12 were the doublets and triplets, while those passed through #22 were the fines. The particle size distribution data obtained from sieve analysis when represented as log-probability plots gave straight lines indicating a log-normal distribution. The geometric mean diameter of the batch which represents the size of 50% of the pellets were found to be 954.99 ± 1.17 μm.
The spheronizing time was found to be a critical processing variable that had an influence on the pellet shape. The pellets spheronized for a short time were clearly elongated (oval or dumb bell shaped). As the residence time in spheronizer was prolonged the pellets became spherical and rounder. It was also noticed that the pellets did not become rounder after 5 min of spheronization. The yield of the pellets (#12/22 mesh fraction) was also reduced as the spheronizing time was further increased. This was accompanied by progressive increase in the yield of fines, which can be ascribed to loss of bind in the pellets by continuous evaporation of water with increase in the spheronizing time. Based on these observations the spheronizing time was set to 5 min to obtain a good yield of spherical pellets (#12/22 mesh fraction). The effect of spheronizing time on the pellet roundness and process yield has been cited in the literature [30].
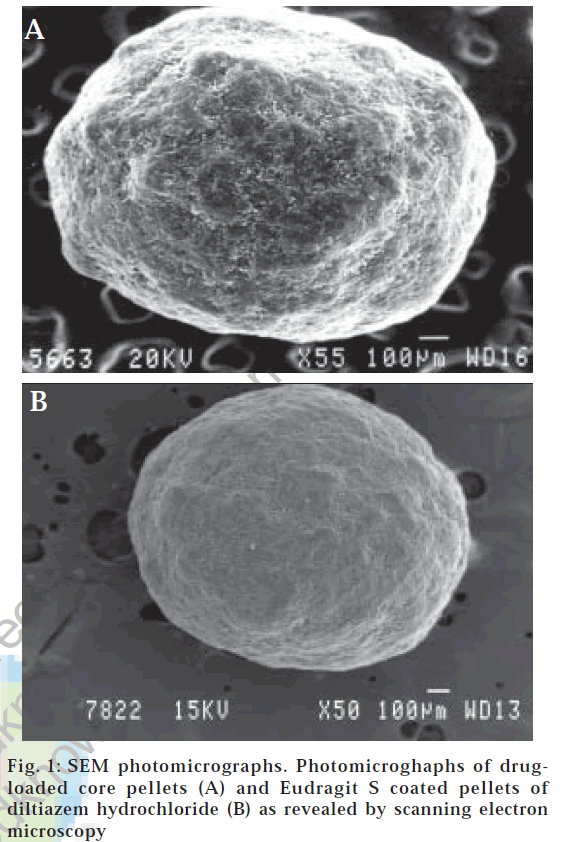
It was evident from SEM photomicrographs (fig. 1A) that the core pellets were discrete, spherical, or oval with a slightly rough surface. The rough surface is caused due to rapid loss of moisture from the wet mass with a high liquid content that results in a porous surface structure. Pellets originating from wet mass with a higher liquid content that are processed by granulation-spheronization tend to have a rough surface [30].
Aspect ratio and pellet circularity were two parameters selected for evaluating the pellet shape. The value of aspect ratio for the core pellets (1.11 ± 0.06) was found to be satisfactory and acceptable since the value of aspect ratio approaches 1.00 as the pellets become more spherical. The aspect ratio was found to be less sensitive to detect a significant difference between visually spherical batches of pellets. Pellet circularity was found to be the more sensitive of the two parameters selected. The circularity value of 1.00 corresponds to a perfect sphere. The circularity for the A batch of core pellets was found to be 0.98± 0.08 which indicated that the aqueous extrusion spheronization process was successful in producing spherical pellets by judicious selection of the processing and the formulation variables. Isopropyl alcohol was used as a solvent for the coating dispersion considering its high flash point (15°), low boiling point (82.3°), low heat of evaporation (667 J/g) and low risk of toxicity [31]. Talc being an important component of the coating dispersion is known to reduce the porosity of the acrylic film coatings and lower their water permeability. The particles of talc are reported to form a lattice structures, which are easily embedded in the polymer layers thereby significantly reducing the sticking during the film forming process31. As Eudragit S is a brittle polymer triethyl citrate was used at a concentration of 10% w/w to lower the glass transition temperature and promote formation of a good elastic film. Since TEC was a hydrophilic plasticizer,water was incorporated (5% w/w) to the spray dispersion to avoid phase separation between the polymer and plasticizer during film formation. The coating dispersion was sufficiently diluted to have 6.00% w/w of the polymer content with the intention to prevent any possible formation of aggregates during the acrylic coating process.
