- *Corresponding Author:
- Gisele Rodrigues Da Silva
School of Pharmacy, Federal University of Ouro Preto, Ouro Preto, Minas Gerais 35400, Brazil
E-mail: giselersilva@ufop.edu.br
| Date of Received | 16 May 2025 |
| Date of Revision | 17 May 2025 |
| Date of Acceptance | 16 June 2025 |
| Indian J Pharm Sci 2025;87(3):119-125 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
This study aimed to develop and validate a high performance liquid chromatography with ultraviolet method for simultaneous quantification of sulfamethoxazole and trimethoprim in rat plasma after parenteral administration of nanoparticles coated with chitosan, supporting preclinical pharmacokinetic studies for toxoplasmosis treatment. The method used a C18 column at 30°, mobile phase (triethylamine, acetonitrile, water; 1:20:79, pH 5.9), flow rate of 1.0 ml/min, and detection at 268 nm (sulfamethoxazole) and 240 nm (trimethoprim). The validation was assessed by selectivity, linearity, precision, accuracy, and stability. Results showed symmetrical peaks with retention times of approximately 3.0 min (caffeine, internal standard), 4.0 min (trimethoprim), and 7.9 min (sulfamethoxazole), confirming no matrix interference and selectivity of the method. Linearity was achieved for sulfamethoxazole (0.5-50 μg/ml) and trimethoprim (0.1-10 μg/ml), with r2>0.97. Precision and accuracy were validated via relative standard deviation (RSD<15 %) and recovery was within the range of 95 to 103 % for both drugs. Stability was confirmed under controlled storage (CV<15 %), ensuring drug integrity. The validated method was applied to simultaneous determination of sulfamethoxazole and trimethoprim released by nanoparticles coated with chitosan in plasma of rats after parenteral administration. It supports preclinical studies for this innovative toxoplasmosis formulation.
Keywords
Bioanalytical method, validation, sulfamethoxazole, trimethoprim, nanoparticles coated with chitosan, toxoplasmosis
The development of novel drug delivery systems has become a critical area in pharmaceutical science, particularly in enhancing therapeutic efficacy and reducing the side effects of existing medications[1]. Among these systems, poly (?-caprolactone) Nanoparticles Coated with Chitosan (NPsCS) have gained significant attention due to their biocompatibility, biodegradability, and ability to improve drug bioavailability[2]. Sulfamethoxazole (SMX) and Trimethoprim (TMP) (fig. 1) are commonly used anti Toxoplasma gondii drugs that are administered together to treat toxoplasmosis. However, their bioavailability and pharmacokinetics can be limited by factors such as poor solubility and rapid metabolism[3].
The use of NPsCS as carriers for SMX and TMP offers a promising approach to overcome these limitations. By encapsulating these drugs within NPsCS, it is possible to achieve controlled release, improve solubility, and enhance therapeutic outcomes[4]. However, the development of such systems requires robust analytical methods to monitor drug release and pharmacokinetics.
Several studies have successfully developed and validated High Performance Liquid Chromatography with Ultraviolet Detection (HPLC-UV) methods for the simultaneous determination of SMX and TMP in plasma. For instance, Sayar et al.[5] developed a method using a reversed-phase column with UV detection at 254 nm, demonstrating high sensitivity and specificity for both drugs in human plasma. Similarly, another study by El Sharkasy et al.[6] optimized an HPLC-UV method that utilized a mobile phase consisting of methanol and phosphate buffer, achieving reliable quantification of SMX and TMP in biological samples. More recently, a study by Singh et al.[7] validated an HPLC-UV method that employed a gradient elution system, ensuring precise and accurate measurement of these antibiotics in plasma.
These examples highlight the effectiveness of HPLC-UV techniques in monitoring SMX and TMP levels in clinical settings. However, to our knowledge, the existing HPLC-UV methods for quantifying SMX and TMP have not been specifically applied to the analysis of these drugs when incorporated into NPsCS. Therefore, there is a need to develop and validate a method tailored to this unique formulation.
