- *Corresponding Author:
- Aruna Jadhav
Department of Quality Assurance, Bharati Vidyapeeth’s College of Pharmacy, Navi Mumbai, Maharashtra 400614, India
E-mail: aruna.jadhav@bvcop.in
| Date of Received | 14 July 2021 |
| Date of Revision | 11 January 2022 |
| Date of Acceptance | 28 October 2022 |
| Indian J Pharm Sci 2022;84(5):1338-1342 |
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms
Abstract
Setebid tablet is the natural antihyperglycemic agent with powerful antioxidant action. A highperformance thin layer chromatography method was developed for simultaneous estimation of mangiferin and berberine from setebid tablets. Camag Linomat 5 sample applicator was used for application of sample. Chromatographic separation of drugs was performed over thin layered chromatography plates pre-coated with silica gel 60F254 using toluene:acetone:formic acid (3.5:5.5:1 v/v/v) as the mobile phase via a linear ascending technique in twin trough chamber. Detection and quantification were carried out at an iso-absorptive wavelength of 261 nm using thin layered chromatography scanner III. This method showed good resolution for both drugs with retention factor 0.39±0.03 and 0.80±0.03 for mangiferin and berberine, respectively. The calibration curves were linear in the range of 400-800 and 100-500 ng/spot for mangiferin and berberine and correlation coefficients (r2) were 0.9995 and 0.9991, respectively. The method was validated according to the international conference on harmonisation Q2 R1 guidelines for accuracy, precision, limit of detection, linearity, limit of quantification, robustness and specificity. The limits of detection were 26.18 and 18.50 ng/spot for mangiferin and berberine, respectively. In conclusion, the developed method was rapid, simple, reliable and specific for the identification and quantification of mangiferin and berberine.
Keywords
High performance thin layered chromatography, simultaneous estimation, mangiferin, berberine
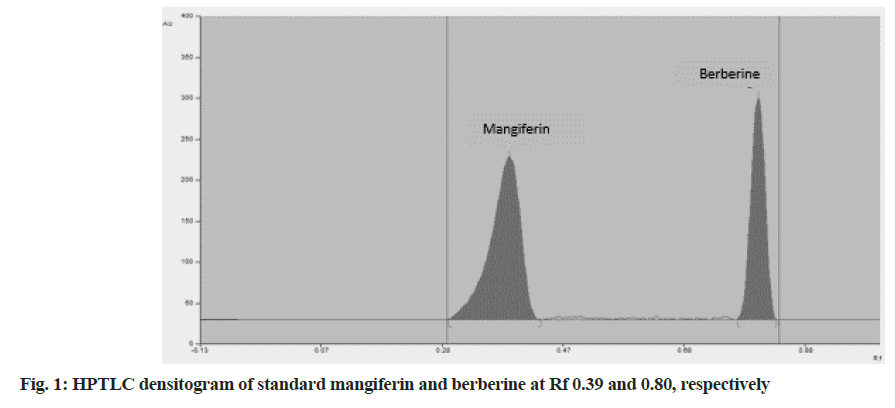
In spite of incredible advances in modern science, technology and allopathic medicine, we are unable to provide quality healthcare to all. Traditional medicine particularly herbal medicine considered as a major healthcare provider around the globe particularly in rural and remote areas[1]. For the past few decades, due to the vast chemical diversity providing by the compounds from natural sources, they are gaining more importance. This has led to an exceptional increase in the demand for herbal medicines within the last 20 y and a requirement has been felt for ensuring the standard, safety, quality and efficacy of herbal drugs[2]. Herbal medicine is still the mainstay of about 75 %-80 % of the world population, mainly in the developing countries, for primary health care because of better cultural acceptability, better compatibility with the human body and lesser adverse effects[3]. Setebid tablet is a polyherbal formulation used in the treatment of diabetes. It helps to control hyperglycemia by enhancing cellular glucose uptake, helps to reduce the need for oral hypoglycemic agent or insulin by augmenting the action of available insulin. It also provides powerful antioxidants to neutralize free radicals, protects against end-organ complications by retarding micro/macro- vascular damage[4]. Each tablet contains 850 mg of aqueous extracts of eleven different herbs in uniform proportions (77.273 mg) namely, Amrasthi (Mangifera indica (M. indica), family: Anacardiaceae), Daruharidra (Berberis aristata (B. aristata), family: Berberidaceae), Nisa (Curcuma longa, family: Zingiberaceae), Amalaki (Emblica officinalis, family: Phyllanthaceae), Jambu (Eugenia jambolana, family: Myrtaceae), Guggulu (Commiphora mukul, family: Burseraceae), Kataka (Strychnos potatorum, family: Loganiaceae), Khadira (Acacia catechu, family: Fabaceae), Jalavedesa (Humboldtia vahliana, family: Leguminosae), Pathya (Terminalia chebula, family: Combretaceae), Musta (Cyperus rotundus, family: Cyperaceae). Mangiferin from M. indica and berberine from B. aristata were selected as markers for simultaneous estimation from Setebid tablet. Mangiferin (1,3,6,7-tetrahydroxyxanth one-C2-β-D glucoside) is the active constituent isolated from various parts of amrasthi, including the leaves, stem bark, fruit peels and root[5]. In the traditional medicine of several countries like China, India and Cuba, mangiferin-rich plants have been grown and actively used to treat many diseases such as cardiovascular diseases, diabetes, many types of infection and cancer. Numerous studies confirm that mangiferin, through different mechanisms, have various biological activities