P. R. Mahaparale*, S. A. Nikam and M. S. Chavan1
Department of Pharmaceutics, Dr. D. Y. Patil College of Pharmacy, Sector 29, Pradhikaran, Akurdi, Pune-411044, India
1Government College of Pharmacy, Aurangabad-431 005, India
- *Corresponding Author:
- P. R. Mahaparale
Department of Pharmaceutics, Dr. D. Y. Patil College of Pharmacy, Sector 29, Pradhikaran, Akurdi, Pune-411044, India
E-mail: sarikadeshmukh1986@gmail.com
| Date of Submission | 01 June 2017 |
| Date of Revision | 02 April 2018 |
| Date of Acceptance | 06 October 2018 |
| Indian J Pharm Sci 2018;80(6):1086-1092 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
The objective of the present study was to develop and evaluate sustained delivery of terbinafine hydrochloride from topical polymeric microsponges. Microsponges of ethyl cellulose containing terbinafine hydrochloride were prepared by quasi emulsion solvent diffusion method. Effect of drug polymer ratio on active drug content, particle size and entrapment efficiency were studied. Drug polymer ratio greatly affects properties (entrapment efficiency, active drug content, particle size) of microsponges. Terbinafine hydrochloride microsponges showed highest actual drug content, entrapment efficiency and smaller particle size, so 1.5:1 ratio of drug and polymer was selected for optimization study. Optimization study was carried out by taking internal phase volume, stirring rate, emulsifier concentration as independent variables and their effects on entrapment efficiency, particle size were studied. It was found that as stirring speed increases, the particle size decreases and entrapment efficiency increases, while as volume of dichloromethane increases, particle size decreases. Morphology of obtained microsponges was revealed by scanning electron microscope and was found to be porous and spherical. Optimized formulation of microsponge was dispersed in Carbopol gel and evaluated for drug content, pH, viscosity and in vitro drug release. Release of drug was found to be sustained through microsponge gel as compared to marketed product and pure drug gel. Ex vivo drug deposition study was carried using rat abdominal skin. Drug deposition was found to be satisfactory. Prepared polymeric microsponges could be a potential topical drug delivery system in antifungal therapy.
Keywords
Microsponges, terbinafine HCl, quasi emulsion solvent diffusion method, microsphere, topical drug delivery system, ethyl cellulose
Topical therapy is one of the attractive modes for the management of the cutaneous infections. Advantages of these delivery systems are; it releases drug to the site of infection and minimization of the risk of systemic side effects. Conventional dermatological products typically provide active ingredients in relatively high concentrations but with a short duration of action. This may lead to a cycle of short term overmedication followed by long-term under medication. Due to this, conventional dermatological products have drawbacks like rash, irritation, itching, redness, allergic reaction. The need exists for system to maximize the amount of time that an active ingredient is present either on skin surface or within the epidermis, while minimizing its transdermal penetration into the body [1,2].
Microsponges are polymeric delivery systems consisting of porous microspheres of an inert polymer that can entrap active ingredients and control their release rate. Microsponges are true sponge like spherical particles containing a large number of interconnecting voids within a non-collapsible structure with large porous surface. Because of interconnected void spaces microsponges get large surface area to hold drug. Microsponge technology allows an even and sustained rate of release, reducing irritation while maintaining efficacy [1,2]. Microsponge can be prepared with two methods i.e. one step process (liquidliquid polymerization method) and two-step process (quasi emulsion solvent diffusion method) [1,2]. Most common and feasible method used for preparation of microsponge is quasi emulsion solvent diffusion method [3].
Terbinafine is an allylamine antifungal agent widely used in the treatment of infections engender by dermatophytes (Trichophyton, Epidermophyton and Microspora) including tinea infections [4-7]. The mode of action of terbinafine includes inhibition of enzyme squalene epoxidase in fungal ergosterol biosynthesis, which induces accretion of intracellular squalene and cells death. Adverse effects of terbinafine hydrochloride include burning, irritation and itching allergic reactions like rashes and swelling at the application site. Thus these adverse effects can be reduced by controlled release of terbinafine hydrochloride. Encapsulation of terbinafine hydrochloride reduces cycle of short term overmedication and thereby reduces side effects. The objective of the present investigation was to prepare terbinafine hydrochloride microsponges using ethyl cellulose by quasi emulsion solvent diffusion method.