This study aims to develop and validate a HPLC-UV method for the simultaneous determination of SMX and TMP released from NPsCS in plasma, allowing for the reliable quantification of both drugs in plasma. This is crucial for understanding the pharmacokinetic profiles of SMX and TMP when administered via NPsCS, which can inform the design of a new therapeutic alternative for the treatment of toxoplasmosis.
HPLC-Waters Alliance, model 2695, UV-visible detector (Waters Alliance, model 2489), and Empower 2 chromatography data software (Waters Corporation) were used (Massachusetts, United States of America (USA)). A C18 column (150 mm × 4.6 mm I.D., 10 µm particle size) (Supelcosil?, Sigma-Aldrich, United States of America (USA)) was used for separation of SMT and TMP from other compounds in plasma. Column temperature was 30°. The mobile phase consisted of HPLC-grade acetonitrile, triethylamine (J.T. Baker, Phillipsburg, NJ, USA), purified water (1:20:79) (Milli-Q® system from Millipore, Billerica, MA, USA), pH 5.9±0.1. Flow rate was 1.0 ml/min. Total analysis time was 10 min. The injected volume was 50 µl. UV detection was performed at 268 nm and 240 nm for SMX and TMP, respectively.
SMX, TMP, and caffeine, used as an Internal Standard (IS), standard stock solutions (1 mg/ml) were individually prepared by dissolving accurately weighed approximately 10 mg of each analyte in 10 ml of acetone (Sigma-Aldrich, MO, USA). The standard stock solutions of drugs were diluted in acetone to achieve the working concentrations required for the experimentation. IS working solution (10 µg/ml) was prepared by diluting the stock standard solution in acetone. All solutions were stored at 4° and equilibrated to room temperature prior to use. The calibration standard solutions and Quality Control (QC) samples were prepared by spiking (5 % of the total plasma volume) with working solutions. Calibration samples were prepared at concentrations of 0.5, 5.0, 10.0, 20.0, 40.0, and 50.0 µg/ml for SMX and 0.1, 2.0, 4.0, 6.0, 8.0, and 10.0 µg/ml for TMP. Quality control samples were prepared at 0.5 and 0.1 µg/ml Low Quality Control (LQC), 20.0 and 4.0 µg/ml Medium Quality Control (MQC), and 50.0 and 10.0 µg/ml High Quality Control (HQC) for SMZ and TMP, respectively.
Biological samples were homogenized by vortexing (1000 rpm, 5 min), centrifuged (14,000 × g, 15 min, 10°), and the supernatant collected, dried under vacuum in a desiccator for 2 h. The supernatant (380 µl) was reconstituted with the mobile phase and mixed with 20 µl of IS at 10 µg/ml. The reconstituted samples were vortexed (1000 rpm, 5 min), filtered through a 0.45 µm membrane, and analysed by injecting 50 µl into the HPLC-UV system using the validated method.
Method validation was conducted in accordance with the bioanalytical method validation ? guidance for Industry[8], with evaluation of selectivity, linearity, precision and accuracy, and stability.
Selectivity was performed using 6 different sources of blank plasma samples comprising four normal, one haemolysed, and one lipemic. They were analysed and their response was assessed at the retention time and Normalized Matrix Factor (NMF) of analytes and IS. In addition, the interference of endogenous compounds was assessed by analysing the normal plasma spiked with 8 samples of SMX and TMP at LQC levels.
Peak areas of SMX and TMP at concentrations of 1.0, 3.0, 31.0, 56.0, 62.0, and 70.0 µg/ml and 1.0, 3.0, 9.0, 15.0, 18.0, and 21.0 µg/ml, respectively, and IS at 10.0 µg/ml spiked into blank plasma were measured. The ratio of peak areas for each analyte to IS was used to plot the calibration curves and for the linear regression analysis. The lowest standard on the calibration curve was accepted as the Lower Limit of Quantitation (LLOQ) if the analyte response was at least five times higher than that of drug-free (blank) extracted plasma. Additionally, for standards other than the LLOQ, the deviation from the nominal concentration should not exceed 15.0%, ensuring the precision and reliability of the calibration curve across the analytical range.
