- Corresponding Author:
- Y. Darwis
Discipline of Pharmaceutical Technology, Universiti Sains Malaysia, 11800 Penang, Malaysia
E-mail: yusrida@usm.my
| Date of Submission | 12 May 2014 |
| Date of Revision | 23 January 2015 |
| Date of Acceptance | 02 August 2015 |
| Indian J Pharm Sci 2015;77(4):422-433 |
This is an open access article distributed under the terms of the Creative Commons Attribution−NonCommercial−ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non−commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Cinnamon leaf oil contains a high percentage of eugenol and has antimicrobial, antioxidant and antiinflammatory properties. However, the undiluted oil can cause irritation to the skin. Therefore, the aims of this study were to develop and evaluate cinnamon leaf oil nanocream using palm oil. Nanocream base was prepared using different ratios of oil, surfactants and water. The surfactant used were mixture of Tween 80:Carbitol or Tween 80:Span 65 at different hydrophile-lipophile balance values. The pseudoternary phase diagrams were constructed to identify the nanocream base areas and the results showed that the nanocream bases using Span 65 as co-surfactant produced bigger cream area. Fifteen formulations using mixtures of Tween 80:Span 65 were further evaluated for accelerated stability test, droplet size, zeta potential, rheological properties and apparent viscosity. The nanocream base which had an average droplet size of 219 nm and had plastic flow with thixotropic behavior was selected for incorporation of 2% cinnamon leaf oil. The nanocream containing cinnamon leaf oil had the average size of 286 nm and good rheological characteristics. Thein vitro release study demonstrated that eugenol as the main constituent of cinnamon leaf oil was released for about 81% in 10 h. The short-term stability study conducted for 6 months showed that the cinnamon leaf oil nanocream was stable at a temperature of 25° and thus, cinnamon leaf oil nanocream is a promising natural based preparation to be used for topical application.
Keywords
Nanocream, cinnamon leaf oil, pseudoternary phase diagram, topical application
Nanocream or semisolid emulsion is one of the pharmaceutical topical formulations that are applied externally[1,2]. The nanocream can be prepared by using high energy methods such as high shear stirring, high pressure homogenizers or ultrasound generators[3]. Generally, a nanocream is very useful in personal care and cosmetics because the small size of the droplets which are in the nano range of 100–600 nm[4] allow them to deposit uniformly onto the skin and enhances the efficient delivery of active ingredients through the skin[5,6]. Basically, the cream contains various drugs for different remedial properties in an appropriate semi solid base either hydrophobic or hydrophilic in character[7].
Cinnamon (Cinnamomum zeylanicum) leaf oil contains a high percentage of eugenol and has characteristically strong astringent properties, antibacterial[8], antiparasitic, antispasmodic[9] and antidiarrhea[10,3]. Thus, these herbs have been used for healing a number of diseases, such as cardiovascular, respiratory, digestive, immune, urinary, lymphatic, reproductive, nervous system complaints and several other disorders. In addition, cinnamon leaf oil also shows very effective mosquito repelling effect[11,12]. However, the undiluted oil can cause irritation if directly applied onto the skin[13,14]. Gosh et al. reported the use of cinnamon leaf oil microemulsion formulation for wound healing[15] , but no study has been done on the preparation of cinnamon leaf oil nanocream. Therefore, the objective of the present study was to prepare cinnamon leaf oil nanocream using palm oil as oil phase. Palm oil has been used mainly in food industry and its application as a pharmaceutical excipient is not widely studied. Palm oil has advantages because it has high content of antioxidants such as tocotrienol which prevent oxidation of oil and triglycerides which may function as natural surface active agents.
Materials and Methods
Palm oil (Seri Murni) was purchased from Tecso hypermarket (Malaysia), polysorbate 80 (Tween 80), cetostearyl alcohol and cinnamon leaf oil were purchased from Euro Chemo−Pharma Sdn Bhd (Malaysia), sorbitan tristearate (Span 65) was purchased from Fluka (USA), propyl paraben, methyl paraben and dethylene glycol monoethyl ether (Carbitol) were purchased from Sigma−Aldrich (USA), Sodium citrate and citric acid were purchased from R & M Chemicals (UK). Cellulose acetate membrane of 0.2 μm was purchased from Sterlitech (USA), potassium dihydrogen phosphate and di−potassium hydrogen phosphate were supplied by R and M Chemicals (UK).
Pseudo ternary phase diagram construction
Phase diagrams of a mixture containing palm oil, surfactants of different HLB values and water were constructed using the water titration method. The surfactants used in this study were mixtures of Tween 80:Carbitol at HLB values of 13.92 (90:10), 12.84 (70:30) and 10.64 (60:40) or Tween 80:Span 65 at HLB values of 13.71 (90:10), 11.17 (70:30) and 9.84 (60:40).
Oil and surfactant mixture were prepared at ratios of 9.0:1.0, 8.0:2.0, 7.0:3.0, 6.0:4.0, 5.0:5.0, 4.0:6.0, 3.0:7.0, 2.0:8.0, and 1.0:9.0 in a separated universal bottle. One ml of distilled water was added every fifteen minutes and the changes in the mixtures were recorded. The mixtures were kept for 24 h at room temperature to achieve equilibrium. Then, the final visual observation was recorded according to the classification shown in the Table 1. The conductivity of resulting mixtures was measured using electrical conductometer to classify them as an O/W emulsion or W/O emulsion. The results were plotted in the pseudoternary phase diagram.
| Classification | Description |
|---|---|
| Microemulsion | It is transparent or translucent and can flow easily |
| Liquid crystal | It is transparent or translucent and nonflowable |
| when inverted at 90° | |
| Emulsion | It is milky or cloudy and can flow easily |
| Emollient gel | It is milky or cloudy and nonflowable when |
| or cream | inverted at 90° |
Table 1: Visual Observation Classification.
Preparation of primary nanocream base
The primary nanocream base formulation was prepared by heating the oil and water phase in the water bath separately in two different beakers at 55° with continuous stirring at 350 rpm for 30 min using a magnetic stirrer. The oil phase consists of palm oil, propyl paraben (0.05%), and Span 65 while the water phase containing Tween 80, buffer pH 5.5 and methyl paraben (0.1%). The oil phase was dispersed in the water phase then continuously mixed using a magnetic stirrer at 350 rpm with the aid of spatula to overcome the formation of a liquid crystalline phase. After a while, the mixture was stirred at 1500 rpm for 30 min and homogenized using T25 Ultra−Turrax (IKA, USA) at 19,100 rpm for 2 min at room temperature for further characterization.
