- *Corresponding Author:
- M. K. Ladola
Department of Pharmaceutics, K. B. H. S. S. Trust’s Institute of Pharmacy, Bhaygaon Road, Opp. Jajuwadi, Malegaon Camp, Malegaon, Nashik-423 105, India
E-mail: ladolamanish@gmail.com
| Date of Submission | 7 October 2013 |
| Date of Revision | 18 August 2014 |
| Date of Acceptance | 21 August 2014 |
| Indian J Pharm Sci, 2014;76(5):423-429 |
Abstract
In the present investigation, a novel multifunctional co-processed superdisintegrants consisting of crospovidone and Kyron T-314 were fabricated by solvent evaporation method to develop melt-in-mouth tablets of metoclopramide hydrochloride with a view to enhance patient compliance by direct compression method. The simple physical blends and co-processed mixture of superdisintegrants were characterized for angle of repose, bulk density, tapped density, Carr's index, Hausner's ratio and compatibility studies by FTIR spectroscopy. Melt-in-mouth tablets of metoclopramide hydrochloride were prepared using the physical blends and co-processed mixture of superdisinterants and were evaluated for hardness, friability, in vitro disintegration time, in vitro dispersion time, wetting time, water absorption ratio, drug content, in vitro drug release and accelerated stability study at 40±2° temperature and 75±5% relative humidity. Among the tablets evaluated, formulation F-X prepared by adding co-processed superdisintegrants in ratio of 1:1 showed minimum in vitro dispersion time of 9.71±0.021 s, in vitro disintegration time of 5.70±0.117 s and higher amount of drug release of 99.695±0.29% at the end of 1 min. Formulation F-X was emerged as the overall best formulation based on drug release characteristics in pH 6.8 phosphate buffer compared with the tablets obtained from conventional method of manufacture as well as with marketed preparation. Analysis of drug release data indicated that formulation F-X followed first order kinetics. This study revealed that the co-processed mixture of superdisintegrants have excellent flow properties, high compressibility, render low disintegration time to tablets and have better binding properties as compared to physical blends of superdisintegrants. These materials can be a good substitute for inert superdisintegrants, which are normally used in tablet manufacturing.
Keywords
Direct compression, co-processed superdisintegrants, solvent-evaporation, crospovidone, Kyron T-314, metoclopramide hydrochloride, wetting time
Formulation of a convenient dosage form for administration and achievement of better patient compliance are the two most important criteria in novel drug delivery system [1]. Oral route of administration is the most safer, convenient and economical to formulate solid dosage form. Tablet dosage form covers 80% of all dosage forms administered to humans and have got high popularity in terms of self-administration, manufacturing convenience, accurate dosing, pain avoidance and high stability as compared to liquid and injectable dosage forms [2,3].
Formulation of certain active pharmaceutical ingredients can not be achieved adequately with the use of single component excipient [4]. Hence, excipients with multifunctional characteristics build into them, such as better flow, a minimum tendency for segregation, high compressibility, rapid disintegration ability and low/no moisture sensitivity have gained most acceptance in current pharmaceutical formulation development studies [5]. The objective of co-processing is to provide the synergy of functionality improvement as well as to provide an excipient with multiple characteristics build into them [6]. Co-processed excipients exhibited improved functionality as compared to their physical mixtures [7]. One of the main objectives of the present study was to prepare co-processed superdisintegrants of crospovidone and Kyron T-314 which avoids the problem of segregation of individual superdisintegrants. The study was also aimed to facilitate rapid disintegration of tablets in oral cavity without need of water and subsequent dissolution of active pharmaceutical ingredient to elicit quick onset of action. The reasons for selection of crospovidone are high capillary activity, pronounced hydration capacity and little tendency to form gels. Unique porous particle morphology facilitates wicking of liquid into the pores of the tablet and makes it suitable for direct compression [8]. The reasons for selection of Kyron T-314 are very high swelling tendency of hydration either in contact with water or gastrointestinal fluids causing very fast disintegration without the lump formation. Thus coprocessing of two superdisintegrants with wicking and swelling properties leads to the rapid disintegration of tablets in contact with saliva within few seconds and also serves as a multifunctional excipient [9]. The coprocessed superdisintegrants were prepared by solvent evaporation method.