Materials and Methods
Terbinafine HCl, ethyl cellulose (20 cps) and Eudragit were obtained as gift samples from Wockhardt Research Centre (Aurangabad, India), Colorcon Asia Pvt. Ltd., and Evonik India Pvt. Ltd., Mumbai, India, respectively. All other ingredients used in this study were of analytical grade and purchased from Research Lab Fine Chemicals Ltd., Mumbai, India.
Microsponge preparation
Terbinafine HCl microsponges were prepared by the quasi emulsion solvent diffusion method. The organic internal phase dichloromethane (DCM) containing terbinafine HCl and ethyl cellulose were gradually added into distilled water (external phase) containing polyvinyl alcohol (PVA) as an emulsifying agent. The mixture was stirred on digital mechanical stirrer at 1000 to 1800 rpm for 60 min in order to remove DCM. The formed microsponges were filtered through Whatman filter paper, dried at 40° and weighed.
Preliminary trial batches were taken for selection of polymers like Eudragit RS 100, Eudragit RL100 and ethyl cellulose [8]. Microsponges formulated with Eudragit were not spherical and rigid. The best result was obtained with ethyl cellulose microsponges. Ethyl cellulose gave spherical, rigid and the required micrometer-size microsponges. Hence for further study ethyl cellulose was used as a polymer. Terbinafine microsponge HCl formulations were prepared with different weight ratios of drug to ethyl cellulose (2:1, 1.5:1, 1:1, 1:1.5 and 1:2).
Optimization of formulation parameters and process variables
Effect of different weight ratios of drug to ethyl cellulose on actual drug content (ADC), particle size and entrapment efficiency of microsponges was determined. Further, Box-Behnken design was adopted to optimize the amount of internal solvent volume (5, 10 and 15 ml), emulsifier concentration (400, 500 and 600 mg) and effect of stirring rate (1200, 1500 and 1800 rpm) as the independent variables affecting the particle size, entrapment efficiency and ADC.
Evaluation of terbinafine microsponges[9]
Weighed sample of terbinafine microsponges were dissolved in ethanol under ultrasonication for up to 1 h. The sample was filtered and absorbance was recorded at 283.5 nm using UV spectrophotometer after suitable dilution with water. The drug content and entrapment efficiency [10-12] were calculated using Eqns., ADC (%) = Mactual/Mmicrosponge×100; entrapment efficiency (%) = Mactual/Mtheoretical×100, % drug loading = (mass of drug in microsponges/mass of microsponges recovered)×100 [13], where, Mactual is the actual terbinafine content in weighed quantity of microsponges, Mmicrosponge is the weighed quantity of powdered microsponges and Mtheoretical is the theoretical amount of terbinafine in microsponges calculated from the quantity added in the process. The particle size was determined using an optical microscope with Pixel Pro software. The average particle size was expressed in terms of micrometre (μm). Microsponges were mounted on slide and placed over stage of microscope. Each determination was carried out on a minimum of 100 particles and their mean was reported [14,15].
Surface morphology of particle was studied with scanning electron microscopy (SEM) [9]. Microsponges were mounted on double-faced adhesive tape and coated with a thin gold-palladium layer by sputter-coated unit and analysed with scanning electron microscope (Jeol JSM-6360 A). Powder X-ray diffraction patterns were recorded on a X-ray diffractometer (PW 1729 Philips, Netherland) for pure drug, ethyl cellulose and microsponges [9].
Formulation of Carbopol gel
Gel forming polymer Carbopol 940 (0.25 %) was soaked in distilled water for 2 h and then dispersed by agitation with digital mechanical stirrer to get a smooth dispersion. The resultant viscous solution was neutralized up to pH 7 by triethanolamine with slow agitation. Prepared terbinafine microsponges (equivalent to 1 % W/W terbinafine HCl) were added into Carbopol gel with continuous stirring to get terbinafine microsponge gel (TBFMG) [9].
TBFMG was evaluated for pH using digital pH meter (Equip-Tronics model EQ-614). Spreadability was determined with wooden block and glass slide apparatus by placing excess amount of sample between two glass slides [9,16-18]. One kilogram weight was placed on the slide for 5 min to get uniform thickness. After addition of weight to the pan, length travelled on the glass slide and time required to separate the slides was measured. Spreadability was calculated as follows, S = WL/T, where, S= spreadability, W= weight added in pan, L= length travelled on the glass slide, T= time taken by slides to separate completely from each other. Viscosity of prepared gel was measured by Brookfield viscometer (DV-II +Viscometer) at a room temperature. Gel sample was placed in the sample holder and the viscosity was measured by inserting spindle in sample with rotating speed of 50 rpm at room temperature.