Preparation of cinnamon leaf oil nanocream
The properties of selected primary nanocream base were further improved by adding cetostrearyl alcohol as a rheological modifier. The nanocream bases were prepared according to the method used to prepare primary nanocream base and subjected to further characterization. The best nanocream base formulation was selected for incorporation of 2% cinnamon leaf oil. The oil phase of cinnamon leaf oil nanocream formulation consisted of cetostearyl alcohol, cinnamon leaf oil, palm oil, Span 65 and propyl paraben (0.05%) while the water phase consisted of Tween 80 and buffer pH 5.5. Similar method as mentioned above was also used for preparing cinnamon leaf oil nanocream formulations.
Accelerated stability study
Two methods were used in the accelerated stability study: centrifugation and heating cooling cycle. In centrifugation method, cream formulation was placed in the graduated tubes and centrifuged at 4000 rpm for 30 min (Eppendorf centrifuge 5702 R). In the heating cooling cycle method, the nanocream base sample was repeatedly subjected to two different temperatures. Firstly, the cream formulation was placed in a graduated tube and freezed at temperature − 8° for 24 h followed by storing at 45° for 24 h to complete 1 cycle. The experiment was repeated for 6 cycles to determine the stability of the nanocream by observing separation and coagulation in the nanocream.
Droplet size measurement
The droplet sizes of the formulation were measured using Zeta Sizer 1000 HSA, (Malvern Instrument, UK) which is based on the basic principle of photon correlation spectroscopy. The sample was diluted with the buffer to get the K count in between 50−200 as required by machine consistency before reading the droplet size.
Zeta potential measurement
Zeta potential of the formulation was measured using Zetasizer Nano ZS (Malvern, UK). Zeta potential of the formulated nanocream was determined to ensure that they are within the limit of ±30 because within this value the droplets usually do not coalesce. The formulations were diluted with the same buffer solution used as the external phase in the formula to fix the ionic strength and reduce the droplet count. Bubbles were eliminated from the samples before measurement to prevent change in the mobility of the droplets in the samples.
Rheological and apparent viscosity measurements
The rheological measurements were carried out using rheometer (rheological instrument AB, Sweden). The system was equipped with a cone and plate measuring head (plate diameter 40 mm). About 0.5 g of the sample was placed on the plate and left to equilibrate with the controlled temperature (25°±0.1) for 3 min before bringing down the cone. Excess sample was swept away with tissue papers. The shear stress was applied in an increasing manner at the rate of 10 Pascal/sec and the rate measurements were recorded. Rheograms were drawn by plotting shear stress on the abscissa and shear rate on the ordinate.
As the creams usually exhibited non Newtonian flow, the rheological behaviors were studied according to the following equation: Log G=N log (S−F)–Log η… (Eq. 1). Where, G is the shear rate in sec−1, S is the shear stress in Pascal, F is the yield value, η is the viscosity and N is the slope of Log (S−F) against log G plot. When N is 1, plastic flow with Bingham model is indicated.
Transmission electron microscopy
The size and morphology of the cinnamon leaf oil nanocream was studied using FEI CM 12 high resolution TEM (Philips, Electron Optics, Eindhoven, Netherlands). The cinnamon leaf oil nanocream sample was placed on collodion formvar carbon film−coated 400 mesh copper grid held with self−locking fine forceps, and then a drop of 2% methylamine tungstate as a negative stain solution was added to the surface of the grid. The excess of stained solution on the sample was gently wiped off using filter paper. The grid was placed on a Petri dish lined with filter paper and left to dry for about 10 min at room temperature before examination under the microscope.
In vitro release study
The in vitro drug transport through the artificial cellulose acetate membrane was carried out using horizontally static type Franz diffusion cell. The Franz diffusion cell consisted of an effective diffusion surface area of 0.636 cm2 and a receptor cell volume of 5 ml. The static receptor cell was filled with 5 ml phosphate buffered saline (pH 5.7) containing 1% Tween 80 and stirred with a small magnetic bar at the speed of 500 rpm for uniform mixing. The receptor compartment was maintained at 37±0.5° using a circulating water bath. Cinnamon leaf oil nanocream (40 mg) was placed on the cellulose membrane surface facing donor compartment and 400 μl samples were withdrawn from the receptor compartment at predetermined time points of 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12 and 24 h. The sample withdrawn was replaced with 400 μl of phosphate buffer saline (pH 5.7) containing 1% Tween 80. The drug content in the collected samples was determined using a validated HPLC method. The mobile phase consisted of methanol and water (75:25 v/v) delivered at 1 ml/min in C18 Phenomenex column (250 mm×4.6 mm, 5 μm). The UV/Vis detector was set at the wavelength of 280 nm and the injection volume was 20 μl.
Stability study
The stability study was conducted at two different temperatures, 40±2°/75±5% RH and room temperature (25±2°/65±5%RH). The samples at temperature 40±2°/75±5% RH were placed in a stability chamber while samples at room temperature were left on a shelf. At periodic intervals of 1, 2, 3 and 6 months, all the samples which were stored at 40±2°/75±5% RH and at room temperature were studied for conductivity, pH, droplet size, apparent viscosity, yield value, flow characteristics, total eugenol content and in vitro release. The eugenol was assayed using HPLC method described above.
Statistical analysis
All parameters except in vitro release study were evaluated using one−way ANOVA and for identification of means that are significantly different from each other, a post hoc Tukey’s HSD test was performed. The difference was statistically significant if P<0.05. The in vitro release study statistical analysis was performed using a post hoc Dunnett test. SPSS version 20.0 software were used for this analysis and all values are expressed as mean±SD.