Materials and Methods
Metoclopramide hydrochloride BP, crospovidone USP-NF, polyvinylpyrrolidone BP (PVP K30), mannitol BP and aspartame were gift samples from Lincoln Pharmaceuticals Limited, Ahmedabad, Gujarat. Direct compressible microcrystalline cellulose NF (Flocel 102) was a gift sample from Gujarat Microwax Pvt. Ltd., Ahmedabad, Gujarat. Kyron T-314 (polacrillin potassium) was a gift sample from Corel Pharm Chem, Ahmedabad, Gujarat. Trusil orange was a gift sample from Medley Pharmaceuticals Limited, Vapi, Gujarat. All other chemicals and reagents used were of analytical reagent grade.
Preparation of co-processed superdisintegrants
The co-processed superdisintegrants were prepared by solvent evaporation method. A blend of crospovidone and Kyron T-314 (in the ratio of 1:1, 1:2 and 2:1) was added to 10 ml of isopropyl alcohol. The contents of the beaker (250 ml capacity) were stirred on a magnetic stirrer. The temperature was maintained between 65° to 70°, and stirring was continued till most of isopropyl alcohol evaporated. The wet coherent mass was granulated through #60-sieve. The wet granules were dried in a hot air oven at 60° for 20 min. The dried granules were sifted on #60-sieve and stored in air tight container till further use [10]. The prepared mixture was evaluated for flow properties and polymer-polymer compatibility studies such as FTIR study.
Flow property
Table 1 shows the parameters associated with flow of physical blend and co-processed mixture of crospovidone and Kyron T-314. For the measurement of angle of repose, a glass funnel was secured with its tip at a given height (h) above a piece of graph paper placed on a horizontal surface. Powder was poured through the funnel until the apex of the conical pile touched the tip of the funnel. The angle of repose was calculated with the formula θ=tan-1 h/r, where θ is the angle of repose and r is the radius of the conical pile. The bulk density was determined as the ratio of weight to the volume of sample. The tapped density was determined as the ratio of weight to the volume of sample after tapping a measuring cylinder for 100 times on an Electrolab Tap density tester. The percentage compressibility (Carr’s index) was calculated as 100 times the ratio of the difference between the tapped density and bulk density to the tapped density. Hausner ratio is equal to the ratio of the tapped density to bulk density [11].
| Parameters | Crospovidone(A) | KyronT‑314 (B) | Physical blends | Co‑processed blends | ||||
|---|---|---|---|---|---|---|---|---|
| 1A: 1B | 1A: 2B | 2A: 1B | 1A: 1B | 1A: 2B | 2A: 1B | |||
| Bulk density* (g/ml)±SD | 0.306±0.003 | 0.844±0.056 | 0.508±0.006 | 0.513±0.013 | 0.508±0.006 | 0.419±0.005 | 0.422±0.005 | 0.413±0.004 |
| Tapped density* (g/ml)±SD | 0.359±0.003 | 0.958±0.092 | 0.545±0.008 | 0.561±0.024 | 0.571±0.016 | 0.437±0.005 | 0.441±0.005 | 0.437±0.005 |
| Angle of repose* (°)±SD | 26.026±1.777 | 30.439±4.392 24.679±1.291 27.641±0.728 | 28.037±1.360 | 27.216±0.744 27.514±0.302 27.122±0.450 | ||||
| Carr’s index* (%)±SD | 14.846±1.456 | 11.370±3.027 | 6.779±1.391 | 8.566±1.721 | 11.055±1.632 | 4.191±0.076 | 4.235±0.076 | 6.201±0.182 |
| Hausner’s ratio*±SD | 1.172±0.683 | 1.126±0.032 | 1.072±0.015 | 1.093±0.020 | 1.124±0.020 | 1.044±0.001 | 1.044±0.001 | 1.066±0.001 |
| Inference | Good | Fair to | Good | Good | Good | Good | Good | Good |
| passable | ||||||||
SD: Standard deviation for n=3 observations
Table 1: Flow Properties Of Physical Blends And Co Processed Mixtures
Drug-excipient compatibility study
Fourier Transform Infra Red (FTIR) spectral data were taken on a Shimadzu (model FTIR-8700) spectrophotometer to find out integrity and chemical stability of the superdisintegrants. FTIR spectra of the crospovidone, Kyron T-314, physical blend and co-processed mixture of superdisintegrants in ratio of 1:1 were obtained. An FTIR spectrum of drug with physical blend and co-processed mixture of superdisintegrants was also recorded. All the samples were crushed with potassium bromide to get pellets at 1 ton/cm2. Spectral scanning was done in the range between 4000-500 cm-1.