In vitro release studies
In vitro release studies were performed using artificial cellophane membrane (molecular weight 12 000) and vertical Franz diffusion cell having 2.5 cm2 area and 15 ml receptor compartment capacity [3,9,12]. The artificial membrane was carefully placed between the two halves of the diffusion cell. The receptor compartment contains phosphate buffer (pH 5.8), its temperature was maintained at 37±0.5° and stirred continuously using magnetic stirrer. A predetermined amount of TBFMG containing 10 mg of terbinafine HCl was placed on the donor side over cellophane membrane. One ml of the sample was withdrawn from the receptor compartment at definite time intervals for 8 h and replaced with equal volume of fresh receptor fluid. The aliquots were suitably diluted with the receptor medium and analysed by UV spectrophotometer. The release of TBFMG was compared to terbinafine marketed cream (TBFMC, 1 % w/w).
Ex vivo drug deposition study
The ex vivo diffusion study was performed on excised Wister rat skin [3]. The abdominal skin of rat was shaved, carefully placed on the Franz diffusion cell with the epidermal side facing the donor compartment and the dermal side in contact with the receptor solution. Receptor compartment of the diffusion cell was filled with phosphate buffer pH 5.8 with continuous stirring. Sample was applied to donor compartment. Diffusion cell was dismantled after 8 h. The skin was removed carefully and washed with distilled water to remove gel present on skin surface. Skin was chopped to small pieces and homogenized with 10 ml methanol and diluted with phosphate buffer. Drug content in the extract was suitably diluted and absorbance was measured using UV spectroscopy. The ex vivo diffusion study was carried on excised Wistar rat skin according to the protocol approved by the Institutional Animal Ethics Committee.
Antifungal activity
Optimized formulation was evaluated for antifungal activity. The activity was tested against Aspergillus niger by cup plate method [5,16,19,20]. Fungal culture was inoculated in Sabouraud dextrose agar medium and uniformly poured into sterile petri dish. The agar medium was allowed to solidify. Under aseptic conditions, the optimized formulation gel, marketed gel, pure drug and placebo were inoculated by cup plate agar method and plates were incubated at 25° for 24 h. The zone of inhibition was measured.
Stability study
Optimized batch of terbinafine microsponge gels were monitored up to 3 mo at 40±2°/75±5 % RH as per International Conference on Harmonisation guidelines. At the interval of 1, 2, 3 mo samples were withdrawn and tested for physical appearance, pH, viscosity and drug content. Stability study is necessary to predict the long term stability of formulation [3].
Results and Discussion
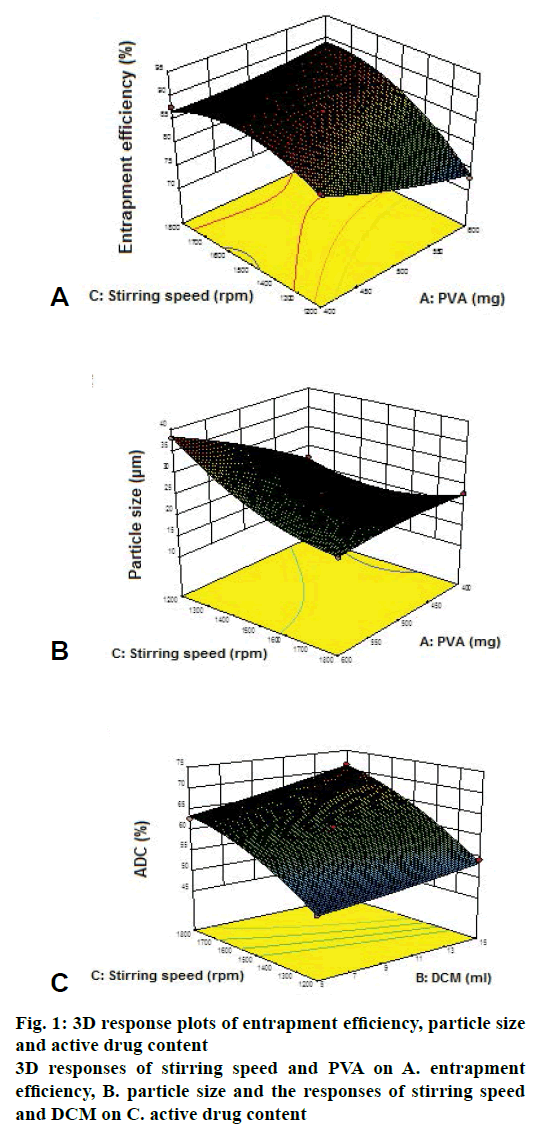
