- *Corresponding Author:
- Sandhya Shenoy
Faculty of Pharmacy, Pacific Academy of Higher Education and Research University, Udaipur-313 003, India
E-mail: sandhya.shenoy@rediffmail.com
| Date of Submission | 03 July 2017 |
| Date of Revision | 21 February 2018 |
| Date of Acceptance | 18 August 2018 |
| Indian J Pharm Sci 2018;80(5):883-891 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Chronomodulated delivery system of metoclopramide hydrochloride was developed for the treatment of morning sickness and diabetic gastroparesis. Drug-excipient compatibility studies revealed no significant degradation of metoclopramide hydrochloride in presence of selected tabletting and compression coating excipients. Immediate release core tablets of metoclopramide hydrochloride were prepared using direct compression technique and various physico-chemical parameters were evaluated. The core tablets were subjected to compression coating with a mixture of glyceryl dibehenate, hydrogenated castor oil and dicalcium phosphate; the levels of which were optimized statistically using face-centred cube design to achieve desired in vitro drug release profile of not more than 10% at 4 h, not less than 50% at 4.5 h and not less than 85 % at 5 h interval. Increase in the concentration of glyceryl dibehenate and hydrogenated castor oil in the formulation significantly decreased drug release at 4.5 and 5 h time intervals in distilled water as dissolution medium whereas quantity of dicalcium phosphate was found to have no influence on drug release characteristics. A quadratic model was suggested for the release profile at 4 h whereas linear model signified the release at 4.5 and 5 h. Formulation containing 100, 92 and 150 mg of glyceryl dibehenate, hydrogenated castor oil and dicalcium phosphate respectively per tablet was considered optimum since it showed the desired release profile. As the formulation showed the desired lag time during in vitro drug release, night time administration of the formulation could be expected to prevent the symptoms of morning sickness among pregnant women and hypoglycaemia upon administration of antidiabetic medication in gastroparetic patients.
Keywords
Chronomodulated delivery, metoclopramide hydrochloride, optimization, glyceryl dibehenate, hydrogenated castor oil, gastric paresis, morning sickness
Certain diseases like arthritis, asthma, myocardial infarction, congestive heart failure, stroke and peptic ulcer exhibit a peak time of activity within a circadian rhythm. The drug therapy of such diseases therefore needs to be optimized by tailoring the dosing schedule based on chronobiological pattern of these diseases. There have been a number of reports in the literature where chronomodulated delivery systems have been developed for the treatment of asthma, hypertension and arthritis in a way that the medication when administered at bed time would release the drug at once, after a lag time of 5-6 h to elicit the therapeutic response early in the morning at which time the disease symptoms are at the peak [1-6]. Such a therapy would improve patient compliance and reduces the necessity of mid-night administering the medication to achieve optimum response early in the morning. Almost 80 % of pregnant women suffer from morning sickness in the first trimester of pregnancy [7]. They have to deal with severe nausea and bouts of vomiting early in the morning, which affects the appetite and eating, resulting in weakness and malnutrition. Thus, morning sickness is a chronological symptom, the treatment or prevention of which, could be handled more efficiently with a chronomodulated delivery system of a drug. Diabetic gastroparesis is a chronic gastrointestinal disorder seen among type I and II diabetic patients in which the gastric contents are emptied at a slower rate than usual. Administration of antidiabetic medicines in such a situation where there is inadequate glucose level in the blood can create serious concerns of hypoglycaemia [8]. Fluctuation in blood glucose levels among diabetic patients with gastroparesis is also a typical chronological event happening mostly early in the morning. Chronomodulated therapy with a gastric prokinetic agent therefore would be most justified in such a situation.
Metoclopramide hydrochloride (MH), a dopamine receptor antagonist is indicated in the treatment of gastro oesophageal reflux disease, nausea and vomiting [9]. Oral therapy with MH is proven safe in relieving the symptoms of morning sickness [10]. It is the only approved drug in USA for managing diabetic gastroparesis by improving gastric emptying rate to allow faster and better systemic absorption of glucose for preventing hypoglycaemia that could result with antidiabetic drugs.
