- *Corresponding Author:
- N. M. Patel
Shri B. M. Shah College of Pharmaceutical Education & Research, Modasa-383 315, India
E-mail: nmp_pharmacist@rediffmail.com
| Date of Submission | 25 June 2005 |
| Date of Revision | 02 March 2006 |
| Date of Acceptance | 19 December 2006 |
| Indian J Pharm Sci, 2006, 68 (6):793-796 |
Abstract
A simple, precise, accurate and rapid high-performance thin-layer chromatographic method has been developed and validated for the estimation of atorvastatin calcium and ezetimibe simultaneously in combined dosage forms. The stationary phase used was precoated silica gel 60F254. The mobile phase used was a mixture of chloroform: benzene: methanol: acetic acid (6.0:3.0:1.0:0.1 v/v/v/v). The detection of spots was carried out at 250 nm. The method was validated in terms of linearity, accuracy, precision and specificity. The calibration curve was found to be linear between 0.8 and 4.0 µg/spot for atorvastatin calcium and 0.1 and 1.0 µg/spot for ezetimibe. The limit of detection and the limit of quantification for atorvastatin calcium were found to be 170 ng/spot and 570 ng/spot respectively, and for ezetimibe, 20 ng/spot and 70 ng/spot respectively. The proposed method can be successfully used to determine the drug content of marketed formulation.
The combination of atorvastatin calcium and ezetimibe has recently been introduced in the market. Atorvastatin calcium [1,2] is a synthetic lipid lowering agent which inhibits HMG CoA reductase, and ezetimibe [3,4] inhibits the absorption of cholesterol, decreasing the delivery of intestinal cholesterol to the liver. Chemically, atorvastatin calcium is [R-(R*,R*)-2-(4-flurophenyl)-β-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H pyrrole-1-heptonic acid-calcium salt (2:1) trihydrate [5-7], and ezetimibe is (3R,4S)-1-(4-flurophenyl)-3-[(3S)-3-(4-flurophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-2-azetidinone [5,8-9]. Atorvastatin calcium and ezetimibe are not official in IP, BP or USP. HPLC method has been reported for the determination of atorvastatin and its impurities in bulk drug and tablets [10]. No analytical method has so far been reported for the simultaneous determination of atorvastatin calcium and ezetimibe in pharmaceutical dosage forms. The objective of the present work was to develop an accurate, specific and reproducible method for the simultaneous estimation of atorvastatin calcium and ezetimibe in pharmaceutical dosage forms.
Atorvastatin calcium and ezetimibe working standards were procured as gift samples from Torrent Research Centre, Ahmedabad. Silica gel 60F254 TLC plates (E. Merck, Mumbai) were used as a stationary phase. Tablets containing 10 mg each of atorvastatin calcium and ezetimibe were purchased from the local market (Zetistat-10, Torrent Pharmaceuticals Ltd.). A Camag HPTLC system comprising of Camag Linnomate V automatic sample applicator, Hamilton syringe, Camag TLC Scanner 3, Camag WinCATS software, Camag twin-trough chamber and ultrasonicator was used during the study.
Working standards of atorvastatin calcium and ezetimibe (10 mg each) were weighed accurately and diluted with methanol to obtain a final concentration of 1 mg/ml for atorvastatin calcium and 100 μg/ml for ezetimibe. The contents of 20 tablets were ground to a fine powder. Weight equivalent to 25 mg each of atorvastatin calcium and ezetimibe was transferred to a conical flask and dissolved in methanol. The solution was sonicated for 15 min. The extract was filtered through Whatman filter paper No. 41, and the residue was washed with methanol. The extract and washing were pooled and transferred to a 25 ml volumetric flask, and volume was made with methanol. Required dilutions were made to obtain 1000 μg/ml of atorvastatin calcium and 100 μg/ml of ezetimibe in two different 10 ml volumetric flasks.
The chromatographic estimations were performed using stationary phase, precoated silica gel 60F254 aluminium sheets (20 × 10 cm, prewashed with methanol and dried in an oven at 50° for 5 min); mobile phase, chloroform: benzene: methanol: acetic acid (6:3:1:0.1 v/vv/v); chamber and plate saturation time of 30 min. Migration distance allowed was 72 mm; wavelength scanning was done at 250 nm (fig. 1).
Aliquots of 0.8, 0.9, 1, 2, 3 and 4 μl of standard solution of atorvastatin calcium and 1, 3, 6, 8 and 10 μl of standard solution of ezetimibe were applied on the TLC plate. The TLC plate was dried, developed and analyzed photometrically as described earlier. The calibration curves were prepared by plotting peak area versus concentration (μg/spot) corresponding to each spot. The method was validated [11–13] by establishing linearity, accuracy, inter-day and intra-day precision, specificity, repeatability of measurement of peak, as well as repeatability of sample application. The limit of detection and limit of quantification were also determined. The related impurities were determined by spotting higher concentration of the drugs so as to detect and quantify them.