Intra-day accuracy and precision were assessed by analysing QC samples at LQC, MQC, and HQC spiked into plasma in sextuplicate on the same day. Inter-day variabilities were assessed by determining three precision and accuracy batches on three consecutive days. Accuracy and precision were expressed as Relative Error (RE) and Coefficient of Variation (CV), respectively, which should not be more than 15%.
The recovery was assessed by comparing the mean peak area response of extracted samples (spiked before extraction) to that of unextracted samples (spiked after extraction) at LQC, MQC, and HQC levels in six replicates. Data were expressed as percentage of recovery (% RE).
The stability of LQC and HQC of each drug, spiked into plasma in sextuplicate, was evaluated in the following conditions: bench top stability (at room temperature for 6 h), autosampler stability (process stability at room temperature), freeze (at -80°) thaw (at room temperature) stability at five cycles, and long-term stability (at -80°) for 15 d. The room temperature was approximately 25°. To meet the acceptance criteria, the analytes were considered stable if the CV of each drug concentration was less than 15% of the initial nominal response.
TMP-SMX-loaded poly (e-caprolactone) (PCL, MW ~80,000?90,000 g/mol, Sigma-Aldrich, USA) nanoparticles (TMP-SMX-NPs) were prepared using the emulsification and solvent evaporation techniques. Briefly, the organic phase was prepared by mixing 50 mg of PCL, 3.5 mg of TMP, and 17.5 mg of SMX in 8 ml of a mixture of chloroform and ethylic ether (1:1) (Synth, Brazil) under magnetic agitation for 10 min. The organic phase was then dropped to the aqueous phase (6 ml) containing 1% (w/v) Tween 80 (Sigma-Aldrich, USA). The resulting NPs were magnetically stirred for 22 h at room temperature to ensure complete evaporation of the organic solvent. The TMP-SMX-NPs were collected by ultracentrifugation (14,000 g, 30 min, 8°) (Tecnal, São Paulo, Brazil) and reconstituted in 2 ml of 0.5 mg/ml of Chitosan (CS, MW ~1,250,000, 50-190 kDa; degree of acetylation: 75%-85%, Sigma-Aldrich, USA) in a 3% (v/v) acetic acid solution (pH 2.35) under magnetic stirring at 16,000 g for 30 min to obtain CS-coated TMP-SMX-NPs (TMP-SMX-NPsCS).
Wistar rats (Rattus novergicus) at 6-8 w of age weighing 120-180 g from the Animal Science Center (CCA) at Federal University of Ouro Preto (UFOP) were housed individually in the Laboratory for Animal Experimentation at UFOP?s Research Centre in Biological Sciences (NUPEB). Animals were provided with food and water ad libitum, with 12 h of light/dark cycles at room temperature. The experimental protocol (Number 572916022) was approved by the Ethics Committee at UFOP.
Animals were anesthetized via intraperitoneal injection of ketamine (100 mg/kg) and xylazine (3 mg/kg). To prevent coagulation, heparin (25,000 IU/kg in 0.9% saline) was also administered intraperitoneally. Aseptic surgical catheterization of the femoral artery was performed. Animals (n=8) received a single bolus of TMP-SMX-NPsCS. Blood samples (0.2 ml) were collected at 0.5, 1, 2, 4, and 8 h post-administration via the femoral catheter into pre-chilled Ethylenediaminetetraacetic Acid (EDTA) tubes. Plasma was immediately separated by centrifugation and stored at -80° until analysis. SMX and TMP released from NPsCS were quantified using the validated HPLC-UV method.
In this study, a HPLC-UV method was developed and validated to simultaneously determine SMX and TMP released from NPsCS in plasma after parenteral administration to support preclinical studies for this innovative toxoplasmosis formulation. To develop this method, two segments of bioanalysis were optimized: (1) chromatographic parameters, and (2) sample extraction.