Terbinafine HCl microsponges were prepared using different polymer ratios of 2:1, 1.5:1, 1:1, 1:1.5 and 1:2. These were evaluated for ADC, entrapment efficiency and particle size as shown in Table 1. TBFMG2 showed highest ADC, entrapment efficiency and smaller particle size, so 1.5:1 ratio of drug and polymer was selected for optimization study. Effect of independent factors (PVA, DCM and stirring speed) on entrapment efficiency, particle size and ADC was shown in Table 2. Eqns. generated for entrapment efficiency, particle size and ADC were as follows. Eqn. 1, entrapment efficiency = +87.17–2.31×A+0.70×B+4.27×C, showed that A (PVA) had inverse relation with entrapment efficiency and B (DCM), C (stirring speed) had direct relation with entrapment efficiency. Eqn. 2, particle size = +23.87+4.09×A–1.85×B–3.74×C, showed that A (PVA) had direct relation with particle size and B (DCM), C (stirring speed) had inverse relation with particle size. Eqn. 3, ADC = +61.39–0.79×A+2.98×B+7.89×C, showed that A (PVA) demonstrated inverse relation with ADC and B (DCM), C (stirring speed) direct relation with ADC.
| Formulation code | Drug:polymer (mg) | Entrapment efficiency (%) | Mean particle size (µm) | Active drug content (%) |
|---|---|---|---|---|
| TBFM1 | 2:1 | 69.67±0.66 | 25.81±3.45 | 52.39±1.49 |
| TBFM2 | 1.5:1 | 81.84±1.65 | 21.87±2.73 | 69.52±5.48 |
| TBFM3 | 1:1 | 78.8±2.05 | 36.07±3.80 | 59.95±2.42 |
| TBFM4 | 1:1.5 | 72.5±3.00 | 35.89±4.47 | 65.25±1.97 |
TBFM2 showed highest actual drug content, entrapment efficiency and smaller particle size
Table 1: Drug polymer ratio selection
| Formulation No. | Factor A PVA (mg) |
Factor B DCM (ml) |
Factor C stirring speed (RPM) |
Response 1 Entrapment efficiency (%) |
Response 2 Particle size (µm) |
Response 3 Active drug content (%) |
|---|---|---|---|---|---|---|
| 1 | 500 | 10 | 1500 | 87.17±1.95 | 23.87±3.23 | 61.39±1.00 |
| 2 | 600 | 10 | 1800 | 88.53±2.48 | 22.3±6.59 | 66±1.94 |
| 3 | 600 | 5 | 1500 | 83.02±2.99 | 23.8±2.65 | 60.96±0.80 |
| 4 | 400 | 5 | 1500 | 85.18±2.84 | 15.54±0.85 | 56.92±2.86 |
| 5 | 500 | 10 | 1500 | 87.17±3.85 | 23.87±5.09 | 61.39±2.49 |
| 6 | 500 | 5 | 1800 | 85.97±3.32 | 20.78±3.85 | 62.7±2.24 |
| 7 | 500 | 5 | 1200 | 72.5±4.99 | 27.7±7.36 | 49.37±2.91 |
| 8 | 400 | 15 | 1500 | 87.7±4.82 | 11.84±2.19 | 66.84±5.58 |
| 9 | 600 | 10 | 1200 | 72.87±4.09 | 38.08±4.01 | 49.41±3.08 |
| 10 | 500 | 15 | 1200 | 78.87±4.09 | 24.13±6.17 | 54.27±4.49 |
| 11 | 500 | 10 | 1500 | 87.17±3.68 | 23.87±2.14 | 61.39±1.10 |
| 12 | 500 | 15 | 1800 | 82.09±6.05 | 16.13±4.64 | 71.25±3.92 |
| 13 | 500 | 10 | 1500 | 87.17±2.01 | 23.87±2.83 | 61.39±4.75 |
| 14 | 600 | 15 | 1500 | 83.59±3.38 | 20.93±4.75 | 61.4±4.32 |
| 15 | 400 | 10 | 1800 | 87.72±0.58 | 22.91±4.32 | 68.26±3.88 |
| 16 | 500 | 10 | 1500 | 87.17±8.05 | 23.87±2.84 | 61.39±2.05 |
| 17 | 400 | 10 | 1200 | 85.89±2.12 | 22.14±2.46 | 52.07±2.02 |
Table 2: Box-Behnken design for optimization
Formulation no. 8 was found to be satisfactory in terms of entrapment efficiency, active drug content and particle size. This optimized batch was subjected to further characterization studies and incorporated into Carbopol gel base.