Results and Discussion
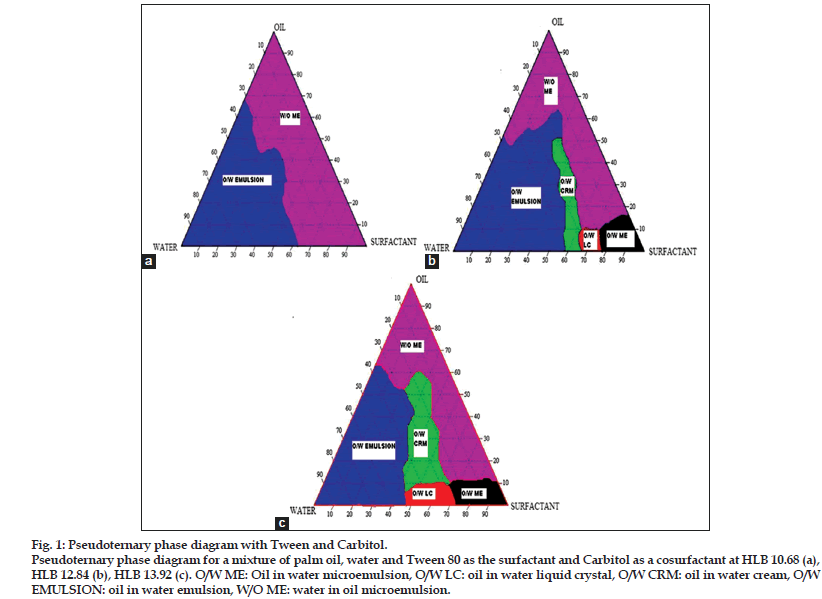
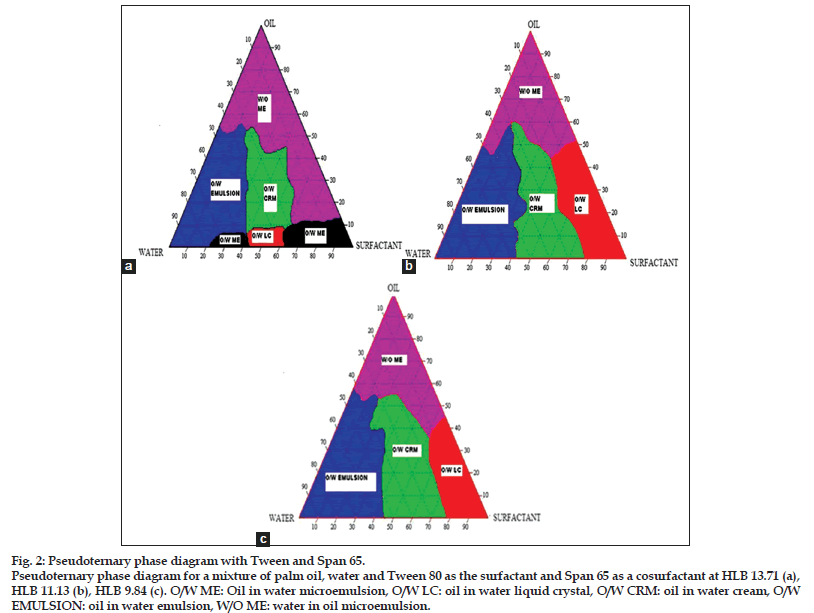
Construction of pseudoternary phase diagrams is the best way to study all kinds of formulations that can be derived from the mixing of surfactants, water and oil because the diagrams can cover all probabilities of mixing ratios and possible areas of finding cream[16]. Figs. 1a−c is the pseudoternary phase diagrams for a mixture of palm oil, water, Tween 80 as the surfactant and Carbitol as a cosurfactant with different HLB values of 10.68, 12.84 and 13.92. Fig. 1a showed no cream area present, but exhibited larger O/W emulsion and W/O microemulsion areas. This could be because the amount of Tween 80 was not enough to form a surfactant layer at the interface that is responsible for producing a cream system[17]. In contrast, the pseudoternary phase diagram represented in fig. 1b and c illustrated formation of small cream areas. Increase in the concentration of surfactant (Tween 80) and reduce the concentration of cosurfactant (Carbitol) resulted in a gradual increase in cream area. However, the combination of Tween 80 and Carbitol was the worst surfactant mixture because it produced a small cream region and the texture of the cream in this region was difficult to spread, sticky and did not have good skin feel. Therefore, it is not a suitable combination of surfactant in cream formulation. These surfactants combinations were excluded from further study. Pseudoternary phase diagrams for mixtures of palm oil, water, Tween 80 and Span 65 with different HLB values of 13.71, 11.13 and 9.84 are depicted in figs. 2a−c. All the phase diagrams of mixtures of Tween 80 and Span 65 showed a larger cream area compared to Tween 80 and Carbitol. The cream area was formed when water content in the system was in the range of 25 to 60%. It was found that water content below 25% was insufficient to hydrate the polyoxyethylene groups which were critical for the swelling of surfactant chains to demonstrate a cream or gel structure[17]. Increase in cosurfactant (Span 65) concentration from HLB 13.71 to HLB 9.84, would increase the interfacial tension of interfacial film and a larger cream area was formed[18,19]. The larger cream area was formed when suitable combination of Tween 80 as surfactant and Span 65 as cosurfactant was used in a cream formulation. Conductivity measurements revealed that each point in the cream area was of the O/W type because it conducted the electricity. Thus, this study suggested that Span 65 showed better cosurfactant action compared to the Carbitol because it produced larger cream area as shown in pseudoternary phase diagrams. Thus, it was a suitable combination with Tween 80 in producing the stable cream formulation. Fifteen cream formulations were randomly selected from the combination of Tween 80 and Span 65 with HLB 13.71, 11.13 and 9.84 and subjected to further study using an accelerated stability test to select the best and most stable formulation.
Figure 1: Pseudoternary phase diagram with Tween and Carbitol.
Pseudoternary phase diagram for a mixture of palm oil, water and Tween 80 as the surfactant and Carbitol as a cosurfactant at HLB 10.68 (a), HLB 12.84 (b), HLB 13.92 (c). O/W ME: Oil in water microemulsion, O/W LC: oil in water liquid crystal, O/W CRM: oil in water cream, O/W EMULSION: oil in water emulsion, W/O ME: water in oil microemulsion.
Figure 2: Pseudoternary phase diagram with Tween and Span 65.
Pseudoternary phase diagram for a mixture of palm oil, water and Tween 80 as the surfactant and Span 65 as a cosurfactant at HLB 13.71 (a), HLB 11.13 (b), HLB 9.84 (c). O/W ME: Oil in water microemulsion, O/W LC: oil in water liquid crystal, O/W CRM: oil in water cream, O/W EMULSION: oil in water emulsion, W/O ME: water in oil microemulsion.