MH is available commercially as immediate release tablets, orally disintegrating tablets, solution, as well as controlled release formulations, but not as a chronomodulated delivery system. There have been no reports related to the development of such a delivery system, though extensive work on sustained release formulations, controlled release matrix tablets, flash release films, melt in mouth tablets, buccoadhesive tablets, gastro-retentive delivery of MH has been reported [11-19]. All these formulations could result in optimum therapeutic effect only after 1 to 2 h of oral administration or in a sustained manner depending on the type of dosage form. This would render the medication almost ineffective in curbing the early morning nausea and vomiting in pregnant women or in preventing blood glucose fluctuations in gastroparetic diabetic patients, typically occurring after taking antidiabetic medication in the morning. To overcome this problem, authors have earlier attempted to develop a chronomodulated delivery system for MH that can be administered at bedtime and which would elicit maximum therapeutic effect early in the morning after a lag time of 6-7 h [20]. Development of such a formulation involved optimization using one-variable-at-a-time (OVAT) approach wherein the quantities of glyceryl dibehenate as a release modulator and dicalcium phosphate as a diluent were optimized to obtain the desired in vitro release profile. Design of experiments (DOE) is a comprehensive multivariate approach for fruitful appraisal and optimization of the formulations allowing extraction of maximal information out of a few well-designed experiments. The current study was aimed at optimizing the chronomodulated formulation of MH using face-centred cubic design (FCCD), a technique under the umbrella of DOE, wherein the quantities of glyceryl dibehenate, dicalcium phosphate and hydrogenated castor oil, an additional release modifier were optimized to achieve the desired release profile.
Materials and Methods
MH was procured from Ipca Laboratories Pvt., Ltd. Microcrystalline cellulose (Avicel PH 102), magnesium stearate and dicalcium phosphate (Di-tab) were procured from Signet Chemicals Ltd. Glyceryl dibehenate (Compritol 888 ATO), hydrogenated castor oil (Kolliwax HCO) were gift samples from Gattefosse Pvt., Ltd., and BASF Ltd., respectively. Lactose spraydried (Supertab) was obtained from DFE Pharma Ltd., and crospovidone (Polyplasdone XL 10) from Ashland Specialties Ltd. All these suppliers were based in Mumbai, India.
Drug-excipient compatibility studies
Binary mixtures of MH and tabletting excipients were prepared as per the ratios given in Table 1. Glass vials filled with mixtures were closed with rubber closures, sealed with aluminium caps and stored at 25 ± 2° as a control. Those glass vials filled with mixtures and stored at 40 ± 2°/75 ± 5 % RH were covered with perforated aluminium foil. The study was carried out for 4 w. The samples were analysed for single maximum impurity and total impurity content using following method.
| Binary mixture | Ratio |
|---|---|
| MH:lactose | 1:1 |
| MH:microcrystalline cellulose | 1:1 |
| MH:dicalcium phosphate | 1:10 |
| MH:colloidal Silicon Dioxide | 1:0.5 |
| MH:crospovidone | 1:0.5 |
| MH:magnesium Stearate | 1:0.5 |
| MH:glyceryl dibehenate | 1:10 |
| MH:hydrogenated castor oil | 1:10 |
Table 1: Ratios of MH and Excipient Binary Mixtures for Compatibility Studies
Each binary mixture equivalent to 50 mg MH was dissolved in the solvent system composed of acetonitrile and water in 9:1 ratio, diluted suitably and 10 μl of the sample solution was subjected to analysis using high performance liquid chromatography (HPLC) of the make Agilent Technologies 1260 Infinity. Reverse phase, C18 column (4.6×250 mm) with 5 μ silica packing was employed at 30° for impurity separation, which were quantified using ultraviolet detector at 276 nm. Gradient analysis was performed using two mobile phases, one of which consisted of a mixture of 5.4 g anhydrous sodium acetate in 1000 ml of water, acetonitrile and tetramethyl ammonium hydroxide 25 % (87:12.8:0.2) and the other one contained a mixture of water and acetonitrile in the ratio of 75:25. The flow rate was maintained at 1.5 ml/min.
Preparation and evaluation of core tablets of MH
Immediate release core tablets of MH were prepared using the compositions given in Table 2. The blend of MH, microcrystalline cellulose, spray-dried lactose, silicon dioxide and crospovidone XL 10 was passed through 40# sieve and mixed well for 5 min in a container blender (RP Products) at 15 rpm. Magnesium stearate was added to the blend and mixed for 3 min. The final blend was compressed into tablets using a Cadmach CMD4 single rotary machine fitted with 4.7 mm standard concave circular plain punches. Average weight, weight variation, hardness using Dr. Schleuniger hardness tester (Model 8M) and thickness using Vernier caliper (Mitutoyo, 500-197-30) of the prepared tablets were evaluated. Disintegration time of the tablets was determined using a disintegration test apparatus (Electrolab, ED2L) containing 900 ml of distilled water maintained at 37 ± 0.5°.
| Ingredients | Quantity mg/ tablet |
|---|---|
| MH (equivalent to 10 mg of base) |
11.82 |
| Microcrystalline cellulose | 22.43 |
| Lactose spray-dried | 12 |
| Crospovidone XL 10 | 2.5 |
| Colloidal silicon dioxide | 0.5 |
| Magnesium stearate | 0.75 |
| Total weight/tablet | 50 |
Table 2: Composition of Immediate Release Tablets of MH
Tablets (n=10) were crushed and powdered using a pestle and mortar. Powder equivalent to 11.82 mg MH was dissolved in a mixture of water and acetonitrile (75:25). After suitable dilution of the solution, a sample volume of 5 μl was injected onto C18 column. A mobile phase consisting of buffer solution (5.4 g/l of sodium acetate in water):acetonitrile:tetramethyl ammonium hydroxide (70:30:0.2) was run at 1 ml/min to quantify MH using a UV detector at the wavelength of 276 nm.
