- Corresponding Author:
- Pandita N
Sunandan Divatia School of Science, SVKM′s NMIMS University, Vile Parle (W), Mumbai-400 056, India
E-mail: nancy.pandita@nmims.edu
| Date of Submission | 11 November 2015 |
| Date of Revision | 03 January 2016 |
| Date of Acceptance | 23 February 2016 |
| Indian J Pharm Sci 2016;78(1):129−135 |
This is an open access article distributed under the terms of the Creative Commons Attribution−NonCommercial−ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non−commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Draksharishta is an ayurvedic polyherbal formulation is prescribed for digestive impairment, respiratory disorders and weakness. Though the formula composition and therapeutic claims of draksharishta are part of the Ayurvedic Formulary of India, the scientific methods for its quality and safety evaluation are yet to be documented. The current work is an attempt to evaluate the quality parameters of draksharishta which has been checked vis a vis herbs used in the formulation by modern scientific control procedures like macroscopic and microscopic study, physico-chemical analysis, preliminary phytochemical analysis, thin layer chromatography and high performance thin layer chromatography to fix the quality standard of this formulation with reference to two marketed formulations i.e. M1 and M2, respectively. The quality control parameters were within the limit as per the Ayurvedic Pharmacopeia of India which signifies good quality and purity of the plant materials. Thin layer chromatography profiles showed the presence of gallic acid, catechin and resveratrol and further it was confirmed by HPTLC fingerprints. The results obtained can be used by pharmaceutical companies as quality control parameters in order to have a proper quality check during processing.
Keywords
Draksharishta, HPTLC fingerprints, pharmacognosy, TLC profile.
Ayurveda “The Science of Life” is the oldest form of healthcare system present in our traditional system of medicine that originated in India thousands of years ago. The aim of Ayurveda is to protect health and prolong life by eliminating diseases and dysfunctions of the body [1]. The Ayurvedic system as an “alternative system of medicine” has become substantial in the post-GATT era [2]. Ayurvedic system of medicine is a plant base, mineral base and animal base system of medicine that meets 70-80% of the healthcare needs of India [3]. The increasing need for safer drugs, efforts are taken to check quality, efficacy and safety of these ayurvedic formulations [2]. Ayurvedic formulations are present in various dosage forms such as solid dosage forms (pills, powders), liquid dosage forms (asavas, aristhas) and semisolid dosage forms (ghritas, avlehas) [4].
Asava and arishta i.e. sadhana kalpana are considered to be the unique and best dosage form discovered by Ayurveda [5]. Asavas are prepared by the fermentation of herbal juices and arishtas are prepared by the fermentation of the decoction of plants. Both are alcoholic medication and are also known as medicinal wine [6]. There are about 79 asavas and arishtas of which 38 are arishtas [7]. They are the high potency polyherbal formulations which are used as appetizers and stimulants [8]. This dosage form has a characteristic feature of self-generating alcohol which contributes to its indefinite shelf life, quick absorption, action and excellent therapeutic efficacy as compared to other ayurvedic herbal medicines [6-9].
Draksharishta is an ayurvedic herbal formulation with draksha (raisins) as chief ingredient prescribed for digestive impairment, respiratory disorders and weakness [10-13]. Draksharishta contains 5–10% of self-generated alcohol in it [10]. This self-generated alcohol and the water present in the product acts as a medium to deliver the water and alcohol soluble active herbal components to the body [14]. The formulation consist of 10 ingredients which are fruit of Vitis vinifera (VV), stem bark of Cinnamomum zeylanicum (CZ), leaf of Cinammomum tamala (CT), fruit of Piper nigrum (PN), fruit of Piper longum (PL), flower of Callicarpa macrophylla (CM), fruit of Embelia ribes (ER), stamen of Mesua ferrrea (MF), seed of Elleteria cardamomum (EC), flower of Woodfordia fructicosa (WF) [10] (Table 1).