The development of the chromatographic method began with a review of existing literature on SMX and TMP quantification in biological matrices for pharmacokinetic studies, including methodologies for human and veterinary samples[9][10]. Initial chromatographic conditions tested a mobile phase composed of triethylamine, acetonitrile, and water (0.1:20:80, pH 5.9), a flow rate of 1.3 ml/min, and a C18 column. However, these conditions proved inadequate due to asymmetric chromatographic peaks and tailing effects for IS, SMX and TMP, alongside excessive analysis times (>15 min), likely caused by prolonged retention of the hydrophobic analytes despite increased flow rates.
Based on these findings, the mobile phase was optimized to triethylamine, acetonitrile, and water (1:20:79, pH 5.9), with a reduced flow rate of 1.0 ml/min, and retention on the same C18 column. The increased proportion of triethylamine in the mobile phase enhanced its interaction with the acidic, free silanol groups on the stationary phase. Consequently, SMX and TMP, both basic drugs, exhibited reduced interaction with the stationary phase, leading to shorter retention times and a total chromatographic runtime compared to initial attempts. Additionally, the interaction between triethylamine and silanol groups minimized tailing effects in the SMX and TMP chromatographic peaks, a common issue in analyses of basic analytes[11]. The pH 5.9 of the mobile phase created a weakly acidic environment that minimized extensive ionization of SMX (pKa=6.16) and TMP (pKa=7.12)[12]. Since both analytes were partially ionized under these conditions, they interacted with the C18 stationary phase, enabling retention. The lower ionization of SMX (due to its lower pKa) compared to TMP resulted in shorter retention times (7.994 min for SMX vs. 3.972 min for TMP), as the non-ionized fraction of SMX exhibited stronger hydrophobic interactions with the column. This pH-dependent retention behaviour aligns with chromatographic principles, where analyte ionization state directly influences elution order and retention times[12]. Finally, to refine separation efficiency and improve resolution between the chromatographic peaks of the analytes, the mobile phase flow rate was reduced from 1.3 ml/min to 1.0 ml/min. Therefore, these modifications in the chromatographic conditions yielded symmetrical peaks with no tailing and a total analysis time of 10 min, resolving issues of peak shape and analytical efficiency while maintaining chromatographic resolution.
The protein precipitation technique proved to be an effective method for extracting plasma samples. This approach resulted in clear samples, efficiently eliminated plasma interferents, and enabled the recovery of SMX and TMP at LQC levels without interference from plasma components, as evidenced by the method's selectivity. Consistent with our findings, previous studies by Mistri et al.[9] and Bedor et al.[10] have also shown that protein precipitation from human plasma ensures consistent quantitation of SMX and TMP with minimal interference from plasma components.
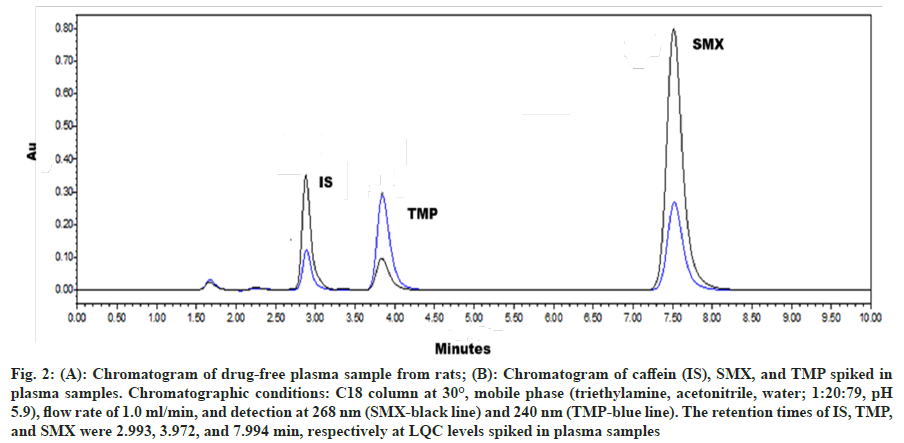
Fig. 2 illustrated the chromatogram of blank plasma (fig. 2A) and the IS, SMX, and TMP at LQC levels in plasma samples (fig. 2B). The chromatographic peaks of the analytes were fully resolved from one another and from plasma interferents, confirming absence of peak overlapping and demonstrating the selectivity of the HPLC method, the effectiveness of the protein precipitation technique to prepare the samples, and finally, no matrix effect. The retention times for IS, TMP, and SMX were determined to be 2.993, 3.972, and 7.994 min, respectively. The precision, expressed as the CV, for eight replicate samples of SMX and TMP at LQC levels spiked in plasma was found to be 6.95% and 13.71%, respectively. These values are below the acceptable limit of 15% as per the validation guidelines[8], thereby validating the method's ability to selectively distinguish the analytes from endogenous substances.