In vitro release study
Tablets (n=6) were subjected to in vitro drug release study using USP type 2 dissolution test apparatus (Electrolab) employing 900 ml distilled water as a medium maintained at 37 ± 0.5° and stirred continuously at 50 rpm [21]. The aliquots were withdrawn at the end of 30 min and analysed for MH by HPLC using the same method as employed for the assay.
Preparation of chronomodulated tablets
Specified quantities of glyceryl dibehenate, hydrogenated castor oil and dicalcium phosphate were passed through 30# sieve and mixed in container blender for 15 min. The blend was transferred to the hopper of Cadmach press coater CPC 900 machine. Compression was done using 9.8 mm circular biconvex punches. The coating blend was fed into the 2 hoppers of the compression machine. The first hopper fed the powder into the die and was pre-compressed to form the lower layer. The core tablets were fed into a vibratory hopper, which were then guided into a rotating wheel. The wheel was synchronized to drop the core tablet accurately at the centre of the pre-compressed lower layer. This was followed by filling the upper layer of coating blend into the die and final compression. Vertical adjustment allowed for centralizing the tablet core to give equal thickness above and below the core. Inspection system allowed for core detection at the point of entry and automatic rejection of a tablet without a core.
Optimization of chronomodulated formulations using DOE
FCCD was used in the optimization of chronomodulated formulation of MH. Design Expert software 8.05 (Stat- Ease Inc., Minneapolis, MN, USA) was employed for this purpose. Glyceryl dibehenate (X1), dicalcium phosphate (X2) and hydrogenated castor oil (X3) were selected as factors (independent variables). Each factor was studied at 3 different levels (–1, 0 and +1) viz. X1 and X2 each at 50, 100, 150 mg/tablet whereas X3 at 42, 92 and 142 mg/tablet. Table 3 gives an account of the 17 formulation runs studied with their factor combinations. In vitro drug release at 4 h (Y1), 4.5 h (Y2) and 5 h (Y3) were selected as response variables (dependent variables). The targets set for response variables were not more than 10 % drug release in 4 h, not less than 50 % drug release in 4.5 h and not less than 85 % release in 5 h.
| Std. | Run | Space type | X1: glyceryl dibehenate (mg) |
X2: dicalcium phosphate (mg) |
X3: hydrogenated castor oil (mg) |
|---|---|---|---|---|---|
| 16 12 13 10 14 5 9 8 7 15 4 1 11 3 2 6 17 |
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 |
Center Axial Axial Axial Axial Factorial Axial Factorial Factorial Center Factorial Factorial Axial Factorial Factorial Factorial Center |
100 100 100 150 100 50 50 150 50 100 150 50 100 50 150 150 100 |
100 150 100 100 100 50 100 150 150 100 150 50 50 150 50 50 100 |
92 92 42 92 142 142 92 142 142 92 42 42 92 42 42 142 92 |
| Verification of design space by repeating the statistically optimized formulation | |||||
| 18 | 18 | Verification | 100 | 150 | 92 |
Table 3: Formulation Trials as per Experimental Design
Evaluation of chronomodulated tablets
The compression-coated tablets prepared using formulae listed in Table 3 were subjected to determination of average weight, hardness, assay and in vitro release in the manner similar to that described for the evaluation of core tablets.
Dissolution studies in various media
Chronomodulated tablets were prepared using optimized conditions suggested by Design Expert software (run 18) and 6 tablets were subjected to in vitro drug release studies separately in various dissolution media, pH 1.2 hydrochloric acid (0.1 N), pH 4.5 acetate buffer and pH 6.8 phosphate buffer. The test was conducted for 5 h using 900 ml of each medium maintained at 37 ± 0.5° stirred continuously at 50 rpm. The aliquots were withdrawn at 4, 4.5 and 5 h and replaced with fresh medium. Concentration of MH in aliquots was analysed by HPLC using the same method that was used for the assay. One way ANOVA at α≤0.05 was applied to the in vitro release values in all the media to identify any difference in the release profiles.
