- *Corresponding Author:
- P. N. S. Pai
Department of Quality Assurance, Al-Ameen College of Pharmacy, Bangalore-560 027, India
E-mail: pnsanjaypai@rediffmail.com
| Date of Submission | 27 November 2003 |
| Date of Revision | 19 August 2005 |
| Date of Acceptance | 26 July 2006 |
| Indian J Pharm Sci,2006, 68 (4):501-502 |
Abstract
A new simple, colorimetric method was developed on the basis of a chemical reaction of amine group in ambroxol hydrochloride with carbon disulphide to form dithiocarbamic acid, which on further reaction with cupric chloride forms a colored copper chelate. The yellowish-orange chromophore has absorption maxima of 448 nm and obeys Beer's law in the concentration range of 10-100 µg/ml. Results of the analysis were statistically validated by recovery studies. The method was found to be suitable for routine determination of ambroxol hydrochloride in tablet formulation.
Chemically ambroxol hydrochloride is trans-(2-amino-3,5dibromobenzyl) amino cyclohexanol hydrochloride. Ambroxol hydrochloride has a significant importance as a potent expectorant and is used as mucolytic agent [1]. It is administered as hydrochloride in a daily dose of 30-120 mg. Literature survey reveals that the drug is determined by spectrophotometry [2-4], HPLC [5-10], GC [11-12] and potentiometry [13] methods. The main objective of the present study was to develop a sensitive and selective colorimetric method for the estimation of ambroxol hydrochloride by exploiting its amino group, using dithiocarbamic acid. A Shimadzu UV/ Vis Spectrophotometer (UV 1601) with 1 cm matched quartz cell was used. A standard solution of ambroxol hydrochloride was prepared by dissolving 100 mg of ambroxol hydrochloride in methanol, the volume was made up to 100 ml with methanol in a 100 ml volumetric flask to get a solution of strength 1 mg/ml. A 10% v/v aqueous acetic acid solution was prepared by diluting 10 ml of glacial acetic acid to 100 ml with distilled water. Carbon disulphide-pyridine-isopropyl alcohol reagent (CPI reagent) was prepared by mixing 35 ml of carbon disulphide, 25 ml of pyridine and 65 ml of isopropyl alcohol in accurate quantities. Cupric chloride solution was prepared by dissolving 0.12 g of cupric chloride in 250 ml of water and further diluted to 500 ml with pyridine.
To 1 ml of the standard ambroxol hydrochloride solution (1 mg/ml) taken in a separating funnel, 4 ml of CPI reagent and 2 ml of cupric chloride reagent were added. The mixture was then allowed to stand for 10 min and then vigorously agitated. Further 3 ml of aqueous acetic acid solution and 3 ml of benzene were added. The mixture was agitated for 5 min and the two liquid phases were allowed to separate. The upper organic layer was collected in a 10 ml volumetric flask, the volume made up with isopropyl alcohol and allowed to stand for 20 min. The solution was scanned in the wavelength region of 380-780 nm against reagent blank. The absorption maxima of the yellowish orange chromogen was found to be 448 nm. The method was validated for fixing optimum concentration and volume required for reagents to show maximum absorbance, stability of color and order of mixing. The standard curve was then plotted for linearity (concentration vs absorbance) and was found to be in the range of 10-100 μg/ml. The molar absorptivity was determined to be 4.03×104 l/mol×cm and Sandell’s sensitivity 10.35 μg/cm2- 0.001 absorption units. A regression equation (y=a+bx) was obtained by linear least squares treatment of the results, established slope (b) of 0.004397 and intercept (a) 1.871, with standard deviation of 0.020 and coefficient of variation 0.005. The accuracy and reliability of the method was proved through recovery studies.
The method was extended for determination of ambroxol hydrochloride in tablet formulation. The marketed tablet formulations Ambrolite (Tablets India, Chennai) and Mucolite (American Remedies, Hyderabad) both containing 30 mg of ambroxol hydrochloride per tablet were used. A total of 20 tablets of Ambrolite each containing 30 mg of ambroxol hydrochloride were crushed and powder equivalent to 100 mg of ambroxol hydrochloride was weighed and dissolved in methanol. The volume was made up to 100 ml with methanol. The solution was then filtered with Whatman filter No 42. To aliquot solutions of sample prepared, the same method as described above was followed. The absorbance values obtained for sample solution were correlated with standard calibration curve to obtain the concentration. Sample analysis at three levels, in the concentration of 20, 40 and 80 μg/ml gave assay of 98.3, 101 and 99.3% indicating conformance of ambroxol content as per the label claim in the formulation. To ensure ruggedness of the method, the proposed method was applied for the analysis of ambroxol hydrochloride in Mucolite tablets. The results conformed to the label claim of ambroxol hydrochloride.