Preparation of melt-in-mouth tablets by direct compression method
All the ingredients (except granular directly compressible excipient) were passed through #60-sieve separately. Then the ingredients were weighed and mixed in geometrical order and compressed into tablets of 100 mg using 6 mm round flat punches on a Rimek mini press 1 (Karnavati). The composition of tablet is presented in Table 2. The punches and die were lubricated with a small amount of magnesium stearate using a cotton swab preceding compression.
| Ingredients (mg/tablet) | F‑I | F‑II F‑III F‑IV F‑V F‑VI | F‑VII (1:1) | F‑VIII (1:2) | F‑IX (2:1) | F‑X (1:1) | F‑XI (1:2) | F‑XII (2:1) | F‑XIII | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metoclopramide hydrochloride | 10.0 10.0 10.0 10.0 10.0 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | |||||
| Crospovidone (A) | 1.0 | 2.0 | 3.0 | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ |
| Kyron T‑314 (B) | ‑ | ‑ | ‑ | 1.0 | 2.0 | 3.0 | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ |
| Physical mixture | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | 1.0 | 1.5 | 1.5 | ‑ | ‑ | ‑ | ‑ |
| Co‑processed mixture | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | ‑ | 1.0 | 1.5 | 1.5 | ‑ |
| PVP‑K‑30 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Talc | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Magnesium stearate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Aspartame | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Trusil Orange | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mannitol | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| MCC (q.s.) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
1:1 indicates 1 part of crospovidone and 1 part of Kyron T-314, 1:2 indicates 1 part of crospovidone and 2 part of Kyron T-314 and 2:1 indicates 2 part of crospovidone and 1 part of Kyron T-314, PVP: polyvinylpyrrolidone, MCC: microcrystalline cellulose
Table 2: Formulations Of Metoclopramide Hydrochloride Melt In Mouth Tablets For Batches From F-Vii To F Xiii
Measurement of tablet breaking force (hardness) and friability
Hardness of the tablet, which is the force applied across the diameter of the tablet to break a tablet into halves, was measured using a Pfizer tablet hardness tester. Tablet’s friability was measured using Roche friabilator (USP) at 25 rpm for 4 min [12].
Measurement of in vitro disintegration time and dispersion time
The in vitro disintegration time was measured using an IP 2007 disintegration test apparatus, with distilled water at 37±2° temperature. The in vitro dispersion time was measured by dropping a tablet in a glass cylinder containing 10 ml of Sorenson’s buffer (pH 6.8) maintained at 37±0.5° temperature [12].
Wetting time and water absorption ratio
A piece of tissue paper folded twice was placed in a small petridish (i.d.=6.5 cm) containing 6 ml of Sorenson’s buffer (pH 6.8). A tablet was placed on the paper and the time required for complete wetting was measured. It was noted as wetting time. The water absorption ratio, R, was determined using the formula- R=((Wa-Wb)/Wb)×100, where Wb is the weight of the tablet before water absorption and Wa is the weight of the tablet after water absorption [13].
Drug content uniformity
Ten tablets were weighed and pulverized to a fine powder. A quantity of powder equivalent to 10 mg of metoclopramide hydrochloride was extracted in pH 6.8 phosphate buffer solution and the liquid was filtered through 0.22 μm whatman filter paper. After appropriate dilution of the filtered solution, the drug content was determined at 272 nm using a UV/Vis spectrophotometer (LabIndia 3000+) [14].
In vitro drug release study and comparison with marketed tablet
In vitro dissolution test was performed according to USP 31, 2008; Type II dissolution apparatus (Electrolab, model TDT-08L) fitted with a paddle rotating at 50 rpm using 900 ml of simulated salivary fluid pH 6.8 solution maintained at 37±0.5°. At a predetermined time interval, 5 ml samples were withdrawn, filtered through a 0.22 μm whatman filter paper to analyze for drug content at 272 nm using a UV/Vis spectrophotometer (LabIndia 3000+). in vitro drug release study was performed on tablet formulations from F-I to F-XIII. The standardized formulation F-X was compared with commercial conventional marketed tablets for percentage metoclopramide hydrochloride dissolved. Cumulative percent drug released was calculated and kinetic study models like zero-order and first-order were applied [15].