| Herbs | Code | Part used | Voucher number |
|---|---|---|---|
| Vitisvinifera | VV | Fruit | F−202 |
| Cinnamomum | CZ | Stem bark | S/B−140 |
| zeylanicum | |||
| Callicarpamycrophylla | CM | Flower | I/F−040 |
| Woodfordiafructicosa | WF | Flower | I/F−041 |
| Piper nigrum | PN | Fruit | F−200 |
| Piper longum | PL | Fruit | F−203 |
| Embeliaribes | ER | Fruit | F−209 |
| Mesuaferrea | MF | Stamens | I/F−042 |
| Cinnamomumtamala | CT | leaves | L−071 |
| Elettariacardamomum | EC | Seed | F−201 |
Table 1: Authentication of 10 plant samples present in the formulation draksharishta.
Draksharishta is a polyherbal ayurvedic formulation. In this Polyherbal formulation the combining effect of different medicinal herbs helps to enhance the potency of the formulation. So the absence of any ingredient or addition of different part or plant alters the therapeutic value of the medicine [15]. The heterogeneous nature of ayurvedic polyherbal medicines like arishtas or asavas necessitate the continuous monitoring of the quality, efficiay and safety of these formulations [16]. Hence there is a need for the quality control check of these ayurvedic products. Thus, the aim of the present study is to evaluate the quality control parameters of the plant samples that are used as ingredients in the preparation of draksahrishta which include organoleptic characters, microscopic analysis, physicochemical parameters, phytochemical analysis, development of TLC profile and HPTLC fingerprints as per the WHO, Indian Pharmacopoeia and Ayurvedic Pharmacopoeia guidelines,
Materials and Methods
The analytical grade of organic solvents and standards were procured from Merck specialities Pvt Ltd. (Mumbai). Resveratrol (≥99% purity) was purchased from Sigma Aldrich, catechin (>95% purity) was purchased from Natural Remedies and gallic acid (≥99.5% purity) was purchased from Loba Chemie.
Plant materials and formulations
The plant samples used as ingredients in draksharishta were procured from ayurvedic Pharmacy from the local market (Mumbai). It was authenticated by Agharkar Research Institute, Pune and voucher numbers were alotted for each plant sample is reported in Table 1. Materials were stored in air tight containers at temperature of 25o. The two marketed formulation of draksharishta were purchased from the local manufacturers. One batch of in house formulation of draksharishta was prepared as per Ayurvedic Formulary of India Part I.
Quality evaluation of raw materials
Following quality control parameters were determined using pharmacopoeial methods and compared with the limits mentioned in the documented reports
Organoleptic characterization
Organoleptic characteristics viz. colour, odour, taste, and texture of the plant samples were carried out as per the procedure given in Indian Pharmacopoeia.
Determination of total ash content
2.0 g of plant powder sample was taken in a pre-weighed empty silica crucible and incinerated at 450° in a muffle furnace till it turned into white showing the absence of carbon. Then the crucible was kept for cooling down in a desiccator to avoid atmospheric moisture. Total ash content was determined with reference to powder plant sample taken initially.
Determination of acid-insoluble ash
Acid-insoluble ash content was performed by adding 25 ml of dilute hydrochloric acid to the ash obtained previously under the ash limit test. It was boiled for 5 min and filtered through ash less filter paper, washed thoroughly with hot water, and the residue together with the filter paper was kept in a muffle furnace and ignited for 3 h in a pre-weighed silica crucible. Crucible was allowed to cool completely in a desiccator and weight was recorded. The procedure was repeated until a constant weight was obtained.
Determination of moisture content
In pre-weighed porcelain 2.0 g of powder sample was taken and dried at 105° in an oven for one hour initially. Subsequently it was weighed at an interval of 30 min and dried till constant weight obtained. The dish was allowed to cool in a dessicator and then the weight of dried sample was recorded. The percentage of moisture content was determined with reference to powder sample taken initially.