Entrapment efficiency was found to be inversely proportional and particle size was directly proportional to quantity of emulsifier (PVA). The emulsifier employed (PVA) was non-ionic and molecules can associate away from the oil-water interface at higher concentrations. Such alternative hydrophobic region can dissolve some portions of the drug resulting in a reduction in entrapment efficiency as per Figure 1A [10,14].
The results showed that at higher concentration of PVA, bigger microparticles were obtained as shown in Figure 1B. An increase in mean particle size of microsponges with an increase in the emulsifier concentration can be attributed to an increase in apparent viscosity. Such increased viscosity would result in larger emulsion droplets and finally in greater microsponge size.
The particle size was found to be inversely proportional to quantity of internal phase (DCM). The negative influence of internal phase volume on mean particle size indicated that increasing the internal phase volume decreased the particle size of microsponges. Particle sizes of microsponges can be directly attributed to apparent viscosity of internal phase. When the internal phase with lower viscosity was poured into continuous phase, the globules of the formed emulsion could easily divide into smaller droplets and mean particle size decreases [3,17,21] (Figure 1C).
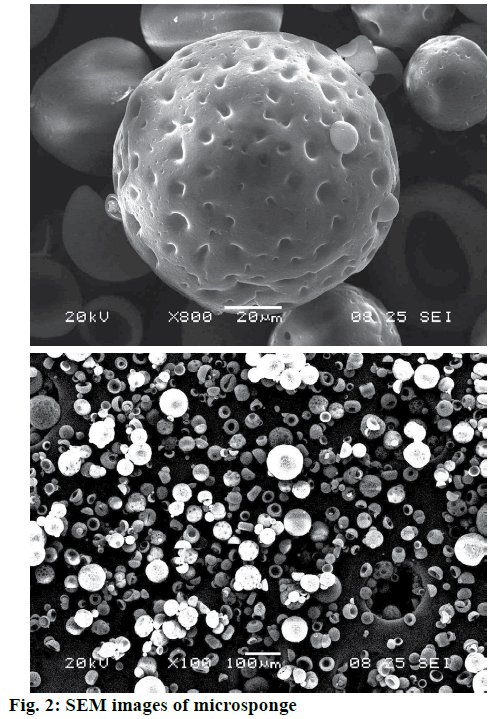
The particle size was found to be inversely proportional to the stirring rate. At higher stirring rate, a vigorous, uniform, increased mechanical shear was imposed and this resulted in a rapid division of the formed droplets, which might have less chance of coalescing into bigger droplets as shown in Figure 2. This led to decrease in particle size with increasing stirring rate [14].