Stability studies
The tablets of the formulation F-X were subjected to accelerated stability studies by storing the tablets in plastic zip bags at 40±2° temperature and 75±5% relative humidity (RH) for 3 months. Stability study was performed in programmable environmental test chamber (REMI). Stability study was conducted as per ICH guidelines. At intervals of 1 month, the tablets were visually examined for any physical changes and evaluated for in vitro drug release studies [16].
Results and Discussion
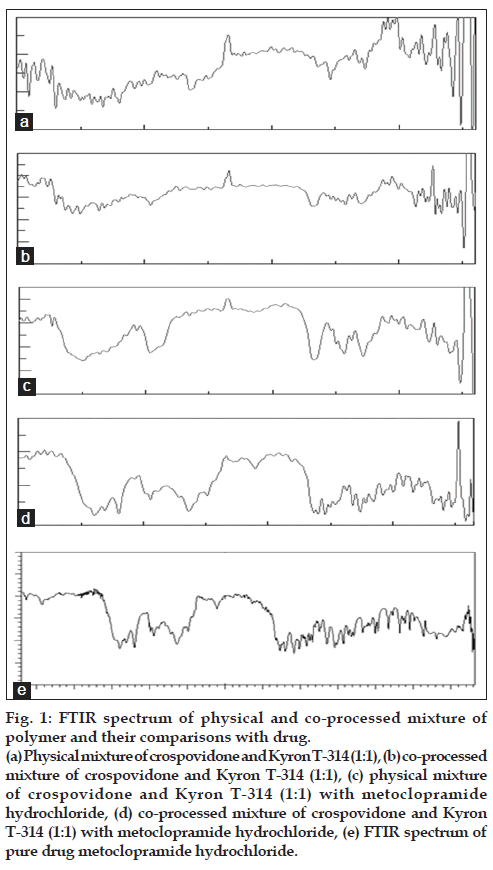
Co-processed superdisintegrants were prepared by incorporating one superdisintegrants into the particle structure of another using solvent evaporation method. The co-processed superdisintegrants were evaluated for their flow and compression properties in comparison with physical mixture of superdisintegrants. The flow properties were graded as per the USP specifications [15]. Table 1 shows the flow property of crospovidone, Kyron T-314, their physical blends and co-processed mixtures with USP specifications. The angle of repose of co-processed superdisintegrants was found be <30° which indicates good flow. The value of Carr’s index between 5-15% indicates excellent flow. Hausner’s ratio in the range of 1.00-1.11 indicates excellent flow as per the USP specifications. This is achieved predominantly because of solvent evaporation method. FTIR studies showed the presence of characteristic peak of C=O stretching of amide of crospovidone at 1685.48 cm-1 in the physical and co-processed blend, thereby indicating that there is no interaction between the two superdisintegrants (fig. 1).
Figure 1: FTIR spectrum of physical and co-processed mixture of
polymer and their comparisons with drug.
(a) Physical mixture of crospovidone and Kyron T-314 (1:1), (b) co-processed
mixture of crospovidone and Kyron T-314 (1:1), (c) physical mixture
of crospovidone and Kyron T-314 (1:1) with metoclopramide
hydrochloride, (d) co-processed mixture of crospovidone and Kyron
T-314 (1:1) with metoclopramide hydrochloride, (e) FTIR spectrum of
pure drug metoclopramide hydrochloride.