To ascertain accuracy of the method, recovery studies were carried out at three levels by adding 0.2, 0.4 and 0.6 ml of standard solutions of ambroxol hydrochloride to previously analyzed samples in a 10 ml volumetric flask. The mixtures were reanalysed by the proposed method. The results are presented in Table 1. The data from the recovery studies indicated no interference of excipients from the formulation. The developed method was thus found to be sensitive, accurate, precise and reproducible and can be used for the routine determination in bulk and in dosage forms.
| Sample ambroxol hydrochloride (µg/10 ml) | Standard ambroxol hydrochloride (µg/10 ml) | Amount of ambroxol from standard graph* (µg) | Recovery of standard (µg/10 ml) | Standard deviation (and coefficient of variance) | % recovery |
|---|---|---|---|---|---|
| 300 | 200 | 504 | 204 | 0.51 (0.01) | 102 |
| 300 | 400 | 694 | 394 | 0.75 (0.06) | 98.5 |
| 300 | 600 | 894 | 594 | 0.98 (0.03) | 99 |
*Average of 3 readings
Table 1: Recovery Studies From Tablet Formulations
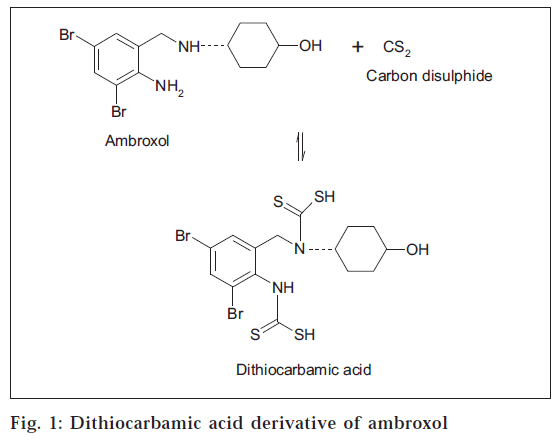
The proposed colorimetric method is based on the principle of chemical reaction [14] of primary and secondary amines in ambroxol hydrochloride with carbon disulphide to form dithiocarbamic acid as shown in fig. 1, which on further reaction with cupric chloride forms a colored copper chelate, which is estimated colorimetrically.
Acknowledgements
The authors thank Prof. B. G. Shivananda, Principal, Al-Ameen College of Pharmacy, Bangalore for providing all necessary facilities and Astra Zeneca Pharmaceuticals, Bangalore for the pure drug sample of ambroxol hydrochloride.
References
- Reynolds, J.E.F., Eds., In: Martindale; The Extra Pharmacopoeia, 30th Edn., The Pharmaceutical Press, London, 1993, 743.
- Perezruis, T., Martinezlozano, C., Sanz, A. and Sanmiguel, M.T., Talanta, 1996, 43, 1029.
- Narayana Reddy, M., KannaRao, K.V., Swapna, M. and Sankar, D.G., Indian J. Pharm. Sci., 1998, 60, 249.
- Indrayanto, G. and Handayani, R., J. Pharm. Biomed. Anal., 1993, 8, 781.
- Nieder, M. and Jaeger, H., J. High. Resolut. Chromatogr. Commun.,1986, 9, 561.
- Botterblom, M.H.A., Janssen, T.J., Guelen, P.J.M. and Vree, T.B., J. Chromatogr. Biomed. Appl., 1987, 65, 211.
- Floresmurrieta, F.J., Hoyovadillo, C., Hong, E. and Castanedahernandez, G., J. Chromatogr. Biomed. Appl., 1989, 82, 464.
- Brizzi, V. and Pasetti, U., J. Pharm. Biomed. Anal., 1990, 8 , 107.
- Kitsos, M., Gandini, C., Massonlini, G., Delorenzi, E. and Caccialanza, G., J. Chromatogr., 1991, 553, 1.
- Nobilis, M., Pastera, J., Svoboda, D., Kvetina, J. and Macek, K., J. Pharm. Biomed. Anal., 1992, 251.
- Schmid, J., J. Chromatogr. Biomed. Appl., 1987, 58, 65.
- Colombo, L., Marcucci, F., Marini, M.G., Pierfederici, P. and Mussini, E., J. Chromatogr. Biomed. Appl., 1990, 95, 141.
- Zarapakar, S.S. and Rana, S.H., Indian Drugs, 2000, 37, 246.
- Siggia, S. and Hanna, J.G., In: Quantitative Organic Analysis, 30th Edn., Wiley Intersciences Publication, New York, 1979, 615.