Determination of alcohol soluble extractive values
Four grams of powder sample was macerated with 100 ml ethanol in a glass stopper closed flask for 12 h. Solution was then filtered and 25 ml for filtrate was transferred to a pre-weighed porcelain dish and kept in the oven at 105° till it evaporated to dryness. The residue was weighed and the percentage of the alcohol extractive value was determined with reference to the filtrate taken.
Determination of water soluble extractive values
Four grams of powder sample was macerated with 100 ml distilled water in a glass stopper closed flask for 12 h. Solution was then filtered and 25 ml for filtrate was transferred to a pre- weighed porcelain dish and kept in the oven at 105° till it evaporated to dryness. The residue was weighed and the percentage of the water extractive value was determined with reference to the filtrate taken.
Microscopic characterization
Plant materials were microscopically characterised with reference to the monographs of the plant material documented in the Ayurvedic Pharmacopeia of India and authorised text book [17-18].
Extract preparation
Fifty grams of each plant material was subjected to soxhlet extraction with n-hexane (300 ml) and ethanol (300 ml) successively and the percentage yield was calculated with reference to the sample taken.
Phytochemical investigation
A preliminary phytochemical test were carried on the extract of each plant sample to check the presence of carbohydrates, proteins, amino acids, steroids, alkaloids, flavonoids, tannins and phenolic compounds [19].
Preparation of draksharishta
The one batch of in-house formulations of draksharishta was prepared by the method as given in Ayurvedic Formulary of India, Part-I. The ingredients of draksharishta were procured from Local market, Mumbai. Identification of all the individual plant material was done as per Ayurvedic Pharmacopoeia of India. All these ingredients were authenticated by Agharkar Research Institute, Pune. According to method given in the standard book inhouse formulation was prepared in lab scale level. About 48.9 g dried fruits of Vitis vinifera after proper crushing were placed in brass vessel with prescribed quantity of distilled water (1L), and allowed to steep overnight. After overnight steeping, this material was boiled until the water for decoction reduced to one fourth of the prescribed quantity (0.25 l), then the heating was stopped and it was filtered through muslin cloth in cleaned vessel and after that 200 g of jaggery was added and stirred properly until it mixed well and filtered again. Then, to this filtrate 8 g of Woodfordia fructicosa (Dhataki flowers) and 1 g of coarsely powdered prakshepa dravyas as Cinnamomum zeyleynicum (stem bark), Eletteria cardamomum (seeds), Cinnamomum tamala (leaves), Mesua ferrea (stamens), Callicarpa macrophylla (flowers), Piper nigrum fruits), Piper longum (fruits), Embelia ribes (fruits) were added, stirred till mixed well and filtered again and this sweet filtered fluid was placed for fermentation. The fermented preparation was then filtered with muslin cloth and kept in cleaned bottles and labelled properly. Microbial analysis of the inhouse prepared formulation of draksharishta was carried out and certified at Bhavan’s Research Centre (Microbiology), Mumbai presented in Table 2.
| Sample | Aerobic viable | Yeast and mold | Escherichia | Pseudomonas | Salmonella | Staphylococcus |
|---|---|---|---|---|---|---|
| count (cfu/g) | count (cfu/g) | coli | aeruginosa | aureus | ||
| In house prepared formulation batch 1 | 15 | <10 | Absent | Absent | Absent | Absent |
Table 2: Microbial analysis of in house prepared formulation draksharishta.
Preparation of test sample
Fifty milliliter each of in house prepared and two marketed formulations were dried on a water bath until the alcohol was completely removed. Then 50 ml of water was added to the residue left behind. It was then subjected to successive solvent extraction, first with n-hexane (150 ml) followed by chloroform (150 ml) and ethyl acetate (150 ml). For further work, ethyl acetate fraction of the inhouse and two marketed formulations was evaporated to dryness and reconstituted with methanol.