As higher temperatures stimulate sticking due to the softening tendencies of the polymer, the bed temperature was maintained close to room temperature or slightly higher by monitoring the inlet air temperature between 40 and 45°. With the aim to get fine spray droplets the spray nozzle of 1 mm was used at an atomization air pressure of 4 kg/cm2. The sticking tendency of the pellets was overcome by setting the pan rotation speed to 20 rpm and empirically controlling the spray dispersion application rate. The coating dispersion flow during the coating process was continuous with no spray system blocking, and the pellets showed no tendency to aggregate forming double or triple units. It was vivid from the photomicrographs (fig. 1B) of the coated pellets that the applied film was smooth, continuous and showed good adhesion to the cores. Coating loads of more than 20% were not employed because lower coat loads have advantages such as lower cost, reduction in processing time, lower weight and smaller size of the dosage formx [16].
The drug content of different batches of coated pellets were found to be 17.34 ± 0.46, 17.00 ± 0.49, 16.69 ± 0.37, and 16.25 ± 0.49 for pellets DH1, DH2, DH3 and DH4, respectively. The drug content was found to slightly decrease with increase in the coat weights applied. The values of the Hausner ratio and Carr Compressibility Index for pellets DH1, DH2, DH3, and DH4 pellets were found to range from 1.02 ± 0.01 to 1.06 ± 0.02 and 2.45 ± 1.09 to 6.36 ± 2.30, respectively which confirmed the free flowing nature of the coated pellets.
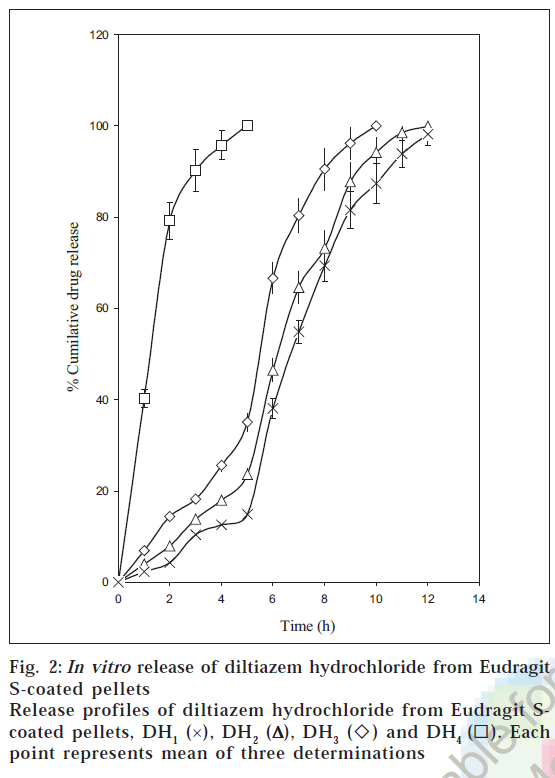
The dissolution profiles of the coated pellets with different weight gains are shown in fig. 2. The studies portrayed that the drug release from coated pellets depended on coat weights applied as well as the pH of the dissolution medium. Although Eudragit S 100 is a pH sensitive polymer with a threshold pH of 7.0 [32], it was not completely resistant to pH values below 7.0. This was due to the fact that pH sensitive Eudragits are ionized and solublised in a range of pH and not at a definite pH [7] . The pellets DH1 released all their contents within 5 h. of dissolution as they failed to have any control over the drug release. This can be attributed to the low coat weights applied (5% w.g).