Accelerated stability studies
The chronomodulated tablets of run 18 were strip packed using aluminium foil and subjected to accelerated stability studies at 40 ± 2°/75 ± 5 % RH for 3 mo. The samples were evaluated for average weight, hardness, assay and in vitro drug release studies at 1, 2 and 3 mo intervals.
Results and Discussion
Earlier work by the authors involved development of a chronomodulated delivery system of MH using OVAT approach [20]. This work employed glyceryl dibehenate as a compression coating agent to achieve the desired lag time. Glyceryl dibehenate has a melting range of 69-74°. Prolonged exposure of the tablets to higher temperatures during stability studies resulted in uneven surfaces. As a result, a need arose to develop a product with better thermal stability, which is needed in a tropical country like India. Based on the evaluation of several fatty materials, hydrogenated castor oil was chosen as a release retardant along with glyceryl dibehenate for its inert properties and a higher melting range of 83-88°.
In order to ascertain any interactions between MH and the excipients, compatibility study was carried out by admixing MH and various excipients in a fixed proportion and subjecting the binary mixtures to accelerated conditions of temperature and humidity. Excipients used for preparing core tablets as well as the ones intended for compression coating were chosen for the study. Impurity analysis at the end indicated absence of incompatibility between MH and any of the excipients thus proving them suitable for the formulation development (Table 4).
| Binary mixture | Impurities | Initial | Control 25° |
40°/75 % RH |
|---|---|---|---|---|
| MH+lactose | Single maximum | 0.04 | 0.04 | 0.05 |
| Total | 0.08 | 0.09 | 0.11 | |
| MH+microcrystalline cellulose | Single maximum | 0.03 | 0.03 | 0.05 |
| Total | 0.06 | 0.08 | 0.08 | |
| MH+dicalcium phosphate | Single maximum | 0.03 | 0.04 | 0.04 |
| Total | 0.05 | 0.06 | 0.08 | |
| MH+colloidal silicon dioxide | Single maximum | 0.04 | 0.04 | 0.05 |
| Total | 0.07 | 0.08 | 0.09 | |
| MH+crospovidone | Single maximum | 0.05 | 0.05 | 0.07 |
| Total | 0.11 | 0.12 | 0.13 | |
| MH+magnesium stearate | Single maximum | 0.05 | 0.05 | 0.05 |
| Total | 0.07 | 0.08 | 0.09 | |
| MH+glyceryl dibehenate | Single maximum | 0.03 | 0.04 | 0.05 |
| Total | 0.08 | 0.08 | 0.08 | |
| MH+hydrogenated castor oil | Single maximum | 0.04 | 0.05 | 0.05 |
| Total | 0.08 | 0.08 | 0.09 |
Table 4: Impurity Analysis of MH-Excipient Binary Mixtures
Core tablets of MH were prepared by direct compression using the formula optimized earlier by the authors [20]. The evaluation parameters of the tablets were given in Table 5. Preliminary trials of the compression-coated tablets of MH were taken with glyceryl dibehenate, dicalcium phosphate and hydrogenated castor oil. The release profile targeted was achieved with a formulation containing 100 mg of glyceryl dibehenate, 100 mg dicalcium phosphate and 92 mg hydrogenated castor oil. Hence, this composition was chosen for further optimization using DOE. FCCD was chosen as an experimental design wherein run nos. 6, 8, 9, 11, 12, 14, 15 and 16 were factorial points to detect the main effects of the factors. Axial points (run nos. 2, 3, 4, 5, 7 and 13) were chosen to optimize the response. Run nos. 1, 10 and 17 (Table 3) were taken as centre point in order to detect the curvature of the response and the replicate trials of the centre point ensured minimization of experimental error. The formulations prepared as per the trial runs given in Table 3 showed average weight in ± 5 % range of the theoretical weight, hardness in the range of 4-7 kp and assay in the range of 98 to 102 %. In vitro drug release data on tablets (n=6) has been furnished in Table 6.