Fig. 2: (A): Chromatogram of drug-free plasma sample from rats; (B): Chromatogram of caffein (IS), SMX, and TMP spiked in plasma samples. Chromatographic conditions: C18 column at 30°, mobile phase (triethylamine, acetonitrile, water; 1:20:79, pH 5.9), flow rate of 1.0 ml/min, and detection at 268 nm (SMX-black line) and 240 nm (TMP-blue line). The retention times of IS, TMP, and SMX were 2.993, 3.972, and 7.994 min, respectively at LQC levels spiked in plasma samples
The linearity of the method was demonstrated over the concentration range of 0.5 to 50 µg/ml for SMX and 0.1 to 10 µg/ml for TMP. Least squares regression analysis was used to calculate the linear equation, where y represented the peak area ratio of the analyte to the IS and x represented the concentration of the analyte. The resulting equations were y = 0.0575x + 0.102 for SMX, with a coefficient of determination (r²) > 0.9759, and y = 0.0625x + 0.0368 for TMP, with r² > 0.9738. The precision of the calibration standards ranged from 0.79 to 8.91% for SMX and 1.63 to 12.63% for TMP.
The intra-day and inter-day precision and accuracy for LQC, MQC, and HQC levels of SMX and TMP spiked in plasma samples were evaluated and presented in Table 1. Precision was expressed as the CV, while accuracy was reported as the RE. These values were lower than 15%, as recommended by validation guidelines[80], showing the precision and accuracy of the HPLC-UV method. Additionally, the recovery of SMX and TMP was determined and expressed as %RE, which was also detailed in Table 1. The %RE were within the acceptance limit of 85% to 105%[10], indicating the suitable recovery of the HPLC-UV method.
| SMX | |||||
|---|---|---|---|---|---|
| Levels | Precision | Accuracy | % RE±SD | ||
| Intra-day | Inter-day | Intra-day | Inter-day | ||
| CV (%) | RSD | ||||
| LQC | 9.39 | 4.59 | 0.83 | 3.83 | 95.36±6.79 |
| MQC | 2.33 | 3.07 | 5.51 | 0.22 | 101.33±7.01 |
| HQC | 1.67 | 3.64 | 2.46 | 1.43 | 102.28±3.93 |
| TMP | |||||
| LQC | 9.16 | 10.76 | 1.29 | 3.95 | 92.73±4.18 |
| MQC | 2.67 | 4.51 | 5.83 | 0.41 | 99.82±5.19 |
| HQC | 2.49 | 8.26 | 2.66 | 11.67 | 99.29±3.17 |
Note: Recovery (% Re) represents the mean percentage of the measured concentration relative to the nominal concentration±SD
Table 1: Intra-Day and Inter-Day Precision, Expressed as CV, and Accuracy, Expressed as Re for SMX and TMP at LQC, MQC, and HQC Quality Control Levels Spiked Into Human Plasma
The stability of SMX and TMP at LQC and HQC quality control levels spiked into plasma was evaluated under various conditions: bench-top stability at room temperature (approximately 25°) for 6 h, auto sampler stability at room temperature, freeze-thaw stability over three cycles (-80° and room temperature), and long-term stability at -80° for 15 d. The stability results, expressed as the CV of the initial nominal response, were detailed in Table 2. Both SMX and TMP demonstrated stability across these conditions, with CV values not exceeding 15%, thereby meeting the acceptance criteria for stability validation[8].