Centrifugation is an excellent tool for the production of phase separation for accelerated stability study of nanocreams. The result of the centrifugation test was shown in Table 2. Some of the formulations underwent phase separation into two phases which was creamy at the top and clear solution at the bottom. It may have occurred due to Ostwald ripening in which molecules move as a monomer, and the coalescence of small droplets resulted in the formation of larger droplets by diffusion processes driven by the gain in surface free energy[19]. Among the formulations tested, the formulations coded with A1, B2, B4, and C1 showed no phase separation, creaming, cracking, coalescence or phase inversion during this centrifugation test. These formulations were considered to have passed the test and were then further examined using another accelerated stability test, heating cooling cycle. After undergoing heating and cooling for six cycles, some samples had separated into two layers, which was creamy at the top and clear solution at the bottom. However, samples B2 and B4 were partially separated (Table 3). This phase separation may have occurred due to the temperature quench during heating and cooling cycles[19].
Lastly, the formulations B2 and B4 were modified using cetostearyl alcohol. The percentages of cetostearyl alcohol were calculated from palm oil content in the primary formulations and the new formulations are shown in Table 4. In this formulation, cetostearyl alcohol acted as a stabilizer and thickening agent[20]. Normally, it is used widely in a variety of cosmetics and pharmaceutical emulsions. The new formulations were coded as B2(1), B2(2), B4(1) and B4(2). The accelerated stability test using centrifugation and heating cooling cycle methods were also carried out on these formulations. All the samples except sample B4(1) were found to be stable as no phase separation occurred. All formulations that passed the accelerated stability test were further analyzed in terms of droplet size, zeta potential and apparent viscosity.
Table 5 shows the results of average droplet size (below 250 nm), polydispersity (less than 1 and zeta potential measurement (about 30 mV) of samples B2(1), B2(2) and B4(2) after homogenizing. Increasing duration of homogenizing at constant speed would reduce the droplet size while prolonging the homogenizing time to 2.5 min would increase the droplet size. Longer homogenizing times may cause instability of particles due to high input of energy that leads to aggregation of the droplets into a larger ones[21]. There was a significant difference in the droplet size of samples B2(2), B2(1) and B4(2). Among the formulations, B2(2) had the smallest droplet size. All the formulations have higher zeta potential values whereby the repulsion force is bigger than the attraction force, so the cream is stable[22]. There were no significant difference in zeta potential of sample B2(2), B2(1) and B4(2).
The rheological characteristic of the prepared creams is important in technical applications including manufacturing, pumping, filling and storage. Yield value is known as the minimum shear stress required to produce flow[23] and below this point the materials will behave as solid. The yield value of pharmaceutical and cosmetic materials should be high, so they do not flow out from the container when placed in an upside−down position[24]. The apparent viscosity was calculated using Eq. 1. The yield values and apparent viscosity of the formulations B4(2), B2(2) and B2(1) are shown in Table 6. Sample B2(2) had the highest yield value compared to the other formulations, which may due to the optimum surfactant concentration and high percentage of the cetostearyl alcohol used in that formulation which formed more hydrogen bonds with the aqueous phase. The apparent viscosity of formulation B2(1) was the lowest compared to the other formulations, hence, it is the most unstable formulation. Low yield value and low apparent viscosity made the formulation easily spill out from the container. Formulations B4(2) had lower yield value and higher apparent viscosity than formulation B2(2). This was due to the higher oil content in formulation B4(2) which would increase the apparent viscosity of the formulation, and hence it would be difficult to remove the nanocream from the container. Among all formulations studied the best nanocream was B2(2) because it had high yield value and good apparent viscosity. Thus, B2(2) was suitable to be used as a nanocream base in cosmetics and pharmaceutical applications because it will not spill out when the container is placed in upside down position and beside that it is also easier to take the nanocream out from the container.
| Smix ratio | Formulation | Oil | Smix | Water | Results |
|---|---|---|---|---|---|
| code | (%) | (%) | (%) | ||
| HLB 13.71 | A1 | 22.2 | 33.3 | 44.5 | No separation |
| A2 | 29.4 | 29.4 | 41.2 | Separated | |
| A3 | 40 | 26.7 | 33.3 | Separated | |
| A4 | 37.5 | 25 | 37.5 | Separated | |
| A5 | 46.7 | 20 | 33.3 | Separated | |
| HLB 11.13 | B1 | 31.3 | 31.3 | 37.4 | Separated |
| B2 | 15.8 | 36.8 | 47.4 | No separation | |
| B3 | 29.4 | 29.4 | 41.2 | Separated | |
| B4 | 23.5 | 35.3 | 41.5 | No separation | |
| B5 | 50 | 21.4 | 28.6 | Separated | |
| HLB 9.84 | C1 | 22.2 | 33.3 | 44.5 | No separation |
| C2 | 31.3 | 31.3 | 37.4 | Separated | |
| C3 | 40 | 26.7 | 33.3 | Separated | |
| C4 | 37.5 | 25 | 37.5 | Separated | |
| C5 | 46.7 | 20 | 33.3 | Separated |
Table 2: Results Of Accelerated Stability Test Using Centrifugation Methods.
| Smix ratio | Formulations | Oil | Smix | Water | Results |
|---|---|---|---|---|---|
| (%) | (%) | (%) | |||
| HLB 11.13 | B2 | 15.8 | 36.8 | 47.4 | Partially separated |
| HLB 11.13 | B4 | 23.5 | 35.3 | 41.5 | Partially separated |
| HLB 9.84 | C1 | 22.2 | 33.3 | 44.5 | Separated |
| HLB 13.71 | A1 | 22.2 | 33.3 | 44.5 | Separated |
TABLE 3: Results of accelerated stability test Using heating cooling cycle method .
| Formulation | Palm | Surfactant | Aqueous | Cetostearyl |
|---|---|---|---|---|
| code | oil (%) | (%) | phase (%) | alcohol (%) |
| B2(1) | 14.8 | 36.8 | 47.4 | 1 |
| B2(2) | 13.8 | 36.8 | 47.4 | 2 |
| B4(1) | 22.5 | 35.3 | 41.5 | 1 |
| B4(2) | 21.5 | 35.3 | 41.5 | 2 |
Table 4: Percentages Of Cetostearyl Alcohol Incorporated In Nanocream Formulations
| Formulation | Droplet | Polydispersity | Zeta |
|---|---|---|---|
| code | size (nm) | index | potential (mV) |
| B2(1) | 240.5±4.57 | 0.197±0.113 | -33.8±0.493 |
| B2(2) | 219.3±2.93 | 0.054±0.039 | -31.3±0.85 |
| B4(2) | 243.13±2.9 | 0.26±0.13 | -29.3±3.86 |
Table 5: Nanocream Formulations.