| Evaluation parameter | Result |
|---|---|
| Average weight (mg) | 50.8 ± 1.7 |
| Thickness (mm) | 2.1-2.2 |
| Hardness (kp) | 2-4 |
| Disintegration time (min) | 2-3 |
| Assay (%) | 100.4 ± 1.3 |
| In vitro drug release at 30 min (%) | 94.3 ± 2.3 |
Table 5: Evaluation of Core Tablets of MH
| Run | Y1: drug release at 4 h (%) | Y2: drug release at 4.5 h (%) | Y3: drug release at 5 h (%) |
|---|---|---|---|
| 1 | 5 | 60 | 90 |
| 2 | 4 | 60 | 90 |
| 3 | 6 | 61 | 91 |
| 4 | 1 | 38 | 72 |
| 5 | 2 | 40 | 75 |
| 6 | 7 | 60 | 90 |
| 7 | 20 | 85 | 98 |
| 8 | 1 | 38 | 68 |
| 9 | 2 | 60 | 90 |
| 10 | 4 | 62 | 94 |
| 11 | 3 | 55 | 85 |
| 12 | 35 | 90 | 92 |
| 13 | 5 | 63 | 89 |
| 14 | 32 | 86 | 90 |
| 15 | 3 | 55 | 86 |
| 16 | 1 | 37 | 70 |
| 17 | 6 | 65 | 96 |
Table 6: Response Parameters of DOE Studies
The aim of the work was to develop a formulation that would elicit the therapeutic effect of MH after 5-6 h of administration of the formulation. To achieve this goal, no drug release was desired up to 4 h of administration following which the tablets were expected to release the drug completely in a conventional manner over the period of 1 h. Hence the constraints put for the responses Y1, Y2 and Y3 were not more than 10 % release (at 4 h), not less than 50 % release (at 4.5 h) and not less than 85 % release (at 5 h), respectively. The responses of in vitro drug release of trial batches were fed into Design Expert software. Polynomial equation generated by the software for the response Y1 as a function of independent variables was as follows: Y1 = 2.22–10.4X1–0.89X2–6.1X3+4.7X1X3+7.2X12+2.7X32. X1 and X3 represent the main effects, X12 and X32 indicate quadratic effects and X1X3 interaction effect of factors glyceryl dibehenate and hydrogenated castor oil. The positive value in the regression equation indicates direct relationship and a negative value an inverse relationship between the factor and the response. Quadratic model was suggested for drug release at 4 h. For estimation of the significance of the model, ANOVA was applied as per the provision of the Design Expert software (Table 7). The model F-value of 179.77 implied that the model was significant and there was only a 0.01 % chance that the F-value this large could occur due to noise. All the model terms shown in the above equation except X2 were found to be significant as indicted by the p values <0.05 (Table 7). Equation for the response Y2 (in vitro release at 4.5 h) generated by the software is as follows. Y2 = 60.94–17.3X1–1.4X2 –10.3X3.
| Source | Sum of squares | Df | Mean square | F value | p-value | Statistical significance |
|---|---|---|---|---|---|---|
| Model | 1960.9 | 5 | 392.2 | 179.77 | 0.000001 | Significant |
| X1 | 1081.6 | 1 | 1081.6 | 495.77 | 0.000001 | Significant |
| X2 | 10.0 | 1 | 10.0 | 13.21 | 0.221012 | Non-significant |
| X3 | 362.1 | 1 | 362.1 | 170.56 | 0.000000 | Significant |
| X1 X3 | 180.5 | 1 | 180.50 | 82.74 | 0.000002 | Significant |
| X12 | 157.3 | 1 | 22.2 | 72.12 | 0.000004 | Significant |
| X32 | 22.2 | 1 | 22.2 | 10.18 | 0.008605 | Significant |
| Residual | 24.0 | 11 | 2.2 | |||
| Lack of fit | 23.3 | 9 | 2.6 | 7.78 | 0.119072 | Non-significant |
| Pure error | 0.7 | 2 | 0.03 | |||
| Core total | 1984.9 | 16 |
Table 7: Anova for Response Surface Reduced Quadratic Model for In Vitro Drug Release at 4 h
Linear model was suggested for the drug release at 4.5 h. Application of ANOVA indicated that the model and main effects X1 and X3were significant whereas X2 was not significant since the p value of its coefficient was more than 0.05 (Table 8). Model reduction was implemented to remove interaction terms and quadratic terms from the regression equation since they were statistically not significant.