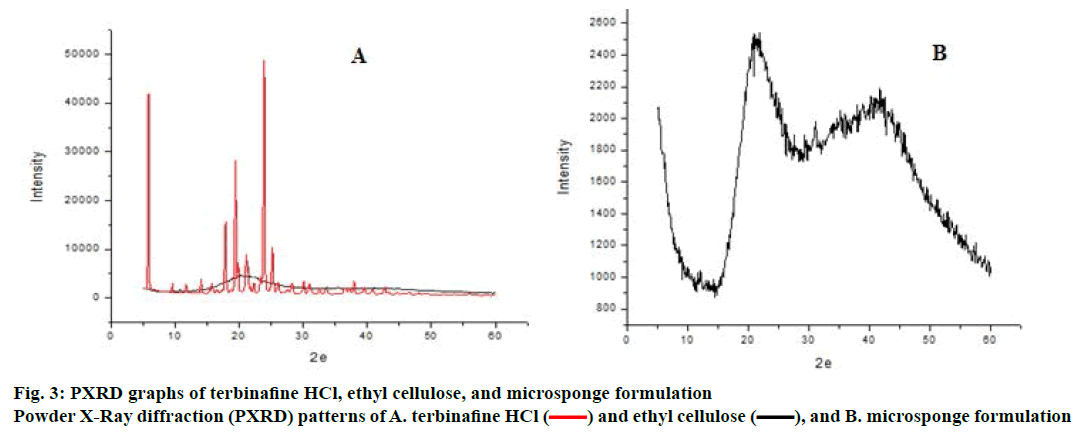
SEM of terbinafine HCl-loaded microsponges shown in Figure 2 revealed that the microsponges were uniform, spherical in shape and porous in nature. The pores were induced by the diffusion of the solvent from the surface of the microparticles. X-ray powder diffractogram of pure drug, ethyl cellulose is shown in Figure 3. Pure drug showed sharp endothermic peak indicating crystalline state while ethyl cellulose not showed sharp peaks indicating amorphous nature. Intensity of peaks was found to be decreased in microsponge, which confirmed that terbinafine HCl was entrapped in ethyl cellulose polymer [9]. Microsponge-loaded gel was white, smooth and homogeneous. The pH, viscosity and spreadability of formulation was found to be 7.1, 2976±1.52 cps and 21.3±0.03 gcm/s, respectively.
In vitro drug release study was carried out using artificial cellophane membrane and phosphate buffer pH 5.8. In vitro release profile of TBFMC and TBFMG were studied. TBFMC showed release within 4 h, while TBFMG showed sustained release up to 8 h with 59.70 % release of drug as shown in Figure 4. Mechanism of drug release was determined using different kinetic models (zero-order, first-order, matrix, Peppas and Hix. Crow. release). The result showed that release kinetics from microsponge gel followed Peppas kinetics (r2: 0.9963).
The amount of drug deposited in rat skin for TBFMG was 0.841 mg/cm2, for TBFMC was 0.357 mg/cm2 at the end of 8 h as shown in Figure 5. The amount of terbinafine HCl deposited in skin from the TBFMG was higher as compared to that with the TBFMC at the end of 8 h. This indicated microsponges improved the drug residence in skin [3,14,15].
The diameters of zone of inhibition were found to be 19.34, 23.66 and 25.12 mm for microsponge gel, marketed gel and pure drug, respectively. Microsponge gel showed relatively small zone of inhibition which could be controlled antifungal activity due to controlled release of terbinafine HCl. The formulation inhibited the growth of A. niger, indicating that the formulation could be used to treat fungal infection.
Microsponges of ethyl cellulose containing terbinafine HCl were prepared by quasi emulsion solvent diffusion method. Drug polymer ratios, internal solvent volume, emulsifier concentration and stirring speed greatly affected physical and chemical properties such as particle size, entrapment efficiency and active drug content of microsponges. Internal phase volume and stirring rate showed negative influence on particle size of microsponges, while concentration of PVA showed direct effect on particle size. X-ray powder diffractogram confirmed entrapment of terbinafine HCl in ethyl cellulose. SEM revealed spherical, porous and rigid nature of microsponges. Optimized microsponge formulation was dispersed into Carbopol gel showed sustained release of terbinafine also showed satisfactory drug deposition within animal skin.
Acknowledgement
The authors thank the institute and Dr N. S. Vyawahare, Principal, Dr D. Y. Patil College of Pharmacy, Akurdi, Pune for providing facilities and also gratefully acknowledge Dr C. Bothiraja, Bharti Vidyapeeth Deemed University, Poona College of Pharmacy, Pune, for useful suggestions.
Conflict of interest
There are no conflicts of interest.
Financial support and sponsorship
References
- Pradhan S. Microsponges as the versatile tool for drug delivery system. Int J Res Pharm Chem 2011;1:243-58.
- Aloorkar NH, Kulkarni AS, Ingale DJ, Patil RA. Microsponges as innovative drug delivery systems. Int J Pharm Sci Nanotech 2012;5:1597-606.
- Nokhodchi A, Jelvehgari M, Siahi MR, Mozafari MR. Factors affecting the morphology of benzoyl peroxide microsponges. Micron 2007;38:834-40.