Melt-in-mouth tablets each containing 10 mg of metoclopramide hydrochloride were prepared by direct compression method employing crospovidone, Kyron T-314, physical blend and co-processed mixture of both the superdisintegrants in different ratios (1:1, 1:2 and 2:1). Directly compressible excipients, Flocel 102 and mannitol were used as diluents to enhance mouth feel. A total of thirteen formulations were designed and evaluated including control formulation without superdisintegrants and also compared with the marketed preparation (Table 2). All the tablet formulations were evaluated for flow property and compression characteristics. The flow prpoperties were shown in Table 3. The angle of repose for blends was found to be 25-35° and Carr’s index of 5-21% indicates good flow. Hausner’s ratio in the range of 1.08-1.19 indicates good flow property. The result of flow property of formulations indicates that the blends were free flowing.
| Evaluated Parameters | Formulation code | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F‑I | F‑II | F‑III | F‑IV | F‑V | F‑VI | F‑VII | F‑VIII | F‑IX | F‑X | F‑XI | F‑XII | F‑XIII | |
| Bulk density (g/ml) | 0.500 | 0.513 | 0.500 | 0.513 | 0.500 | 0.513 | 0.526 | 0.513 | 0.500 | 0.513 | 0.500 | 0.526 | 0.500 |
| Tapped density (g/ml) | 0.571 | 0.588 | 0.555 | 0.555 | 0.588 | 0.571 | 0.571 | 0.571 | 0.555 | 0.588 | 0.606 | 0.588 | 0.625 |
| Angle of repose (°) | 29.17 | 24.94 | 29.05 | 25.04 | 28.09 | 25.35 | 27.86 | 25.78 | 29.62 | 25.14 | 27.86 | 29.28 | 32.51 |
| Carr’s index (%) | 12.43 | 12.75 | 9.91 | 7.57 | 14.96 | 10.16 | 7.88 | 10.16 | 9.91 | 12.75 | 17.49 | 10.54 | 20.00 |
| Hausner’s ratio | 1.14 | 1.14 | 1.11 | 1.08 | 1.17 | 1.11 | 1.08 | 1.11 | 1.11 | 1.14 | 1.21 | 1.12 | 1.25 |
Table 3: Evaluation Of Physical Properties Of Powder Blends Of Melt In Mouth Tablets Formulations F I To F Xiii Before Direct Compression
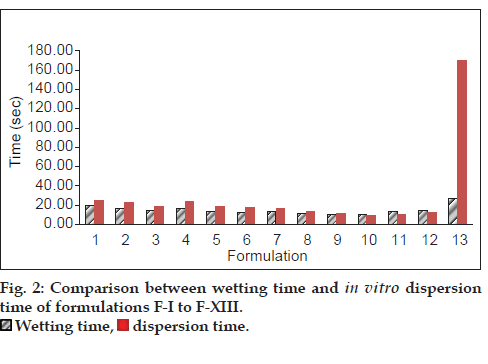
The prepared tablets were evaluated for physical parameters such as hardness, thickness, diameter, friability, weight variation, in vitro dispersion time, in vitro disintegration time, wetting time and water absorption ratio. Table 4 and 5 depicts all the tablet parameters evaluated. The thickness of the prepared tablets was found to be in the range of 3.562±0.004 to 3.602±0.008 mm, while the weight of all the tablets was found to be in the range of 99.45±2.19 to 105.30±1.38 mg. Since mechanical integrity is of paramount importance in successful formulation of melt-in-mouth tablets, the hardness of the tablets was found to be in the range of 3.20±0.141 to 4.04±0.167 kg/cm2. Friability was observed between 0.246±0.002 to 0.652±0.002%, which was below 1% indicating sufficient mechanical strength of the prepared tablets. Wetting time and water absorption ratio which is an important criteria for understanding the capacity of superdisintegrants to swell in the presence of little amount of water was found to be in the range of 10.77±0.025 to 26.76±0.082 s and 76.588±0.999 to 102.474±0.565%, respectively. Results of wetting time and water absorption ratio determined are tabulated in Table 4 and 5. Disintegration time is of much importance in formulation of melt-inmouth tablets which was found to be in the range of 5.70±0.117 to 12.05±0.065 s for formulated tablets. It was observed that the tablets with shortest wetting time showed minimum disintegration time. Comparison between wetting time and in vitro dispersion time of all the prepared formulations is shown in fig. 2. The tablets containing co-processed superdisintegrants (in a ratio of 1:1) showed faster disintegration than tablets containing crospovidone alone, Kyron T-314 alone and their physical blends. As seen from Table 4 and 5, all the tablets subjected to uniformity of drug content revealed the tablets to contain metoclopramide hydrochloride equivalent to metoclopramide between 98.055±0.555 to 100.740±0.698% of the labelled claim.