| In vitro drug release at 4.5 h | ||||||
| Source | Sum of squares | Df | Mean square | F value | p-value | Statistical significance |
| Model | 4053.80 | 2 | 2026.90 | 97.47 | 0.00001 | Significant |
| X1 | 2811.50 | 1 | 2811.50 | 143.92 | 0.00001 | Significant |
| X2 | 181.40 | 1 | 181.40 | 2.87 | 0.32456 | Non-significant |
| X3 | 1060.90 | 1 | 1060.90 | 51.02 | 0.00000 | Significant |
| Residual | 291.14 | 14 | 20.80 | |||
| Lack of fit | 278.47 | 12 | 23.21 | 3.66 | 0.23421 | Non-significant |
| Pure error | 12.67 | 2 | 6.33 | |||
| Core total | 4344.94 | 16 | ||||
| In vitro drug release at 5 h | ||||||
| Source | Sum of squares | Df | Mean square | F value | p-value | Statistical significance |
| Model | 1490.60 | 2 | 745.30 | 25.85 | 0.00002 | Significant |
| X1 | 1116.70 | 1 | 1116.70 | 14.21 | 0.00002 | Significant |
| X2 | 71.40 | 1 | 71.40 | 1.18 | 0.27041 | Non-significant |
| X3 | 302.50 | 1 | 302.50 | 10.49 | 0.00594 | Significant |
| Residual | 403.50 | 14 | 28.83 | |||
| Lack of fit | 384.97 | 12 | 32.08 | 3.44 | 0.24731 | Non-significant |
| Pure error | 18.67 | 2 | 9.33 | |||
| Core total | 1894.24 | 16 | ||||
Table 8: Anova for Response Surface Reduced Linear Model for In Vitro Drug Release at 4.5 and 5 h
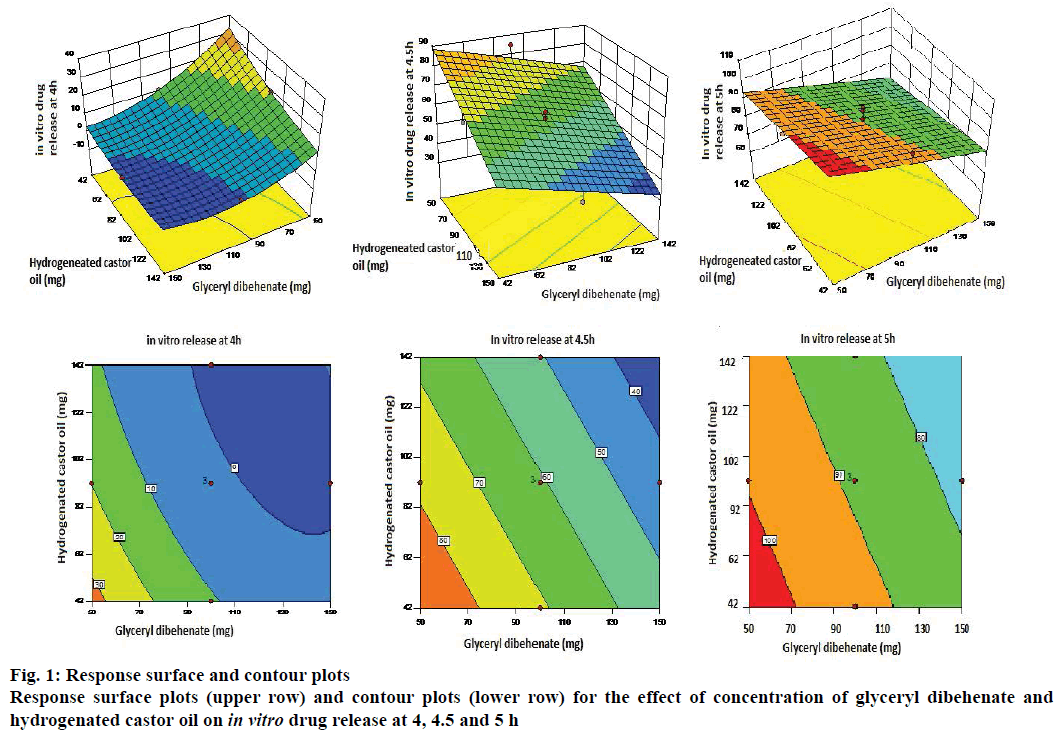
Y3 = 88.47–10.9X1–0.2X2–5.5X3 was the polynomial linear equation generated by the software for the response Y3 (in vitro drug release at 5 h). As per ANOVA, the model and the model terms X1 and X3 were significant. However, X2 in this case also was not significant (Table 8). Statistical treatment during optimization studies thus indicated that all the three responses were influenced by only two factors X1 and X3 and/or their interactions, whereas factor X2 did not have any significant impact on the responses. Hence the response surface plots and contour plots were generated considering only two variables viz. X1 and X3 (Figure 1).
The first response surface plot in Figure 1 indicated that the in vitro release at 4 h was inversely proportional to the quantities of factors X1 and X3 i.e. as the concentration of glyceryl dibehenate and hydrogenated castor oil increases the drug release decreases. The curvature of the lines on the contour plot showed quadratic relationship between the response and the factors. In vitro drug release at 4.5 and 5 h also indicated inverse impact of concentrations of glyceryl dibehenate and hydrogenated castor oil on the amount of drug release. The straight lines on the contour plots of these responses indicated linear relationship between the response and the variables.