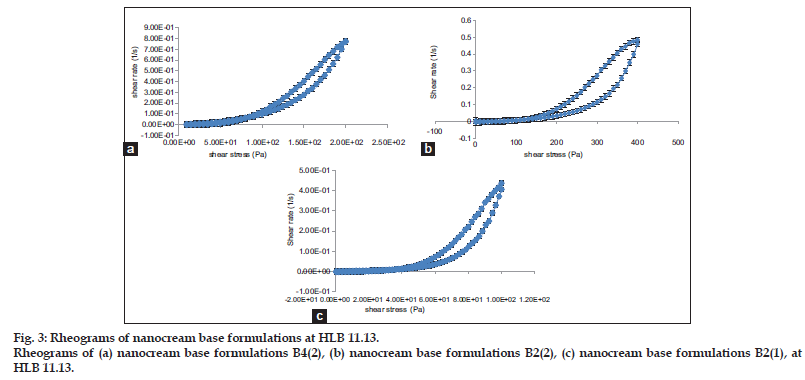
The rheological properties of samples B4(2), B2(2) and B2(1) are shown in fig. 3 and all the rheograms have yield value, which mean all formulations have plastic flow properties. All the rheograms of formulations studied have the same curve pattern which formed hysteresis−loop type with the down curves to the left of the up curves. This curve pattern is called thixotropic behaviour. The thixotrophic behavior is a favourable characteristic of cosmetics, pharmaceutical creams and gel emulsions[25]. Since formulation B2(2) had the best characteristics, it was chosen as the nanocream base for cinnamon leaf oil. The cinnamon leaf oil nanocream was further examined in terms of droplet size, zeta potential, apparent viscosity and flow characteristics.
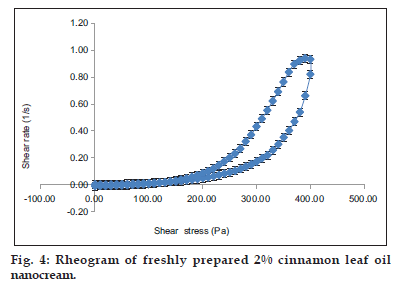
The droplet size of the nanocream after incorporation of cinnamon leaf oil was increased from 219.3±2.93 nm to 286.4±2.15 nm and the apparent viscosity was reduced from 11812±128.22 Pa.s. to 10473.14±230.39 Pa.s. This occurance may be due to 2% of palm oil being replaced by cinnamon leaf oil in the formulation. Different types of oil may affect droplets size and apparent viscosity of nanocream, however the yield value and zeta potential were still quite high. Thus, it still produced a stable cinnamon leaf oil nanocream. The rheogram curve of cinnamon leaf oil nanocream showed in fig. 4 demonstrated plastic flow properties as it has yield value. It also has hysteresis−loop with the down curves to the left of the up curves where it is called thixotrophy that is important for cream application.
| Formulation code | Cetostearyl alcohol (%) | Oil (%) | Smix (%) | Water (%) | Apparent viscosity (Pa.S) | Yield value (Pa) |
|---|---|---|---|---|---|---|
| B2(1) | 1 | 14.8 | 36.8 | 47.4 | 8020.17±1333 | 60±10 |
| B2(2) | 2 | 13.8 | 36.8 | 47.4 | 11,812.42±128.22 | 286±15 |
| B4(2) | 2 | 21.5 | 35.3 | 41.5 | 59,407.78±6134.33 | 120±20 |
Table 6: Rheological Parameter Of The Formulated Nanocream
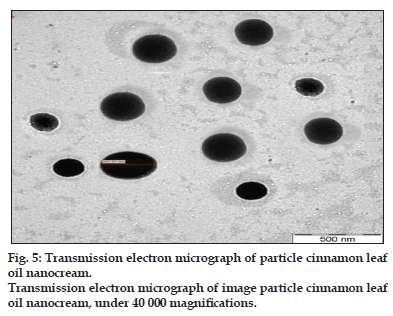
Fig. 5 shows the image of oil droplets in cinnamon leaf nanocream taken using high resolution transmission electron microscope (TEM). The oil droplets in nanocream formulation are of a dark colour and have a spherical shape with average size less than 300 nm. Thus, this finding further supports the results obtained using Zeta Sizer 1000 HSA, (Malvern Instrument, UK) that the droplet size is in the nano range.
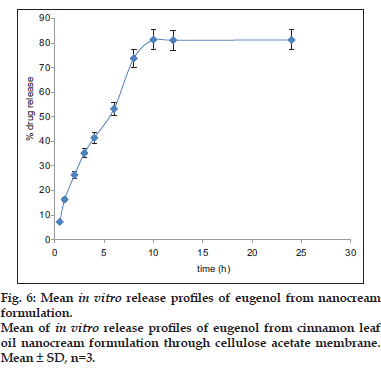
Fig. 6 shows the in vitro release profile of eugenol from the optimized cinnamon leaf oil nanocream formulation through the cellulose acetate membrane. Almost 81% of eugenol is released from the cinnamon leaf oil nanocream formulation after 24 h. The percentage of eugenol released from cinnamon leaf oil nanocream increased with time until 10 h and did not increase thereafter. The prolonged eugenol release could be attributed to embedment of eugenol in the cream. Increased released of eugenol may be contributed by the large surface area of nanosized particles and high solubility of eugenol in the permeation medium. The small size of particles is one of the factors which contribute to the increased penetration of skin[7]. Besides that, the presence of a surfactant (i.e Tween 80) in the formulation also contributed to the higher percentage of eugenol released. The surfactant can act as achemical enhancer in the penetration of eugenol into the skin where 50% of eugenol was released from the formulation within 5 h.
The cinnamon leaf oil nanocream formulation was packed into 30 g glass ointment jars with tight−fitting closures. This container was selected for use since it was easy to measure formulation parameters such as viscosity and pH directly from the ointment jar following storage of the samples for the required time[26].
Based on visual observation, there was no change of the milky yellow colour of the cinnamon leaf oil nanocream upon storage at 40±2°/75±5% RH and room temperature (25±2°/65±5% RH) for 6 months. After storage of the nanocream in the stability chamber at either of the two temperatures, the strong odour of cinnamon leaf oil was still present.