| Parameters | Formulation code | |||||
|---|---|---|---|---|---|---|
| F‑I | F‑II | F‑III | F‑IV | F‑V | F‑VI | |
| Weight variation (mg) ±SD | 103.30±1.128 | 103.35±1.039 | 103.75±1.292 | 105.30±1.380 | 103.65±1.424 | 101.25±1.860 |
| Hardness* (kg) ±SD | 3.20±0.141 | 3.84±0.089 | 4.00±0.141 | 3.96±0.167 | 4.04±0.167 | 3.36±0.089 |
| Thickness* (mm) ±SD | 3.576±0.005 | 3.582±0.004 | 3.598±0.008 | 3.598±0.004 | 3.602±0.008 | 3.594±0.005 |
| Diameter* (mm) ±SD | 6.026±0.005 | 6.028±0.004 | 6.022±0.008 | 6.026±0.005 | 6.024±0.005 | 6.026±0.008 |
| Friability (%) ±SD | 0.246±0.002 | 0.338±0.004 | 0.385±0.002 | 0.284±0.002 | 0.384±0.002 | 0.288±0.001 |
| In vitro dispersion time* (sec) ±SD | 24.66±0.292 | 23.37±0.031 | 18.68±0.041 | 23.79±0.021 | 18.71±0.040 | 17.76±0.112 |
| Wetting time* (sec) ±SD | 19.94±0.890 | 16.61±0.242 | 14.54±0.262 | 16.88±0.479 | 13.45±0.339 | 12.73±0.481 |
| Water absorption ratio* (%) ±SD | 76.58±0.999 | 86.31±1.531 | 92.68±1.992 | 92.64±1.558 | 97.15±1.295 | 96.48±2.019 |
| In vitro disintegration time* (sec) ±SD | 12.05±0.065 | 11.06±0.063 | 10.07±0.027 | 08.81±0.017 | 08.65±0.021 | 08.65±0.034 |
| Drug content (%) ±SD | 99.07±1.122 | 98.98±0.578 | 99.26±0.892 | 98.15±0.698 | 99.16±0.555 | 100.27±0.555 |
SD: Standard deviation for n=6 observations
Table 4: Evaluation parameters of metoclopramide hydrochloride melt in mouth tablets Formulations F-I to F-IV
| Parameters | |||||||
|---|---|---|---|---|---|---|---|
| F‑VII | F‑VIII | F‑IX | F‑X | F‑XI | F‑XII | F‑XIII | |
| Weight variation (mg) ±SD | 101.85±1.136 | 100.10±1.071 | 100.05±1.316 | 100.65±2.007 | 99.45±2.187 | 100.15±1.598 | 101.80±1.005 |
| Hardness* (kg) ±SD | 3.56±0.089 | 3.76±0.089 | 3.80±0.141 | 3.64±0.089 | 3.80±0.141 | 3.86±0.089 | 3.84±0.167 |
| Thickness* (mm) ±SD | 3.592±0.008 | 3.578±0.004 | 3.576±0.005 | 3.574±0.008 | 3.562±0.004 | 3.574±0.008 | 3.584±0.005 |
| Diameter* (mm) ±SD | 6.022±0.008 | 6.028±0.004 | 6.024±0.008 | 6.022±0.008 | 6.028±0.004 | 6.024±0.008 | 6.028±0.004 |
| Friability (%) ±SD | 0.392±0.001 | 0.447±0.002 | 0.652±0.002 | 0.500±0.002 | 0.462±0.002 | 0.555±0.004 | 0.395±0.002 |
| In vitro dispersion time* (sec) ±SD | 16.68±0.035 | 14.02±0.036 | 11.20±0.033 | 09.71±0.021 | 10.49±0.037 | 12.28±0.034 | 169.90±0.375 |
| Wetting time* (sec) ±SD | 13.79±0.124 | 11.48±0.036 | 10.77±0.025 | 10.79±0.315 | 13.5±0.108 | 14.54±0.056 | 26.76±0.082 |
| Water absorption ratio* (%) ±SD | 94.12±0.649 | 93.22±1.241 | 96.58±0.499 | 100.24±2.772 | 102.47±0.565 | 93.95±1.506 | 79.53±0.671 |
| In vitro disintegration time* (sec) ±SD | 08.22±0.016 | 08.12±0.017 | 07.66±0.026 | 05.70±0.117 | 06.40±0.013 | 06.64±0.018 | 139.0±0.483 |
| Drug content (%) ±SD | 98.05±0.555 | 99.72±0.555 | 99.07±0.578 | 99.62±0.699 | 100.74±0.698 | 100.37±0.69 | 98.240±0.42 |
SD: Standard deviation for n=6 observations
Table 5: Evaluation Parameters Of Metoclopramide Hydrochloride Melt In Mouth Tablets Formulations F-I To F-Xiii