Chromatographic analysis
A pre-coated TLC plates of Silica Gel 60 F254 (Merck) was used. Plates were developed in a glass twin trough chamber (CAMAG) pre-saturated with mobile phase for 10 min. The TLC system was optimized as per the three standards used i.e. gallic acid (toluene:ethyl acetate:formic acid 6:4:0.8) [20], catechin (toluene:ethyl acetate:formic acid 5:6:1) [21] and resveratrol (chloroform:ethyl acetate:formic acid 5:4:1) [22] and was observed at wavelengths 254, 254 and 306 nm, respectively. Further, the samples were subjected to HPTLC fingerprints using Camag Linomat V using a syringe of 100.0 μl capacity. Camag Scanner V equipped with win winCATS Planar Chromatography manager software version 1.4.6 was used as a scanning device.
Results and Discussion
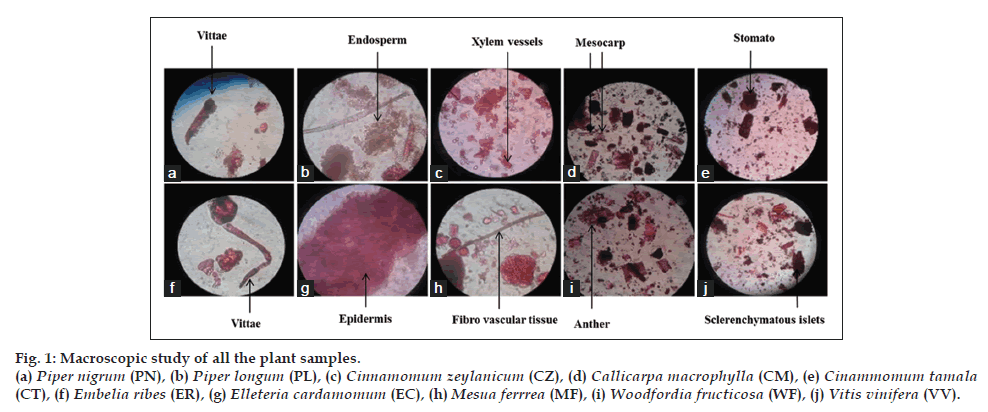
Macroscopic characterization of each plant sample used in formulation was done in terms of color, odour, taste and texture that authenticate the identity and quality of a plant samples Table 3. Microscopic inspection revealed similar observations in plant material as reported in the Ayurveda Pharmacopeia of India (fig. 1).
| Plants | Colour | Odour | Taste | Texture |
|---|---|---|---|---|
| PN | Blackish grey | Pungent | Pungent | Smooth |
| PL | Green | Pungent | Pungent | Coarse |
| CZ | Dark brown | Aromatic | Sweet | Smooth |
| CT | Green | Aromatic | Astringent | Smooth |
| CM | Light brown | Odourless | Bitter | Smooth |
| ER | Dark brown | Pungent | Pungent | Smooth |
| EC | Light brown | Aromatic | Astringent | Coarse |
| MF | Dark brown | Aromatic | Sweet | Smooth |
| WF | Light brown | Aromatic | Sweet | Smooth |
| VV | Light brown | Pleasant | Sweet | Coarse |
PN: Piper nigrum, PL: piper longum, CZ: cinnamomumzeylanicum, CT: cinammomumtamala, CM: callicarpamacrophylla, ER: embeliaribes, EC: elleteriacardamomum, MF: mesuaferrrea, WF: woodfordiafructicosa, VV: vitisvinifera
Table 3: Organoleptic characteristics of all the plant samples.