In addition, there was no contamination of fungi and molds in the nanocream at both conditions (25± 2°/65±5% RH) and 40±2°/75±5% RH). It might be due to the presence of preservatives (methyl and propyl paraben) in the nanocream. The results suggested that the nanocream was stable in both conditions over the specified time of observation.
The results of the conductivity of cinnamon leaf oil nanocream at two different temperatures, 40±2°/75±5% RH and room temperature (25±2°/65±5% RH) after 6 months storage are shown in Table 7. The initial conductivity of the nanocream stored at room temperature was 1240.6±3.1 μS and after 6 months, conductivity was 1244.7±3.79 μS. It was found that there was no significant change in the conductivity measurement after 6 months storage at room temperature. This indicated that the bottom of the container contains the same amount of oil phase within the time frame of the stability study[27]. Thus, the results suggested that the cinnamon leaf oil nanocream was stable at this temperature as no creaming or sedimentation in the nanocream was detected during the period of the study. In contrast, a significant change occurred for the nanocream stored in the stability chamber at 40°±2°/75±5% RH for 6 months whereby the conductivity increased to 1260.7±7.5 μS. The significant change was due to the upward movement of the oil phase. Thus, the conductivity increased because of the lower number of oil droplets at the bottom of the nanocream container.
Figure 7: Rheogram of cinnamon leaf oil nanocream after 6 months.
Rheogram of cinnamon leaf oil nanocream stored at 25±2°/65±5% RH (a) and at 40±2°/75±5% RH (b) for 6 months, Mean±SD, n =3.
Figure 8: In vitro release profiles of eugenol from nanocream during stability period. In vitro release profiles of eugenol from cinnamon leaf oil nanocream at 25±2°/65±5% RH (a) and at 40±2°/75±5% RH (b) for 0 month( ![]() ), 1 month (
), 1 month (![]() ), 2 month (
), 2 month (![]() ), 3 month (
), 3 month (![]() ), 6 month (
), 6 month (![]() ) through cellulose acetate membrane, Mean±SD, n =3.
) through cellulose acetate membrane, Mean±SD, n =3.
The pH of a freshly prepared formulation was 5.70 and after 6 months, the pH of the nanocream stored at room temperature (25±2°/65±5% RH) was 5.63±0.05 while the pH of the nanocream stored at 40±2°/75±5% RH was 5.66±0.06 (Table 7). There were no significant changes found for both nanocreams stored either at 40±2°/75±5% RH or 25±2°/65±5% RH. The pH values of both nanocreams which were unchanged could be due to the stability of the compounds in the cinnamon leaf oil nanocream formulation. Thus, this indicated that there was no degradation or ionization of chemicals in the formulation at both temperatures during the period of study.
The mean droplet sizes of nanocream during 6 months stability study at two different temperatures is shown in Table 7. The freshly prepared nanocream had an average droplet size of 285.33±1.06 nm and after six months of storage at room temperature (25±2°/65±5% RH) or at 40±2°/75±5% RH, the droplets sizes increased to 292.47±4.81 nm and 505.73±16.85 nm, respectively. The droplet size of the nanocream stored at room temperature (25±2°/65±5% RH) did not change significantly during the duration of the stability study. This may be due to minimal free energy available in the system, hence no aggregation and coalescence occurred. The size of droplets in the nanocream stored at temperature 40±2°/75±5% RH increased gradually with time and there was a significant change after 6 months stability study. These results suggested that this increase of droplet size could be due to the free energy available which caused free moveable droplets to collide and coalesce with each other in the system and hence increase the droplet size. Thus, it can be concluded from this study, that the nanocream stored at room temperature (25±2°/65±5% RH) was more stable compared to the nanocream stored at 40±2°/75±5% RH. Moreover, no significant changes in zeta potential values were observed in all samples throughout the study at this temperature (25±2°/65±5% RH). However, the value of zeta potential at 40±2°/75±5% RH significantly decreased to 24.43 mV after 6 months. Low zeta potential may be due to the coalescence of droplets in the nanocream.
| Nanocream properties | 25°±2°/65±5% RH | 40°±2°/75±5% RH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 month | 1-month | 2 months | 3 months | 6 months | 1-month | 2 months | 3 months | 6 months | ||
| Conductivity (µS) | 1240.6±3.1 | 1241±3.1 | 1246.3±8.1 | 1247±7.5 | 1244.7±3.79 1248.7±6.1 | 1249±4.36 | 1247.7±4.16 | 1260.7±7.5 | ||
| pH | 5.70±0.05 | 5.71±0.04 | 5.62±0.09 | 5.65±0.07 | 5.63±0.05 | 5.71±0.02 | 5.62±0.05 | 5.61±0.04 | 5.66±0.06 | |
| Droplet size (nm) | 285.3±1.06 | 287.67±3.01 | 295.5±5.66 | 294.33±3.8 | 292.47±4.81 | 294.3±6.05 | 308.0±7.08 316.63±4.23 505.73±16.85 | |||
| Zeta potential (mV) | -28.93±1.24 | -29.33±1.11 | -29.8±0.95 | -29.67±1.1 | -28.6±1.63 | -27.9±1.21 | -28.6±1.48 | -25.03±4.06 | -24.43±1.3 | |
| Apparent viscosity (Pa.s) | 10,499.98± | 10,723.27± | 10,751.48± | 10,367.71± | 10,847.64± | 10,711.33± | 10,791.19± | 10,038.47± | 94,40.63± | |
| 381.42 | 13.99 | 326.77 | 38.17 | 84.03 | 197.63 | 235.53 | 26.71 | 21.74 | ||
| Yield value (Pa) | 293±11.5 | 280±10 | 283±15 | 290±10 | 290±10 | 270±10 | 286±11.5 | 286±11.5 | 290±10 | |
| Eugenol content (%) | 101.04±0.78 | 100.49±0.45 | 100.43±0.51 | 99.82±1.38 | 99.36±0.16 | 97.51±1.38 | 98.99±0.54 | 97.12±2.34 | 91.1±1.06 | |
| T50% (h) | 5.65±0.39 | 5.00±0.16 | 5.29±0.16 | 5.39±0.05 | 6.63±0.06 | 5.65±0.30 | 6.91±0.37 | 5.61±0.17 | 6.87±0.03 | |
Table 7: Stability Results Of Cinnamon Leaf Oil Nanocream
The apparent viscosity of the freshly prepared formulation was 10499.98 Pa.s and after 6 months of storage at different temperatures of 25±2°/65±5% RH and 40±2°/75±5% RH, the apparent viscosities were 10847.63 Pa.s and 9440.63 Pa.s, respectively (Table 7). There was no significant change in apparent viscosity at room temperature but, at 40±2°/75±5% RH the apparent viscosity decreased significantly. The insignificant change in apparent viscosity at 25±2°/65±5% RH might be due to the intactness of hydrogen bonds between the polyoxyethylene chains of the surfactants[28]. A significant drop in the apparent viscosity value after storage for six months at a temperature 40±2°/75±5% RH may be caused by the movement of a small number of surfactant molecules from the interface to the surface, which affected the structure of the nanocream[29] or owing to the free movement of droplets resulting in collision with each other (Brownian movement) and coalescence. Thus, this study, suggested that cinnamon leaf oil nanocream was more stable at lower temperature (25±2°/65±5% RH) compared to the higher temperature (40±2°/75±5% RH).