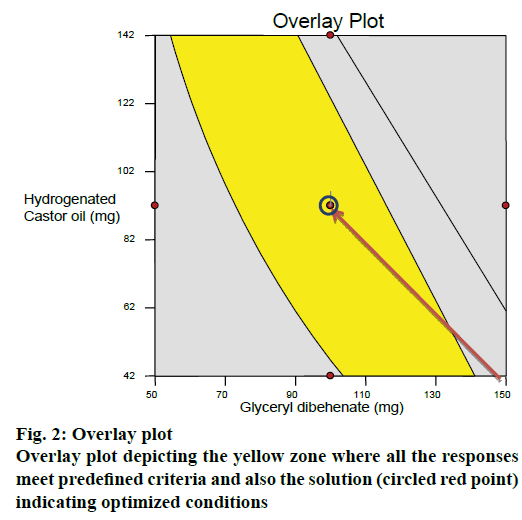
After generating the response surface plots, optimization process was undertaken with desirable characteristics of responses to probe the optimal solution. An overlay plot was obtained by overlapping the contour plots for the three responses. Yellow zone in the Figure 2 indicated the design space fulfilling the criteria of <10 % release at 4 h, >50 % and >85 % drug release at 4.5 and 5 h, respectively. The optimized formulation (red point circled in yellow zone of the overlay plot) suggested by the software was prepared (run 18) and subjected to in vitro release studies. For all the responses, the relative error was found to be less than 5 % between the observed values and the predicted values thus verifying the predictability of the model (Table 9). Hence the formulation of run18 suggested by the model containing 100 mg of glyceryl dibehenate, 150 mg of dicalcium phosphate and 92 mg of hydrogenated castor oil was considered as optimum.
| Response | Predicted results | Actual results | Relative error (%) |
|---|---|---|---|
| % Drug release at 4 h | 3.85 | 4.10 | 3.75 |
| % Drug release at 4.5 h | 59.10 | 62.04 | 4.67 |
| % Drug release at 5 h | 85.83 | 89.11 | 3.56 |
Table 9: Comparison of Predicted and Observed Responses of Optimized Run 18
Formulation of run18 was subjected to dissolution studies in various media to compare the effect of pH on the in vitro drug release as against that of distilled water which was earlier used as the dissolution medium (Table 10). Drug release profiles from the optimized formulation in different media when compared using one way ANOVA test confirmed no significant difference thus indicating the robustness of the formulation in terms of response parameters.
| Time point | Distilled water | pH 1.2 (0.1 N HCl) | pH 4.5 buffer | pH 6.8 buffer |
|---|---|---|---|---|
| 4 h | 4.10 ± 2.6 | 0 | 2.8 ± 0.8 | 0 |
| 4.5 h | 62.04 ± 1.4 | 72.3 ± 2.3 | 63.9 ± 2.6 | 67.1 ± 4.1 |
| 5 h | 89.11 ± 2.7 | 95.5 ± 1.9 | 90.4 ± 3.5 | 93.1 ± 3.4 |
Table 10: In Vitro Release of MH from Optimized Chronomodulated Formulation in Various Media
Tablets of run 18 when subjected to accelerated conditions of temperature and humidity did not show any remarkable changes in the parameters like appearance, hardness, assay or in vitro release profile over the storage period of 3 mo (Table 11). Application of one way ANOVA at α≤0.05 showed no significant difference among the assay values and in vitro release profiles thus indicating good stability of the formulation.
| Test | Specification | Initial | 1 month | 2 months | 3 months |
|---|---|---|---|---|---|
| Description | White to off-white, circular, biconvex tablets | White to off-white, circular, biconvex tablets | White to off-white, circular, biconvex tablets | White to off-white, circular, biconvex tablets | White to off-white, circular, biconvex tablets |
| Average weight | 392 mg ± 3 % | complies | Complies | Complies | Complies |
| Hardness | 4–7 kp | 5–7 kp | 4–7 kp | 4–6 kp | 4–6 kp |
| Assay | 90-110 % | 99.5 % ± 0.8 | 99.3 % ± 1.8 | 98.9 % ± 1.3 | 99.1 % ± 1.7 |
| In vitro drug release | 4 h:NMT 10 % | 4.1 % ± 2.6 | 0 % | 1.5 % ± 2.3 | 2.6 % ± 3.1 |
| 4.5h:NLT 50 % | 62 % ± 1.4 | 67.1 % ± 2.7 | 65.4 % ± 3.5 | 68.9 % ± 3.8 | |
| 5 h:NLT 85 % | 89.1 % ± 2.7 | 87.7 % ± 2.5 | 92.2 % ± 3.2 | 91.4 % ± 2.7 |
Table 11: Accelerated Stability Studies of Optimized Chronomodulated Formulaion of MH
In conclusion, chronomodulated formulation of MH was developed and optimized statistically using FCCD as a DOE approach. The formulation comprised of fast disintegrating core tablets of MH, compression coated with mixture of glyceryl dibehenate, hydrogenated castor oil and dicalcium phosphate. Glyceryl dibehenate and hydrogenated castor oil were the key factors that controlled the in vitro drug release at all the time points. Night-time administration of the optimized formulation could curb the early morning symptoms of nausea and vomiting in pregnant women and also prevent hypoglycaemia among the diabetic patients with gastroparesis.