The total ash content determines the presence or absence of foreign matter such as metallic salt or silica. A high ash value indicates the presence of impurities and adulteration in the plant samples [23]. Acid insoluble ash indicates contamination with silicious materials e.g. earth and sand [24]. The evaluation of ash content and acid insoluble ash was within the limit as reported in Ayurvedic Pharmacopeia of India indicates the quality and purity of the plant samples as shown in Table 4.
| Plants | Total ash | Acid insoluble | Loss on drying | Alcohol soluble | Water soluble extractive |
|---|---|---|---|---|---|
| content (% w/w) | ash (% w/w) | (% w/w) | extractive values (% w/w) | values (% w/w) | |
| PN | 6.90 ± 0.06 | 0.01 ± 0.01 | 10.35 ± 0.05 | 6.20 ± 0.1 | 17.60 ± 0.6 |
| PL | 7.50 ± 0.25 | 0.01 ± 0.006 | 11.95 ± 0.1 | 18.60 ± 0.15 | 29.20 ± 0.3 |
| CZ | 3.00 ± 0.15 | 0.40 ± 0.1 | 7.85 ± 0.25 | 13.60 ± 0.25 | 6.40 ± 0.3 |
| CT | 2.33 ± 0.15 | 0.60 ± 0.1 | 6.77 ± 0.28 | 9.00 ± 0.05 | 10.60 ± 0.25 |
| CM | 7.70 ± 0.1 | 0.80 ± 0.1 | 5.72 ± 0.15 | 12.00 ± 0.15 | 12.40 ± 0.3 |
| ER | 5.40 ± 0.15 | 0.80 ± 0.11 | 7.28 ± 0.15 | 12.60 ± 0.15 | 13.20 ± 0.36 |
| EC | 5.70 ± 0.15 | 1.60 ± 0.26 | 14.28 ± 0.3 | 3.80 ± 0.2 | 19.80 ± 0.25 |
| MF | 5.10 ± 0.31 | 2.10 ± 0.26 | 7.92 ± 0.15 | 18.00 ± 0.05 | 16.00 ± 0.3 |
| WF | 9.70 ± 0.31 | 0.60 ± 0.15 | 9.18 ± 0.2 | 14.00 ± 0.06 | 34.80 ± 0.26 |
| VV | 2.80 ± 0.40 | 0.20 ± 0.007 | 3.09 ± 0.1 | 28.80 ± 0.35 | 86.60 ± 0.2 |
Table 4: Physicochemical parameters for all the plants samples.
The time duration for deterioration of the raw materials depends upon the amount of water present in it. If the water content is high, then the raw materials can be easily deteriorated due to fungus. The objective of drying fresh materials is to check hydrolytic reaction that might alter the chemical composition of the plant materials [23-25]. The moisture content of all the raw materials was found to be within the limit that indicates they were properly dried and stored Table 4.
The solubility of active constituents present in plant samples are determined by the extractive value. The alcohol soluble extractive values indicate the presence of polar components and the water soluble extractive values indicates the presence of non-polar components of the plant samples which were found within the limit as per the Ayurvedic Pharmacopeia of India Table 4.
The plant materials were subjected to successive soxhlet extractions and the percentage yield for ethanol extract for VV (w/w) was highest i.e. 26.42% while the hexane extract for CM (w/w) was highest i.e. 11.76% Table 5.
| Plants | Total yield of hexane | Total yield of alcoholic |
|---|---|---|
| extract (% w/w) | extract (% w/w) | |
| PN | 3.34 | 4.65 |
| PL | 4.44 | 22.40 |
| CZ | 2.74 | 10.51 |
| CT | 5.09 | 7.48 |
| CM | 11.76 | 2.44 |
| ER | 6.72 | 4.83 |
| EC | 5.33 | 1.26 |
| MF | 5.51 | 14.49 |
| WF | 1.40 | 10.03 |
| VV | 2.85 | 26.42 |
PN: Piper nigrum, PL: piper longum, CZ: cinnamomumzeylanicum, CT: cinammomumtamala, CM: callicarpamacrophylla, ER: embeliaribes, EC: elleteriacardamomum, MF: mesuaferrrea, WF: woodfordiafructicosa, VV: vitisvinifera
Table 5: Percentage yields of all the plant materials Percentage yields of all the plant materials.