such as anti-cancer, antioxidant, anti-inflammatory, anti-diabetic, cardiovascular protection, neuroprotective, antiviral, analgesic activity, enhanced immunity, gastro protective effect and radioprotection. Some preliminary studies indicate that mangiferin has the effect of reducing insulin resistance as well as reducing hyperglycemia in animal models that already have diabetes[6]. Berberine is a yellow quaternary protoberberine alkaloid found in many medicinal plants[7]. It is extracted from the roots, rhizome and stem bark of daruharidra. It is most widely used since thousands of years in Ayurveda and traditional Chinese medicine for its antiprotozoal, antidiarrheal anti-inflammatory and antimicrobial properties. Recent research has reported that berberine possesses potential therapeutic effects such as antidiabetic, hypolipidemic, anticancer, antiarrhythmic, antifungal, neuroprotective, as well as an anti-atherosclerotic action and improves treatment of polycystic ovary syndrome. In addition, berberine has attracted great interest due to its wide therapeutic applications, cost economy and low toxicity profile[8]. Literature survey showed that mangiferin and berberine were found to possess glucose lowering activity; hence an additive therapeutic effect can be expected in the formulation[9-11]. Numerous studies reported methods for estimation and quantification of mangiferin and berberine individually as well as in combination with other drugs from various formulations using High- Performance Thin-Layer Chromatography (HPTLC) and High Performance Liquid Chromatography (HPLC), but no method was reported for the simultaneous estimation of mangiferin and berberine together from any formulation[12]. The objective of the present investigation was the development of a simple, precise and reproducible HPTLC method for the simultaneous estimation of mangiferin and berberine in the pure form and a commercially marketed formulation. As method validation is an essential constraint in analytical method development, the presented method has been validated following the guidelines of the International Conference on Harmonisation (ICH)[13]. Reference standards Mangiferin and Berberine were procured from Yucca enterprises, Mumbai. Analytical grade reagents and solvents used were toluene, acetone, formic acid and methanol (S. D. Fine-Chem Limited, Mumbai). The marketed formulation (Setebid Tablets) used in this investigation was obtained from the local ayurvedic shop (Patwardhan Ayurved Bhandar) at Panvel, Navi Mumbai, Maharashtra, India. The HPTLC system used consisted of a Camag Linomat 5 sample applicator containing a 100 µl Hamilton syringe. The samples and standard solutions of markers were spotted in the form of bands (6 mm width) at the bottom of the chromatographic plates at a distance of 10 mm. Aluminium TLC plates (10×10 cm, 250 mm thickness Merck) precoated with silica gel 60F254 were used for spotting. The slit dimension was kept at 5 mm×0.45 mm and a scanning speed of 10 mm/s. Analysis was carried out using Camag TLC scanner-III for scanning at 261 nm, which was found to be an absorptive wavelength of both mangiferin and berberine. Camag Win CATS software was used for application and scanning. Camag glass twin-trough is a glass chamber (10×10 cm) saturated with mobile phase was used for the development of the plates. The mobile phase consisted of toluene:acetone:formic acid (3.5:5.5:1, v/v/v) was used for chromatogram development. Prior to the study, the chamber was saturated with the mobile phase for 20 min at room temperature. A separate standard stock solution of 1000 µg/ml was prepared by dissolving 10 mg of each marker (mangiferin and berberine) in 10 ml of methanol. A total of 10 tablets were weighed and powdered. To accurately weighed powder methanol was added and the mixtures were sonicated for 30 min and then filtered through Whatman filter paper no. 42 into a 10 ml volumetric flask. Volume was made up to 10 ml using methanol. The proposed analytical method was validated as per the ICH guidelines Q2 (R1). The parameters checked were linearity, specificity, Limit of Detection (LOD), Limit of Quantification (LOQ), precision, accuracy (recovery) and robustness. The linearity of an analytical procedure is defined as the ability of the analytical procedure to obtain test results within a given range, which are directly proportional to the concentration of the analyte in the sample. Linearity was observed by the plotting graph of drug concentration against peak area for each standard. The Standard Deviation (SD), coefficient of determination (r2), slope, and intercept of the calibration curves