For the analysis of the marketed formulations, 2 μl (for atorvastatin) and 5 μl (for ezetimibe) of filtered solutions of the marketed formulations were spotted onto the same plate, followed by development scanning. The analysis was repeated six times. The spots were resolved into two peaks in the chromatogram of drug samples extracted from the marketed formulations. The content of the drug was calculated from the peak areas recorded.
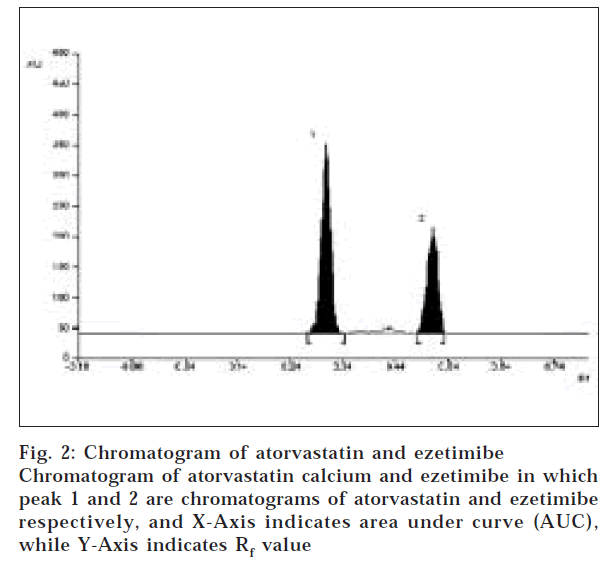
A solvent system that would give dense and compact spots with appropriate and significantly different Rf values was desired for quantification of atorvastatin calcium and ezetimibe in pharmaceutical formulations. The mobile phase consisting of chloroform: benzene: methanol: acetic acid (6:3:1:0.1 v/v/v/v) gave Rf values of 0.3 (±0.04) and 0.53 (±0.04) for atorvastatin and ezetimibe respectively (fig. 2). Linearity range for atorvastatin calcium and ezetimibe was found to be in the range of 0.8-4.0 μg/spot and 0.1-1.0 μg/spot, with a correlation coefficient of 0.9992 and 0.9995, respectively. The LOD and LOQ for atorvastatin calcium were found to be 170 ng/spot and 570 ng/spot for ezetimibe, 20 ng/spot and 70 ng/spot respectively.
The intra-day and inter-day precision (RSD) values were determined for standard atorvastatin calcium (0.8-4.0 μg/ spot) and ezetimibe (0.1-1.0 μg/spot) six times on the same day and over a period of 1 w. The intra-day and inter-day coefficients of variation are given in Table 1.
| Parameter | Result | |
|---|---|---|
| Atorvastatin calcium | Ezetimibe | |
| Linearity range (µg/spot) | 0.8-4.0 | 0.1-1.0 |
| Correlation coefficient | 0.9992 | 0.9995 |
| Precision (% CV) | 1.05-1.15 | 1.12-1.32 |
| Intra day (n=6) | 1.39-1.50 | 1.45-1.89 |
| Inter day (n=6) | 1.09 | 1.17 |
| Repeatability of sample | 0.14 | 0.07 |
| application (n=6) | 170 | 20 |
| Repeatability of peak area (n=6) | 570 | 70 |
| Limit of detection (ng/spot) | Specific | Specific |
| Limit of quantification (ng/spot) | ||
| Specificity | ||
Table 1: Validation Parameters Of Atorvastatin Calcium And Ezetimibe
Repeatability of sample application was assessed by spotting 2 μl of atorvastatin calcium and 5 μl of ezetimibe solution six times on a TLC plate, followed by development of plate and recording the peak area for 6 spots. The % RSD for peak area values of atorvastatin calcium and ezetimibe was found to be 1.09 and 1.17 respectively.
Repeatability of measurement of peak area was determined by spotting 2 μl of atorvastatin calcium and 5 μl of ezetimibe solution on a TLC plate and developing the plate. The separated spot was scanned five times without changing the position of the plate and % RSD for measurement of peak area of atorvastatin and ezetimibe was found to be 0.143 and 0.072 respectively. To confirm the specificity of the proposed method, the solution of the formulation was spotted on the TLC plate, developed and scanned. It was observed that the excipients present in the formulation did not interfere with the peaks of atorvastatin calcium and ezetimibe.