Phytochemical evaluation showed the presence of medicinally active components in all the 10 plant samples. Flavonoids, Phenols and tannins were found to be present in the ethanol extract of all the plants and absence of all these phytoconstituents in the hexane extract as it is nonpolar solvent Tables 6 and 7.
| Plants | Test for | Test for | Test for | Test for | Test for | Test for | Test for tannins and |
|---|---|---|---|---|---|---|---|
| carbohydrates | proteins | amino acids | steroids | alkaloids | flavonoids | phenolic compouds | |
| PN | – | – | – | – | – | – | – |
| PL | – | – | – | – | – | – | – |
| CZ | + | – | – | – | – | – | – |
| CT | – | – | – | – | – | – | – |
| CM | – | – | – | – | – | – | – |
| ER | – | – | – | – | – | – | – |
| EC | – | – | – | – | – | – | – |
| MF | – | – | – | – | – | – | – |
| WF | – | – | – | – | – | – | – |
| VV | – | – | – | – | – | – | – |
+: Presence, –: absent. PN: piper nigrum, PL: piper longum, CZ: cinnamomumzeylanicum, CT: cinammomumtamala, CM: callicarpamacrophylla, ER: embeliaribes, EC:elleteriacardamomum, MF:mesuaferrrea, WF:woodfordiafructicosa, VV:vitisvinifera
Table 6: Qualitative screening of the hexane extracts of all the plants.
| Plants | Test for | Test for | Test for | Test for | Test for | Test for | Test for tannins and |
|---|---|---|---|---|---|---|---|
| carbohydrates | proteins | amino acids | steroids | alkaloids | flavonoids | phenolic compouds | |
| PN | – | – | – | + | + | + | + |
| PL | – | – | – | + | + | + | + |
| CZ | – | – | – | – | + | + | + |
| CT | – | – | – | – | + | + | + |
| CM | + | – | – | – | – | + | + |
| ER | – | – | – | – | + | + | + |
| EC | + | – | + | + | + | + | + |
| MF | – | – | – | – | + | + | + |
| WF | – | – | – | – | – | + | + |
| VV | + | – | – | – | – | + | + |
+: Presence, –: absent. PN: piper nigrum, PL: piper longum, CZ: cinnamomumzeylanicum, CT: cinammomumtamala, CM: callicarpamacrophylla, ER: embeliaribes, EC:elleteriacardamomum, MF:mesuaferrrea, WF:woodfordiafructicosa, VV:vitisvinifera
Table 7: Qualitative screening of the alcoholic extracts of all the plants.
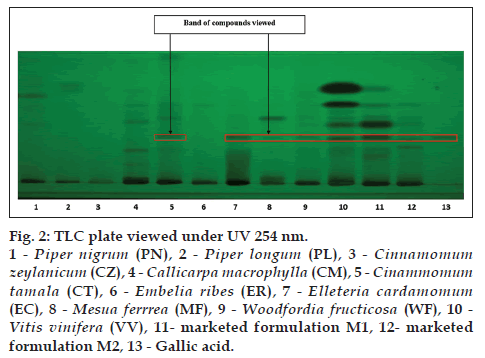
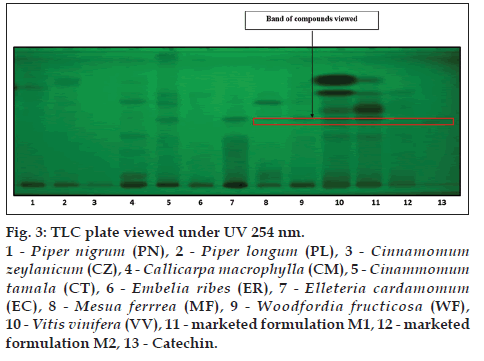
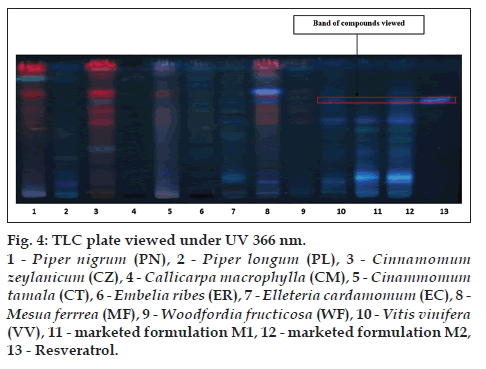
The alcoholic extracts of all the plant samples were chosen for the further analysis on the basis of phytochemical evaluation. TLC is specific method for the identification of chemical constituents present in the plant materials TLC was developed for all the plant samples along with two marketed formulation M1 and M2 by using gallic acid, catechin and resveratrol as standards. CT (lane 5), EC (lane 7), MF (lane 8), WF (lane 9), VV (lane 10), M1 (lane 11), M2 (lane 12) shows corresponding bands with gallic acid (lane 13) at 254 nm (fig. 2). MF (lane 8), WF (lane 9), VV (lane 10), M1 (lane 11), M2 (lane 12) shows corresponding bands with catechin (lane 13) at 254 nm (fig. 3). VV (lane 10), M1 (lane 11), M2 (lane 12) shows corresponding bands with resveratrol (lane 13) at 366 nm (fig. 4).