In vitro dissolution studies on all the prepared metoclopramide hydrochloride melt-in-mouth tablets were performed in pH 6.8 phosphate buffer solution. The cumulative percent drug released from formulation F-X was compared with the tablets obtained from conventional method of manufacture as well as with commercial conventional formulation (CCF) (figs. 3 and 4). In comparison to direct compression tablets containing the physical blends of crospovidone and Kyron T-314, a faster drug release was observed from the tablets made from coprocessed superdisintegrants which might be attributed to increased porosity. In order to describe the kinetics of the release process of drug in all the prepared formulations, zero-order and first-order rate equations were used. Zero-order rate equation describes the one whose rate is independent of the concentration of drug undergoing reaction, while the first-order rate equation describes the one whose rate is directly proportional to the concentration of drug undergoing reaction. It is evident from Table 6, that a linear relationship was obtained with ‘r’ (correlation coefficient) value close to unity and higher than ‘r’ value obtained from the zero-order rate equation. This indicates that the drug release process is first order in nature. Accelerated stability studies of the prepared formulations indicated that there are no significant changes in drug content and in vitro dispersion time at the end of 3 months period (P<0.05).
| Formulation code | r* | |
|---|---|---|
| Zero order plot | First order plot | |
| F‑I | 0.9737 | −0.7698 |
| F‑II | 0.9758 | −0.8941 |
| F‑III | 0.9421 | −0.9955 |
| F‑IV | 0.9542 | −0.9827 |
| F‑V | 0.8985 | −0.9922 |
| F‑VI | 0.9049 | −0.9735 |
| F‑VII | 0.8714 | −0.9908 |
| F‑VIII | 0.8390 | −0.9891 |
| F‑IX | 0.7036 | −0.9696 |
| F‑X | 0.9316 | −0.9989 |
| F‑XI | 0.3437 | −0.9470 |
| F‑XII | 0.8379 | −0.3949 |
| F‑XIII | 0.9567 | −0.9815 |
| CCF | 0.9564 | −0.9371 |
r* is correlation coefficient, CCF: commercial conventional formulation
Table 6: Kinetic Data Of Metoclopramide Hydrochloride Melt In Mouth Tablets Formulations F-I To F-Xiii And Ccf
Tablets formulated using a combination of coprocessed crospovidone (A) and Kyron T-314 (B) (1:1) as superdisintegrants (formulation F-X) had shown shortest disintegration time and very fast dissolution profile. Undoubtedly, all the formulations showed very short disintegration time, apart from fulfilling all compendial and other standard specifications, and exhibited faster release rates of metoclopramide hydrochloride.
Acknowledgements
The authors thank the Lincoln Pharmaceuticals Limited, Ahmedabad for providing gift samples of metoclopramide hydrochloride, crospovidone, polyvinylpyrrolidone and mannitol. The authors would also like to thank Corel Pharm Chem, Ahmedabad for gifting Kyron T-314. The authors are also grateful to Gujarat Microwax Pvt. Ltd., Ahmedabad for providing gift sample of microcrystalline cellulose.
References
- Tiwari G, Pathak A, Goyal R, Jadaun CS, Shivhare R, Sharma K. Fast dissolving tablets: A novel approach to drug delivery. World J Pharm Res 2012;1:478-99.
- Gohel MC, Patel TM, Parikh RK, Parejiya PB, Barot BS, Ramkishan A. Exploration of novel co-processed multifunctional diluents for the development of tablet dosage form. Indian J Pharm Sci 2012;74:381-6.
- Banker GS, Anderson NR. Tablets. In: Lachman L, Lieberman HA, Kaning JL, editors. The theory and practice of industrial pharmacy. 3rd ed. Philadelphia, PA: Lea and Febiger; 1986. p. 293-345.