The pellets DH2 released 35.08 ± 2.01% of the drug during the first 5 hours of dissolution in pH below 7.0. The acrylic coat applied was found to be permeable at pH values below 7.0, which could be ascribed to the limited thickness of the acrylic coat corresponding to 10% w.g. These pellets released 64.92% of the drug in pH 7.5 by the end of 10 h of dissolution. An amount of 23.65 ± 1.15% was found to be released at the end of 5 hours of dissolution from DH3 below pH 7.0. These pellets released 76.32% of the drug in pH 7.5 by the end of 12 h of dissolution.
The pellets DH4 exhibited a limited drug release (14.92 ± 0.72%) at the end of the first five hours of dissolution below pH 7.0 and at the same time released the entire drug by 12 h. The limited drug release during the first five hours can be attributed to the gastro resistant property of the acrylic film at the increased coat loads. The release rates were slower at higher coat weights, which could be due to increased diffusional path length and tortuosity at higher coating loads [7]. The pellets DH4 was found to be the best formulation as minimum drug was released below pH 7.0 and at the same time released the entire content (83.29%) at the colonic pH. The rapid release phase (burst effect) in the pH 7.5 can be ascribed to the enterosoluble nature of Eudragit S100 coupled with high drug solubility. The burst effect is reported to be characteristic of drug release from acrylic coated systems containing drugs with high solubility [33]. The rapid drug release following an initial lag phase would ensure adequate protection in the early mornings when the myocardial risk appears to be the highest.
The IR spectrum of diltiazem hydrochloride exhibited the characteristic absorption peaks at 3055.66 cm-1 (aromatic CH stretching), 3034.44 cm-1 (aromatic C-H stretching), 2965.98 cm-1 (aliphatic C-H stretching), 2837.74 cm-1 (O-CH3C-H stretching), 2389.37 cm-1 (amine HCl N-H stretching), 1743.33 cm-1 (acetate C=O stretching), 1679.69 cm-1 (lactum C=O stretching) 839.85 cm-1 (o-substituted aromatic C-H out-of-plane deformation), and 781.03 cm-1 (p-substituted aromatic C-H out-of-plane deformation). The IR spectrum of the coated pellets displayed the characteristic peaks of diltiazem hydrochloride at 2837.74 cm-1stretching), 1743.33 cm-1 (acetate C=O stretching), 1678.73 cm-1 (lactum C=O stretching) and 839.85 cm-1 (o-substituted aromatic C-H out of plane deformation). These IR spectral observations confirmed the lack of chemical interaction between the drug and other excipients used.
The results collectively establish the industrial feasibility of the combination of aqueous extrusion spheronization and pan coating to develop pH sensitive multi-particulate systems. The coated multi-particulates produced by precisely monitoring the coat weights applied can be used to effectively target the actives to the colon in response to circadian rhythms. Bed-time dosing of these multi-particulates ensures that, the patients with angina and hypertension can be better protected in the early mornings (6 AM to 12 noon) when the cardiovascular risks appear to be greatest and the effect of traditional medication tends to wane.
Acknowledgements
The authors are grateful to Prof. B. G. Shivananda, Principal, Al-Ameen college of Pharmacy for his constant support during the present research. The authors would like to thank Dr. Suresh Venkataram, Vice President, Pharma Operations and Mr. Srinivas S Manager, Formulation development, Zydus Health Care Ltd.,Bangalore for the technical support provided during the research. They are also thankful to Anglo French Drug Co., Bangalore, for providing the gift sample of diltiazem hydrochloride.
References
- Smolensky, M.H. and Labreque, G., Pharmaceutical News, 1997, 4, 10.
- Pepine, C.G., J. Amer. Med. Assoc., 1991, 265, 386.
- Fisherman, W.H., Glasser, S., Stone, P., Deedwania, P.C., Johnson, M. and Fakouhi, D., Amer. J. Cardiol, 1999, 83, 507.
- Fukui, E., Miyamura, N., Uemura, K. and Kobayashi, M., Int. J. Pharm., 2000, 204, 7.
- Sangalli, M.E., Maroni, A., Foppoli, A., Zema, L., Giordano, F., Giordano, F. and Gazzaniga, A., Eur. J. Pharm. Biopharm., 2004, 22, 469.