The HPTLC fingerprint has potential to determine authenticity and reliability of chemical constituents present in the plant samples. HPTLC analysis was performed which confirmed the presence of gallic acid (Rf-0.32), catechin (Rf-0.44), resveratrol (Rf-0.58) in the marketed formulation and ethanol extract of all plant materials (figs. 2-4), respectively and tabulated in Table 8, thereby showing that the marketed formulations contains all the plant ingredients and the standards as an authentic markers.
| Herbs | Standards |
|---|---|
| CT | Gallic acid (Rf=0.32) |
| EC | |
| MF | |
| WF | |
| VV, two marketed formulation | |
| CT | Catechin (Rf=0.44) |
| ER | |
| EC | |
| MF | |
| WF | |
| VV, two marketed formulation | |
| VV, two marketed formulation | Resveratrol (Rf=0.58) |
CT: Cinammomumtamala, EC: elleteriacardamomum, MF: mesuaferrrea, WF: woodfordiafructicosa, VV: vitisvinifera, ER: embeliaribes
Table 8: Presence of marker compounds in herbs present in the formulation Draksharishta.
From the present investigation it can be concluded that the study like macroscopic and microscopic analysis, physicochemical analysis and phytochemical analysis can be used as a first line for quality control study at industry level for raw material. As per the label claims of the two marketed formulations, TLC and HPTLC study confirmed the presence of gallic acid, catechin and resveratrol and the fingerprint match with the plant materials. The results obtained from this study could be used for routine monitoring of raw materials, formulations and the finished product which can lead to batch to batch consistency of ayurvedic polyherbal medicines like arishtas for its safety and efficacy.
Acknowledgements
The author is grateful to Dr. Aparna Khanna, Dean of School of Science, Mumbai for providing the necessary facilities for carrying out the experimental work and Herbal Research Lab, Ramarian Ruia College, Mumbai for extending laboratory competence and providing necessary amenities to carry out this work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Mishra AK, Gupta A, Gupta V, Sannd R, Bansal P. Asava and aristha?: An ayurvedic medicine – An overview. IntJ Pharm Biol Arch 2010;1:24-30.
- Vidyapeeth S, Sailor G, Seth A, Parmar K, Patel M, Shrirang P. Standardization of marketed drakshasava – A polyherbalayurvedic. Pharm SciMonit 2013;4:363-70.
- Viswanathan MV, Unnikrishnan PM, Komatsu K, Fushimi H, Basnet P. Brief introduction to ayurvedic system of medicine and some of its problems. Indian J TraditKnowl 2003;2:159-69.