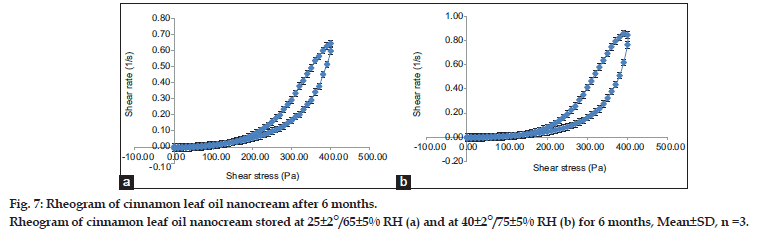
The yield value measurement of freshly prepared cinnamon leaf oil nanocream at room temperature was 293±11.5 Pa. After 6 months storage at two different temperatures 25±2°/65±5% RH and 40±2°/75±5% RH, the values were 290±10 Pa for both temperatures (Table 7). There were no significant changes in yield values at both temperatures after 6 months storage. Even though the apparent viscosity was changed significantly at 40±2°/75±5% RH after 6 months stability study, the yield value of the cinnamon leaf oil nanocream was not affected. In addition, there were no changes of the flow characteristic of the nanocream after 6 months stability study at 25±2°/65±5% RH and 40±2°/75±5% RH (fig. 7). The unchanged plastic flow characteristics and insignificant difference in yield value of cinnamon leaf oil nanocream might be due to insignificant physical changes attributed to the nanocream over the entire stability study for both temperatures. Therefore, the slight liquefaction of the nanocream stored at 40±2°/75±5% RH did not affect its rheological flow.
The eugenol content in the samples (91−101%) was within the range of the original eugenol content. There was no significant change in the eugenol content in the nanocream after 6 months stability study at room temperature (25±2°/65±5% RH). However, at a temperature of 40±2°/75±5% RH there was a significant difference in eugenol content in the nanocream after 1 month compared to the freshly prepared sample (0 month). The eugenol content in the formulation dropped from 101 to 97% after 1 month (Table 7). Therefore, it can be concluded that the cinnamon leaf oil nanocream is stable at room temperature (25±2°65±5% RH) while at higher temperature it started to degrade after 1 month of the stability study. Reduction of the eugenol content in the nanocream may be due to increased degradation of volatile cinnamon leaf oil constituents at the temperature of 40±2°/75±5% RH.
During the stability study, the in vitro release of eugenol from formulations of cinnamon leaf oil nanocreams were observed at 0, 1, 2, 3 and 6 months at two different temperatures 25±2°/65±5% RH and 40±2°/75±5% RH. The percentage of eugenol release was calculated based on the total drug content at the evaluated point.
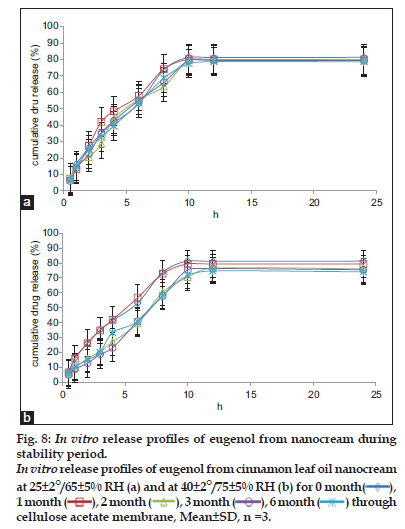
Fig. 8 shows the graph of the in vitro release profiles of eugenol from cinnamon leaf oil nanocream through the cellulose acetate membrane at different temperatures (25±2°/65±5% RH and 40±2°/75±5% RH) over the entire test period (0, 1, 2, 3 and 6 months). It was revealed that the cumulative release of eugenol from freshly prepared nanocream at room temperature was 81% for 24 h. After 6 months stability study at room temperature, the cumulative release of eugenol was 78.6%. There was no significant difference in the cumulative release of eugenol from the nanocream formulation after 6 months storage. In contrast, at the temperature of 40±2°/75±5% RH, the cumulative release of eugenol from the nanocream formulation decreased significantly to 74.1% after 6 months storage. The in vitro release profile of eugenol from cinnamon leaf oil nanocream remained relatively constant at room temperature (25±2°/65±5% RH) but shows a slight decrease at 40±2°/75±5% RH after 6 months storage. Based on this study, it was confirmed that the release of eugenol from the cinnamon leaf oil nanocream was not affected at room temperature compared to 40±2°/75±5% RH.
Table 7 shows the mean time of 50% eugenol releases (T50%) across the cellulose acetate membrane over the time period (0, 1, 2, 3 and 6 months) of stability study. The T50% of eugenol release from freshly prepared cinnamon leaf oil nanocream was 5.65±0.39 h. After 6 months stability study at room temperature, the T50% of eugenol release was 5.61±0.17 h. There was no significant change in theT50% of eugenol in the formulation when stored at 25±2°/65±5% RH after 6 months stability study. However, T50% of eugenol was significantly increased to 6.87±0.03 h after 6 months stability study at 40±2°/75±5% RH storage conditions. A decrease in cumulative release of eugenol from the nanocream formulation after 6 months stability study and increasedT50% of eugenol at 40±2°/75±5% RH might be attributed to the physical changes of the droplet size and viscosity of the formulation which affected the penetration of eugenol across the cellulose acetate membrane.