- Ozcan I, Abaci O, Uztan AH, Aksu B, Boyacio?lu H, Güneri T, et al. Enhanced topical delivery of terbinafine hydrochloride with chitosan hydrogels. AAPS PharmSciTech 2009;10:1024-31.
- Charyulu NR, Satveek M, Harish NM, Patil AB. Comparative study of terbinafine ethosomal formulations: A Novel Approach. Nitte Univ J Health Sci 2013;3:23-9.
- Chakraborti P. A Textbook of Microbiology. London: New Central Book Agency (P) Ltd.; 2013. p. 616-19.
- Tortora GJ, Funke BR, Case CL. Microbiology an Introduction. 9th ed. San Francisco: Pearson Benjamin Cummings; 2004. p. 629-32.
- Rajeshree M, Harsha P, Vishnu P. Photostability enhancement of miconazole nitrate by microsponge Formulation. Int J Curr Trends Pharm Res 2014;2:437-58.
- Bothiraja C, Gholap AD, Shaikh KS, Pawar AP. Investigation of ethyl cellulose microsponge gel for topical delivery of eberconazole nitrate for fungal therapy. Ther Deliv 2014;5:781-94.
- Jain V, Singh R. Dicyclomine-loaded eudragit®-based microsponge with potential for colonic delivery: preparation and characterization. Trop J Pharm Res 2010;9:67-72.
- Como?lu T, Gönül N, Baykara T. Preparation and Invitro evaluation of modified release ketoprofen microsponges. Farmaco 2003;58:101-6.
- Swetha A, Gopal Rao M, Venkata Ramana K, Niyaz Basha B. Formulation and in vitro evaluation of etodolac entrapped in microsponge based drug delivery system. Int J Pharm 2011;1:73-80.
- Dora CP, Singh SK, Kumar S, Datusalia AK, Deep A. Development and characterization of nanoparticles of glibenclamide by solvent displacement method. Acta Pol Pharm 2010;67;3:283-90.
- Jain V, Singh R. Development and characterization of Eudragit RS 100 loaded microsponges and its colonic delivery using natural polysaccharides. Acta Pol Pharm 2010;67:407-15.
- Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 2006;318:103-17.
- Vij NN, Saudagar RB. Formulation, development and evaluation of film-forming gel for prolonged dermal delivery of terbinafine hydrochloride. Int J Pharm Sci Res 2014;5:537-54.
- Abdelmalak NS, el-Menshawea SF. New topical fluconazole microsponge loaded hydrogel: preparation and characterization. Int J Pharm Pharm Sci 2011;4:460-8.
- D’souza J, More HN. Topical antiinflammatory gels of fluocinolone acetonide entrapped in Eudragit based microsponge delivery system. Res J Pharm Tech 2008;1:502-10.
- Delahaye C, Rainford L, Nicholson A, Mitchell S, Lindo J, Ahmad M. Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. J Med Biol Sci 2009;3:1-7.
- Kim DM, Suh MK, Ha GY, Sohng SH. Fingernail onychomycosis due to Aspergillus niger. Ann Dermatol 2012;24:459-63.
- Maity S, Kaity S, Ray S, Sa B. Development and evaluation of xanthan gum-facilitated ethyl cellulose microsponges for controlled percutaneous delivery of diclofenac sodium. Acta Pharm 2011;61:257-70.
 ) and ethyl cellulose (
) and ethyl cellulose ( ), and B. microsponge formulation
), and B. microsponge formulation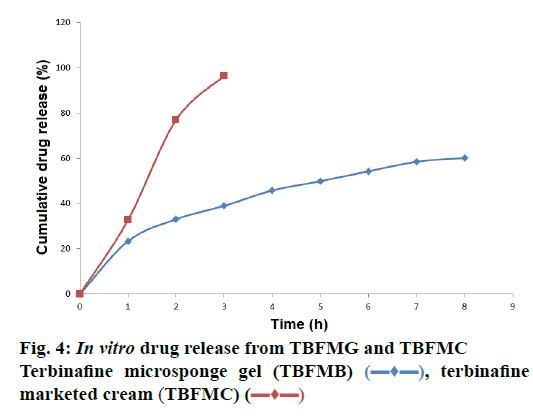
 ), terbinafine marketed cream (TBFMC) (
), terbinafine marketed cream (TBFMC) ( )
)