| Stability conditions | SMX | TMP | ||
|---|---|---|---|---|
| LQC | HQC | LQC | HQC | |
| CV (%) | CV (%) | |||
| 1 | 0.56 | 0.51 | 0.24 | 0.19 |
| 2 | 1.60 | 1.17 | 1.85 | 1.07 |
| 3 | 6.66 | 7.13 | 5.18 | 7.14 |
| 4 | 13.27 | 13.10 | 11.09 | 13.81 |
Note: The stability results were expressed as the coefficient of variation (CV %) of the initial nominal response
Table 2: Stability of SMX and TMP at LQC and HQC Quality Control Levels Spiked Into Plasma Evaluated Under The Following Conditions: (1) Bench-Top Stability At Room Temperature (Approximately 25°C) For 6 H; (2): Autosampler Stability At Room Temperature; (3): Freeze-Thaw Stability Over Three Cycles (-80° And Room Temperature); (4): Long-Term Stability At -80° For 15 D
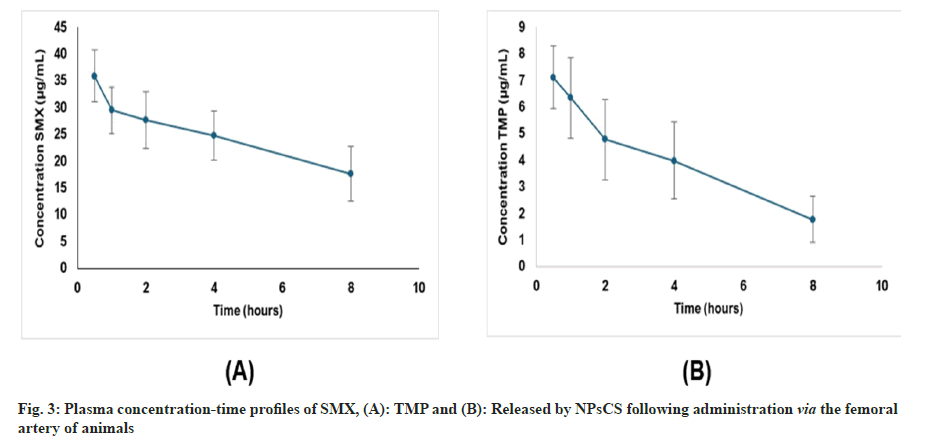
SMX-TMP-NPsCS were administered to animals via the femoral artery, and plasma samples were collected at predetermined time points to quantify drug release using the validated HPLC-UV method. Fig. 3 illustrated the mean plasma concentration-time profiles of SMX and TMP following administration of the formulation, highlighting their distinct pharmacokinetic behaviours. The development of this specific, sensitive, and reliable method enabled accurate quantification of both drugs in plasma, serving as a critical analytical tool to support further pre-clinical studies of CS-coated NP formulations.
In conclusion, the developed HPLC-UV method was validated, demonstrating selectivity, linearity, precision, accuracy, and stability for the determination of SMX and TMP in plasma. By meeting rigorous bioanalytical validation criteria, this method ensured reliable quantitation of both drugs in biological matrices. Notably, the method was successfully applied to assess the plasma concentrations of SMX and TMP released from NPsCS following parenteral administration in rats. This application highlighted the method's utility in preclinical pharmacokinetic studies and supports the optimization of SMX-TMPNPsCS as a novel formulation for toxoplasmosis treatment.