In conclusions, the nanocream base formulation B2(2) consisted of aqueous phase (pH 5.5), palm oil as the oil phase and the mixture of Tween 80:Span 65 (70:30) HLB 11.13 at the ratio of 47.4:15.8:36.8 was chosen as the best nanocream base formulation. The selected formulation had rheological characteristics suitable for topical application. Droplet size after incorporating cinnamon leaf oil determined by zeta sizer was around 286.4 nm and the zeta potential of −29 millivolts which could hinder the coalescence and aggregation of the oil droplets and produced stable nanocream formulation. Cinnamon leaf oil nanocream was most stable at room temperature compared to the higher temperature (40±2°/75±5% RH).
Acknowledgements
The authors would like to thank Universiti Sains Malaysia for providing a short term research grant (304/PFarmasi/6312023) to support this work. The author (Nor Azah Zainol) gratefully acknowledges Majlis Amanah Rakyat for the granting of a scholarship.
Financial support and sponsorship
The author (Nor Azah Zainol) gratefully acknowledges Majlis Amanah Rakyat for the granting of a scholarship.
Conflicts of interest
There are no conflicts of interest.
References
- Fakhry KR, Mohammed Hassan KA. Formulation and evaluation of diphenhydramine HCL release from different semi-solid bases (cream, gel and ointment). World J Pharm Res 2013;2:1306-24.
- Sonje A, Thube R, Parmar V, Kumari G, Deshpande P. A review on penetration enhancer for semisolids. Asian J Pharm Res Dev 2013;1:94-107.
- Farahpour MR, Habibi M. Evaluation of the wound healing activity of an ethanolic extract of Ceylon cinnamon in mice. Vet Med 2012;1:53-7.
- Sharma N, Bansal M, Visht S, Sharma P, Kulkarni G. Nanoemulsion: A new concept of delivery system. Chron Young Sci 2011;1:2-6.
- Bouchemal K, Briançon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: Solvent, oil and surfactant optimisation. Int J Pharm 2004;280:241-51.
- Rajalakshmi R, Mahesh K, Kumar CK. A critical review on nanoemulsions. Int J Innov Drug Discov 2011;1:1-8.
- Aulton ME. Aulton’s Pharmaceutics: The Design and Manufacture of Medicines. 3rd ed. London: Churchill Livingstone; 2007.
- Kamatou GP, Vermaak I, Viljoen AM. Eugenol – From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012;17:6953-81.
- Amr RA, Maysa EM. Antiulcer effect of cinnamon and chamomile aqueous extracts in rats models. J Am Sci 2010;6:209-16.
- Jakhatia V, Patel R, Khatri P, Pahuja N, Garg S, Pandey A, et al. Cinnamon: A pharmacological review. J AdvSci Res 2010;2:19-23.
- Barceloux DG. Cinnamon (Cinnamomum species). Dis Mon 2009;55:327-35.
- Cheng SS, Chung MJ, Chen YJ, Chang ST. Antipathogenic activities and chemical composition of Cinnamomumosmophloeum and Cinnamomumzeylanicumleaf essential oils. J Wood ChemTechnol2011;31:73-87.
- Kumar S, Vaudeva N, Sharma S. Pharmacological and pharmacognostical aspects of CinnamomumtamalaNees and Eberm. J Pharm Res 2012;5:480-4.
- Sangal A. Role of cinnamon as beneficial antidiabetic food adjunct: A review. AdvApplSci Res 2011;2:440-50.
- Ghosh V, Saranya S, Mukherjee A, Chandrasekaran N. Antibacterial microemulsion prevents sepsis and triggers healing of wound in Wistar rats. Colloids Surf B Biointerfaces 2013;105:152-7.
- Alam MS, Ali MS, Alam N, Alam MI, Anwer T, Imam F, et al. Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. J Appl Pharm Sci 2012;2:148-58.
- Abdulkarim MF, Abdullah GZ, Sakeena MH, Chitneni M, Yam MF, Mahdi ES, et al. Study of pseudoternary phase diagram behaviour and the effect of several tweens and spans on palm oil esters characteristics. Int J Drug Deliv 2011;3:95-100.
- Ali MS, Alam MS, Alam N, Qadry SA, Alam MI, Shamim M, et al. Formulation, characterization and in-vivo assessment of topical nanoemulsion of betamethasone valerate for psoriasis and dermatose. Int J Pharm 2013;3:186-99.
- Baboota S, Alam MS, Sharma S, Sahni JK, Kumar A, Ali J. Nanocarrier-based hydrogel of betamethasone dipropionate and salicylic acid for treatment of psoriasis. Int J Pharm Investig 2011;1:139-47.
- Ghosh TK, Jasti BR. Theory and Practice of Contemporary Pharmaceutics. Boca Raton: Taylor and Francis; 2010.
- Gardouh AR, Ghorab MM, Abdel-Rahman SG. Effect of viscosity, method of preparation and homogenization speed on physical characteristics of solid lipid nanoparticles. ARPN J SciTechnol 2012;2:996-1006.
- Al-Edresi S, Baie S. Formulation and stability of whitening VCO-in-water nano-cream. Int J Pharm 2009;373:174-8.
- Dapcevic T, Dokic P, Hadnadev M, Pojic M. Determining the yield stress of food products-importance and shortcomings. J Inst Food Technol Novi Sad 2013;40:143-50.
- Mahdi ES, Noor AM, Sakeena MH, Abdullah GZ, Abdulkarim MF, Sattar MA. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthusurinaria for skin antiaging. Int J Nanomedicine 2011;6:2499-512.
- Davis SS. Viscoelastic properties of pharmaceutical semisolids. I. Ointment bases. J Pharm Sci 1969;58:412-8.
- Kasongo KW. Development and In Vitro Evaluation of a Clobetasol 17-Propionate Topical Cream Formulation. Grahamstown: Rhodes University; 2007.
- Ghassan ZA, Abdulkarim FM, Salman MI, Ameer ZO, Chitneni M, Mahdi ES, et al. Stability studies of nano-scaled emulsions containing ibuprofen for topical delivery. Int J Drug Deliv 2011;3:74-82.
- Abdulkarim MF, Abdullah GZ, Chitneni M, Salman IM, Ameer OZ, Yam MF, et al. Topical piroxicamin vitro release and in vivo anti-inflammatory and analgesic effects from palm oil esters-based nanocream. Int J Nanomedicine 2010;5:915-24.
- Akhtar N, Khan BA, Khan MS, Mahmood T, Khan HM, Iqbal M, et al. Formulation development and moisturising effects of a topical cream of Aloe veraextract. World AcadSciEngTechnol 2011;51:172-80.