- Block LH, Moreton RC, Apte SP, Wendt RH, Munson EJ, Creekmore JR, et al. Co-processed excipients. In: Pharmacopoeial forum. vol. 35. Maryland, USA: United States Pharmacopoeia Convention Inc; 2009. p. 1026-8.
- Danish M, Kottke MK. Pediatric and Geriatric aspects of Pharmaceutics. In: Banker GS, Rhodes CT, editors. ModernPharmaceutics. 4th ed. New York: Marcel Dekker; 2005. p. 667-94.
- Masareddy R, Kokate A, Shah V. Development of OrodispersibleTizanidineHCl tablets using spray dried co-processed excipient bases. Indian J Pharm Sci 2011;73:392-6.
- Jacob S, Shirwaikar AA, Joseph A, Srinivasan KK. Novel co-processed excipients of mannitol and microcrystalline cellulose for preparing fast dissolving tablets of glipizide. Indian J Pharm Sci 2007;69:633-9.
- Bala R, Khanna S, Pawar P. Polymers in fast disintegrating tablets: A review. Asian J Pharm Clin Res 2012;5:8-14.
- Augsburger LL, Hahm HA, Brzeczko AW, Shah V. Superdisintegrants: Characterization and function. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. 2nd ed. New York: Marcel dekkerInc; 2002. p. 2623-38.
- Gohel MC, Parikh RK, Brahmbhatt BK, Shah AR. Preparation and assessment of novel co-processed superdisintegrant consisting of crospovidone and sodium starch glycolate: A technical note. AAPS Pharmscitech 2007;8:E1-7.
- Carr R. Evaluating flow property of solids. ChemEng 1965;72:163-8.
- Gandhi PP, Vaidhya KA, Shelake GT, Yadav JD, Kulkarni PR. Formulation and evaluation of metformin hydrochloride fast disintegrating tablets by using polacrillin potassium NF from different sources as superdisintegrants. Int J Pharm PharmSci 2010;2:55-7.
- Swathi S, Neeharika V, Lakshmi PK. Formulation and evaluation of fast dissolving tables of freely and poorly soluble drug with natural and synthetic superdisintegrants. Drug Invention Today 2011;3:250-6.
- Shirsand SB, Ramani RG, Swamy PV. Novel co-processed superdisintegrants in the design of fast dissolving tablets. Int J Pharm Bio Sci 2010;1:1-12.
- The United States Pharmacopoeia-31/National Formulary-26. Asian Edition; Rockville, MD: US Pharmacopoeial Convention. Inc; 2008. p. 2692-3.
- Shirsand SB, Sarasija S, Jodhana LS, Swamy PV. Formulation design and optimization of fast disintegrating Lorazepam tablets by effervescent method. Indian J Pharm Sci 2010;72:431-6.
 Wetting time,
Wetting time, 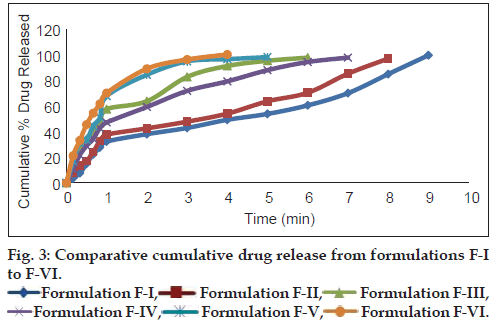
 Formulation F-I,
Formulation F-I, Formulation F-II,
Formulation F-II, Formulation F-III,
Formulation F-III, Formulation F-IV,
Formulation F-IV,  Formulation F-V,
Formulation F-V, Formulation F-VI.
Formulation F-VI.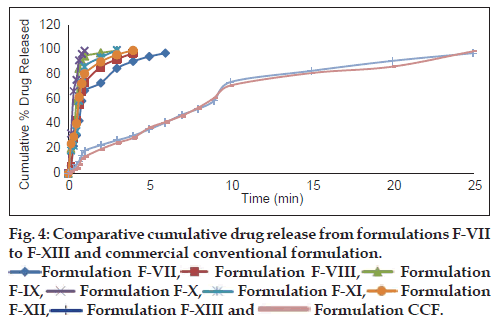
 Formulation F-XIII and
Formulation F-XIII and  Formulation CCF.
Formulation CCF.