Recovery studies of drugs were carried out for accuracy parameters. These studies were carried out at three levels, i.e., multiple level recovery studies. Sample stock solution from tablet formulation of 1 mg/ml and 100 μg/ml of atorvastatin and ezetimibe respectively was prepared. To the above prepared solution, 50%, 100%, 150% of the standard atorvastatin solution and 20%, 40% and 60% of the standard ezetimibe solution were added. Dilutions were made and recovery studies were performed. Percentage recovery was found to be within limits, as listed in Table 2. For the detection of the related impurities, atorvastatin calcium and ezetimibe (0.1 g each) were dissolved separately in 10 ml of methanol, and these solutions were termed as sample solutions (10 mg/ml). One millilitre of each sample solution was diluted to 10 ml with methanol, and these solutions were termed as standard solutions (1000 μg/ml). Aliquots of both the standard solutions (2 μl) and sample solutions (20 μl) were spotted on the plate and chromatography performed as described earlier. The spot other than the principal spot and the spot of the starting point from the sample solution were not intense than the spot from the standard solution. The sample solution of atorvastatin calcium showed three unknown additional spots at Rf of 0.06, 0.41 and 0.47. The sample solution of ezetimibe showed three unknown additional spots at Rf of 0.37, 0.70 and 0.76. However, the areas of these spots were found to be less than 0.04% as compared to the areas of standard solution spots.
| Label claim mg/tablet | Amount added (%) | Total amount added (mg) | Amount recovered* (mg) ± SD | % recovery ± SD |
% RSD |
|---|---|---|---|---|---|
| Atorvastatin calcium 10 | 50 | 15 | 15.36 ± 0.20 | 102.4 ± 1.36 | 1.36 |
| 100 | 20 | 19.60 ± 0.33 | 98.00 ± 1.63 | 1.63 | |
| 150 | 25 | 25.68 ± 0.29 | 102.7 ± 1.16 | 1.16 | |
| Ezetimibe 10 | 20 | 12 | 11.98 ± 0.12 | 99.87 ± 1.02 | 1.02 |
| 40 | 14 | 14.37 ± 0.22 | 102.65 ± 1.59 | 1.59 | |
| 60 | 16 | 16.20 ± 0.16 | 101.23 ± 0.72 | 0.72 |
Recovery study of atorvastatin calcium and ezetimibe. *indicates that each value is a mean ± standard deviation of three determinations
Table 2: Recovery Of Atorvastatin Calcium And Ezetimibe
The assay value for the marketed formulation was found to be within the limits, as listed in Table 3. The low RSD value indicated the suitability of the method for routine analysis of atorvastatin and ezetimibe in pharmaceutical dosage forms.
| Label claim | Amount | % of drug | % RSD |
|---|---|---|---|
| (mg/tablet) | found* | found* | |
| Atorvastatin calcium 10 | 10.04 | 100.40 | 1.96 |
| Ezetimibe 10 | 9.84 | 98.40 | 0.767 |
*Each value is mean of six determinations
Table 3: Analysis Of Atorvastatin Calcium And Ezetimibe
The developed HPTLC technique is simple, precise, specific and accurate, and the statistical analysis proved that method is reproducible and selective for the analysis of atorvastatin and ezetimibe simultaneously in bulk drug and tablet formulations.
Acknowledgements
Authors sincerely thank the Torrent Research Centre, Ahmedabad, for providing gift samples of atorvastatin and ezetimibe.
References
- Cilla, D. D., Gibson, B. S., and Posvar, M. D., Clin. Pharmacol. Ther., 1996, 60, 687.
- Bakker-Arkema, R. G., Best, J., Fayyad, R., and Marais, A. D., Atherosclerosis, 1997, 131, 17.
- VanHeek, M., France, C. F., and Compton, D. S., J. Pharmacol. Exp. Ther., 1997, 283, 157.
- VanHeek, M., Farley, C., Compton, D. S., Hoos, L., and Alton, K. B., Brit. J. Pharmacol., 2000, 129, 1748.
- Budavari, S., Eds., In; The Merck Index, 13th Edn; Merck and Co.Inc., Whitehouse Station, NJ. 2001, 148, 693.
- Baumann, K. L., Butter, D. E., and Deering, C. F., Tetrahedron Lett., 1992, 33, 2283.
- Kearney, A. S., Crawford, L. F., Mehta, S. C., and Radebaugh, G. W., Pharm. Res., 1993, 10, 1461.
- Rosenblum, S. B., Hvynh, T., Afonso, A., and Yumibe, N., J. Med. Chem., 1998, 41, 973.
- Wu, G., Wong, Y., Chen, X., and Ding, Z., J. Org. Chem., 1999, 64, 3714.
- Erturk, S., Sevinc, Aktas, E., Ersoy, L., and Ficicioglu, S., J. Pharm. Biomed. Anal., 2003, 33, 1017.
- United States Pharmacopoeia XXIV, National Formulary XIX, Assian Edn., US Pharmacopoeial Convention, Inc; Rockville, MD 2000, 2149.
- Shethi, P. D., Eds., In; HPTLC Quantitative Analysis for Pharmaceutical Formulations, 1st Edn., CBS Publishers & Distributors, New Delhi, 1996, 3.
- ICH Guideline Q B, Validation of Analytical Procedures, Methodology, 1996, 1.