Acknowledgements
The authors are grateful to Gattefosse India Pvt., Ltd. and BASF India Pvt., Ltd. for providing gift samples of glyceryl dibehenate and hydrogenated castor oil respectively.
Conflict of interest
We declare that we have no conflict of interest.
References
- Sokar M, Hanafy A, Elkamel A, El-Gamal S. Design of chronomodulated drug delivery system of valsartan: an in vitro characterization. Indian J Pharm Sci 2015;77:470-7.
- Quereshi J, Amir M, Ahuja A, Baboota S, Ali J. Chronomodulated drug delivery system of salbutamol sulphate for the treatment of nocturnal asthma. Indian J Pharm Sci 2008;70:351-56.
- Patil S, Pund S, Joshi A, Shishoo C, Shahiwala A. Chronomodulated press-coated pulsatile therapeutic system for aceclofenac: optimization of factors influencing drug release and lag time. Chronophysiol Ther 2011;1:1-10.
- Gangane PS, Mahajan NM, Danao KR, Pawde GN. Formulation and evaluation of chronomodulated pulsatile therapeutic system for early morning surge in blood pressure. Int J Pharm Sci 2015;7:337-41.
- Masareddy RS, Gupta AL, Danish K. Development and characterization of diltiazem hydrochloride pulsatile drug delivery system for chronomodulated therapy. Asian J Pharm Clin Res 2015;7:168-73.
- Neeharika MS, Jyothi BJ. Chronotherapeutics: an optimizing approach to synchronize drug delivery with circardian rhythm. J Crit Rev 2015;2:31-40.
- Patient information leaflet, Reglan®. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017854s062lbl.pdf.
- Lee A, Kuo B. Metoclopramide in the treatment of diabetic gastroparesis. Expert Rev Endocrinol Metab 2010;5:653-62.
- Patrick A, Epstein O. Review article: gastroparesis. Aliment Pharmacol Ther 2008;27:724-40.
- Pasternak B, Svanström H, Mølgaard-Nielsen D, Melbye M, Hviid A. Metoclopramide in pregnancy and risk of major congenital malformations and fetal death. JAMA 2013;16:1601-11.
- Nargis M, Islam MS, Naushin F, Haider SS. Development of sustained release preparations of metoclopramide hydrochloride based on fatty matrix. Dhaka Univ J Pharm Sci 2012;11:129-36.
- Khidr SH, Niazy EM, el-Sayed YM. Preparation and in vitro evaluation of sustained release metoclopramide hydrochloride microspheres. J Microencapsul 1995;12:651-60.
- Savaser A, Tas C, Bayrak Z, Ozkan CK. Effect of different polymers and their combinations on the release of metoclopramide hydrochloride from sustained release hydrophilic matrix tablets. Pharm Dev Technol 2013;18:1122-30.
- Patel MH, Kumar PA, Kulkarni SV, Rao BS. Formulation and in vitro evaluation of controlled release matrix tablets of metoclopramide hydrochloride: influence of fillers on hydrophilic natural gums. Int J Pharm Sci 2012;4:181-7.
- Raju S, Reddy PS, Kumar VA, Deepthi A, Reddy KS, Reddy PVM. Flash release oral films of metoclopramide hydrochloride for pediatric use: formulation and in vitro evaluation. J Chem Pharm Res 2011;3:636-46.
- Lakshmi AG, Patel R, Kumar DS. Formulation and evaluation of fast dissolving tablets of antiemetic drug metoclopramide. World J Pharm Pharm Sci 2014;3:2080-90.
- Ladola MK, Gangurde AB. Development and evaluation of melt in mouth tablets of metoclopramide hydrochloride using novel co-processed superdisintegrants. Indian J Pharm Sci 2014;76:423-9.
- Yadav DR, Ayyappan T, Shanmugam K, Sundermoorthy K, Vetrichelvan T. Development and in vitro evaluation of buccoadhesive metoclopramide hydrochloride tablet formulation. Int J PharmTech Res 2011;3:516-25.
- Jindal S, Sharma A, Jindal K. Development of metoclopramide floating tablets based on HPMC matrices: a comparison study with marketed formulation. IJTCS 2015;5(3):2146-50.
- Shenoy SR, Jain P, Kulkarni MC. Development and Evaluation of chronomodulated delivery system of metoclopramide hydrochloride. Int J App Pharm 2016;8:28-32.
- USP: United States Pharmacopoeia. Vol. 39. Rockville, Maryland: United States Pharmacopoeial convention Inc.; 2016. p. 4847-48.