Acknowledgements:
This research was supported by the National Council for Scientific and Technological Development (CNPq, Brazil) (309559/2021-9) and the Minas Gerais State Research Support Foundation (FAPEMIG, Brazil) (APQ-00182-22 and APQ- 04983-22). We acknowledge FAPEMIG and the Research and Project Financing (FINEP, Brazil) for the improvements and expansion of the Animal Science Center (CCA, APQ-02511-22) at UFOP. We also acknowledge UFOP Multiuser Laboratory 1 of the Graduate Program in Pharmaceutical Sciences (CiPharma) by the analysis performed.
Conflict of interests:
The authors declared no conflict of interests.
Ethical approval:
Also, authors declare that this paper has not been submitted elsewhere in similar form, and all authors have contributed significantly to the publication and are aware of the submission and agree with it.
Funding:
This research received no specific grant from any funding agency in the public, commercial, or not-for- profit sectors.
Conflict of interests:
The authors declared no conflict of interests.
References
- Cheng X, Xie Q, Sun Y. Advances in nanomaterial-based targeted drug delivery systems. Front Bioeng Biotechnol 2023;11:1177151.
[Crossref] [Google Scholar] [PubMed]
- Miku?ová V, Miku? P. Advances in chitosan-based nanoparticles for drug delivery. Int J Mol Sci 2021;22(17):9652.
[Crossref] [Google Scholar] [PubMed]
- Aerts R, Mehra V, Groll AH, Martino R, Lagrou K, Robin C, et al. Guidelines for the management of Toxoplasma gondii infection and disease in patients with haematological malignancies and after haematopoietic stem-cell transplantation: guidelines from the 9th European Conference on Infections in Leukaemia, 2022. Lancet Infect Dis 2024;24(5):e291-306.
[Crossref] [Google Scholar] [PubMed]
- Akbari S, Khalili NR, Akbari M, Zarrabi A, Maleki Dizaj S, Sharifi S. Chitosan-based nanoparticles as efficient drug delivery systems for parenteral administration: A review of recent advances in controlled release and therapeutic applications. Int J Biol Macromol 2022;206:1-18.
- Sayar E, Sahin S, Cevheroglu S, Atilla Hincal A. Development and validation of an HPLC method for simultaneous determination of trimethoprim and sulfamethoxazole in human plasma. Eur J Drug Metab Pharmacokinet 2010;35(1-2):41-6.
[Crossref] [Google Scholar] [PubMed]
- El Sharkasy ME, Aboshabana R, Tolba MM, Walash MI, Belal F. A factorial-design/assisted HPLC method for simultaneous monitoring of phenytoin, trimethoprim, and sulphamethoxazole in plasma samples to optimize therapy and mitigate toxicity risks. Microchem J 2024;206:111462.
- Singh A, Kumar R, Sharma P, Gupta S. Development and validation of a gradient HPLC-UV method for the simultaneous determination of sulfamethoxazole and trimethoprim in human plasma. J Chromatogr Sci 2020;58(6):531-538.
- National Health Surveillance Agency (ANVISA). Collegiate Board Resolution-RDC No. 941, November 18, 2024. Provides for the validation of bioanalytical methods and analysis of study samples for regulatory submissions of industrialized human-use medicines. Official Gazette of the Union; 2024.
- Mistri HN, Jangid AG, Pudage A, Shah A, Shrivastav PS. Simultaneous determination of sulfamethoxazole and trimethoprim in microgram quantities from low plasma volume by liquid chromatography?tandem mass spectrometry. Microchem J 2010;94(2):130-8.
- Bedor DC, Goncalves TM, Ferreira ML, De Sousa CE, Menezes AL, Oliveira EJ, et al. Simultaneous determination of sulfamethoxazole and trimethoprim in biological fluids for high-throughput analysis: Comparison of HPLC with ultraviolet and tandem mass spectrometric detection. J Chromatogr B 2008;863(1):46-54.
[Crossref] [Google Scholar] [PubMed]
- Snyder LR, Kirkland JJ, Dolan JW. Introduction to modern liquid chromatography. 3rd ed. Hoboken: John Wiley & Sons; 2010.
- DrugBank. 2025.