- Kinget, R., Kalala, W., Vervoort, L. and Van den. Mooter, G., J. Drug.Target.1998, 6 (2), 129.
- Akhgari, A., Garekani, H.A., Sadeghi, F. and Azimaie, M., Int. J. Pharm., 2005, 305, 22.
- McNeill, M., Rashid, A., Stevens, H., Dispensing device, WO Patent No. 90/09168, 1990.
- Stevens, H., Rashid, A., Bakshaee, M., Dispensing device, US Patent No. 5, 474, 784, 1995.
- McNeill, M., Rashid, A., Stevens, H., Drug dispensing device, US Patent No. 5, 342, 624, 1994.
- Hiorth, M., Versland, T., Heikkila, J., Tho, I. and Sande, S.A., Int. J. Pharm., 2006, 308, 25.
- Adkin, D.A., Davis, S.S., Sparrow, R.A. and Wilding, I.R., J. Control. Release, 1993, 23, 147.
- Rubinstein, A., Drug Discovery Today: Technologies, 2005, 2(1), 33.
- Lamprecht, A., Yamamoto, H., Takeuchi, H. and Kawashima, Y., Eur. J. Pharm. Biopharm., 2005, 59, 367.
- Krogars, K., Heinamak, J., Vesalahti, J., Marvola, M., Antikainen, O., Yliruusi, J., Int. J. Pharm., 2000, 199, 187.
- Gupta, V.K., Beckert, T.E., Price, J.C., Int. J. Pharm., 2001, 213, 83.
- Lehmann, K and Hoss, W., European Patent No. 704, 207, 2001.
- Evans, D.F., Pye, G., Branley, R., Clarke, A.G., Dyson, T.J., and Hardcastle, J.D., astroenterology, 1982, 83, 1062.
- Follonier, N. and Doelker, E., S.T.P. Pharm. Sci., 1992, 2, 141.
- 20. Sweetman, S.C., In: Martindale, The Complete Drug Reference, 33rd Edn., Pharmaceutical Press, London, U.K., 2002, 874.
- Watts, P. J. and LisbethIllum, Drug Develop. Ind. Pharm., 1997, 23 (9), 893.
- Shivakumar, H.N, Sarasija, S., Venkataram, S., Indian J. Pharm. Sci., 2002, 64, 133.
- Alvarez, L., Concheiro, A., Gomez-Amoza, J. L., Souto, C. and Martinez, P. R., Eur. J. Pharm. Biopharm., 2003, 55, 291.
- Deasy, P.B. and Gouldson, M.P., Int. J. Pharm., 1996, 132, 133.
- Martin, A., Bustamante, P., Chun, A.H.C., In: Physical Pharmacy, 4th Edn., Waverly Pvt. Ltd., New Delhi 1995, 423.
- Goskonda, S.R., Hileman, G.A. and Upadrashta S.M., Drug Develop. Ind. Pharm., 1994, 20 (3),279.
- Davies, P., In; Gibson, M., Eds., Pharmaceutical Preformulations and Formulations. Interpharm, CRC, New York, 2004, 379.
- Rodriguez, M., Jose, L., Vila-Jato and Dolores, T., J. Control. Release, 1998, 55, 67.
- Chowdary, K.P.R. and GirijaSankar, G., Drug Develop. Ind. Pharm., 1997, 23, 325.
- Ranjana Chopra, Newton J.M., Goran, A. and Fridrun, P., Pharm. Dev. Tech., 2001, 6, 495.
- Lehmann, K., In; IssacGhebreSellassie, Eds., Multiparticulate Oral Drug Delivery, Marcel Dekker, New York, 1994, 51.
- Information sheets (Basic info 1/E), Eudragit® acrylic polymers for controlled release of active ingredients, Rohm Pharma, 1995.
- Kao, C-C., Chen, S-C. andSheu. M-T., J. Control. Release, 1997, 44, 263.
 ) and DH4 (
) and DH4 ( ). Each point represents mean of three determinations
). Each point represents mean of three determinations