- Kumar T, Larokar YK, Jain V. Standardization of different marketed brands of Ashokarishta? : An ayurvedic formulation. J SciInnov Res 2013;2:993-8.
- Chaudhary A, Singh N, Dalvi M, Wele A. A progressive review of Sandhanakalpana(Biomedical fermentation): An advanced innovativedosage form of Ayurveda. Ayu 2011;32:408-17.
- Sekar S, Mariappan S. Traditionally fermented biomedicines,arishtas and asavas from Ayurveda. Indian J TraditKnowl 2008;7:548-56.
- Kushwaha R, Karanjekar S. Standardization of ashwagandharishta formulation by TLC method. Int J Chemtech Res 2011;3:1033-6.
- Singh H, Mishra S, Pande M. Standardization of Arjunarishta formulation by TLC method. Int J Pharm Sci Rev Res 2010;2:25-8.
- Sayyad SF, Randive DS, Jagtap SM, Chaudhari SR, Panda BP. Preparation and evaluation of fermented ayurvedic formulation: Arjunarishta. J Appl Pharm Sci 2012;2:122-4.
- Anonymous. Ayurvedic Pharmacopoeia of India Monographs. Part II. Vol. 2. New Delhi: Government of India; 2009. p. 36-8.
- Tiwari P, Patel RK. Total phenolics and flavanoids and antioxidant potential of draksharishta prepared by traditional and modern methods. Res J PharmacognPhytochem 2012;4:244-9.
- Tiwari P, Patel RK. Chances of reduction in cardiovascular risk by induced diabetic condition. Pharmacologyonline 2010;2:583-90.
- Tiwari P, Patel RK. Development and validation of HPTLC method for quantification of quercetin and rutin in draksharishta. Asian J Pharm SciRes 2012;2:7-18.
- Chakrabortty S, Ahmed Z. Study of antimicrobial activity of ayurvedicand unani medicine and their comparative analysis with commercial antibiotics. Impact Int J Res Appl 2013;1:63-74.
- Gupta P, Tiwari AK, Chaturvedi A. Research article standardization of ayurvedic formulation – Kutajadikashaychurna. J Chem Pharm Res 2013;5:34-8.
- Tiwari S. Plants: A rich source of herbal medicine. J Nat Prod 2008;1:27-35.
- Anonymous. The Ayurvedic Pharmacopoeia of India. Part II. Vol. 1. New Delhi: Government of India; 2010. p. 140-1.
- Khandelwal KR. Practical Pharmacognosy. 4th ed. New Delhi: NiraliPrakashan; 2005.
- Vaghasiya Y, Dave R, Chanda S. Phytochemical analysis of some medicinal plants from western region of India. Res J Med Plant 2011;5:567-76.
- Leela V, Saraswathy A. Quantification of pharmacologically active markers gallic acid, quercetin and lupeol from acacia leucophloea wild flowers by HPTLC method. J Anal Bioanal Tech 2013;4:2-5.
- Dharmender R, Madhavi T, Reena A, Sheetal A. Simultaneous quantification of bergenin, (+)-catechin, gallicin and gallic acid; and quantification of b-sitosterol using HPTLC from bergeniaciliata(Haw.) sternb. Forma Ligulata Yeo (Pasanbheda). Pharm Anal Acta2010;1:1-9.
- Rolfs CH, Kindl H. Stilbene synthase and chalcone synthase 1. Plant Physiol 1984;75:489-92.
- Jarald EE. Textbook of Pharmacognosy and Phytochemistry. 1st ed. New Delhi: CBS Publication; 2007. p. 57-64.
- Mukherjee PK. Quality Control of Herbal Drugs. 1st ed. New Delhi: Business Horizons Publishers; 2003. p. 701-25.
- World Health Organization (WHO). Quality Control Methods for Medicinal Plant Materials. New Delhi: AITBS Publishers; 2002. p. 46-51.