were estimated to determine the method linearity. The precision of an analytical procedure is the closeness between a series of measurements that are obtained from multiple sampling of that same homogeneous sample under the prescribed conditions. The current method was validated for intraday and interday precision. Intraday precision was determined in triplicate with the same method on the same day for three different concentrations of mangiferin (400, 600 and 800 ng/spot) and berberine (100, 300 and 500 ng/ spot). The interday precision of the method was done by performing a similar method on different days under the same set of experimental situations. The results were reported as percentage Relative Standard Deviation (% RSD). The accuracy of an analytical procedure is termed as trueness which expresses the closeness of agreement between the conventional true value or an accepted reference value and the value found. Recovery experiments were carried out using a method of spiking of standard. Sample of known concentrations was applied and then spiked with 80, 100 and 120 % w/w amount of analytic in triplicate and the accuracy was calculated as percent of analytic recovered. The robustness of an analytical procedure is a measure of its capacity to stay unchanged by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage. The robustness was studied in triplicate at 400 and 800 ng/spot for mangiferin and 100 and 500 ng/ spot for berberine by deliberately making small changes in the mobile phase composition (3.3:5.3:1 v/v/v) and (3.7:5.7:1 v/v/v) and variation in saturation time (±5 min). Specificity is the ability to assess unequivocally the analytic within the presence of components that can be expected to be present. The specificity of the method was checked by overlaying the spectra of standards with the spectra of the marketed formulation. The method was found to be specific since the spectra of both standards matched with the spectra of the spots in the formulation at the same Retention factor (Rf) values as that of standards. The LOD of an analytical procedure is the lowest amount of analytic present in a sample which can be detected but not necessarily quantitated as an exact value. The Detection Limit (DL) may be expressed as: DL=3.3 σ/S (σ is a standard deviation and S is the slope of the calibration curve). The LOQ of an analytical procedure is defined as the lowest amount of analytic within a sample which can be quantitatively determined with suitable precision and accuracy. The Quantitation Limit (QL) may be expressed as: QL=10 σ/S (σ is a SD and S is the slope of the calibration curve). Chromatographic separation was performed with standard solutions of mangiferin and berberine. The spots of mangiferin and berberine were applied on TLC plates. For the development of the mobile phase, several trials were made using many solvents in different proportions by the linear ascending development method. The optimized mobile phase consisting of toluene:acetone:formic acid (3.5:5.5:1, v/v/v) showed satisfactory resolution at Rf 0.39 for mangiferin and 0.80 for berberine (fig. 1) respectively. Densitometry measurements were obtained with Camag TLC scanner III at 261 nm that was operated by win CATS software. A five-point calibration curve was obtained by plotting peak area against concentrations. Linearity was evaluated by applying different concentrations 400-800 ng/spot for mangiferin and 100-500 ng/spot for berberine in triplicates. A good linearity relationship was found with r2 values of 0.9995 (y=12.05x+399.47) and 0.9991 (Y=10.949x+408.47) for mangiferin and berberine, respectively (Table 1). Both intra-day and inter-day precision of the presented method were calculated for each drug. The outcomes of intra-day and inter-day repeatability are shown in Table 2, which shows % RSD; the low % RSD indicated the method is precise for the analysis. Results from accuracy studies, provided in Table 3, were in an acceptable range (98 %-102 %), indicating that the recovery of the proposed method was good. The effect of intentional changes in the composition of mobile phase and saturation time of mobile phase was studied as % RSD. Low % RSD indicated the method is robust (Table 1). The LOD values for mangiferin and berberine were 26.18 and 18.50 ng, respectively and the LOQ values were 79.35 and 56.08 ng respectively, which shows the sensitivity of the method (Table 1). The specificity of the method was determined by analyzing standard drugs and sample. The spots for mangiferin and berberine in the sample were confirmed by comparing Rf and spectra of spot with that of standard. The purity of the proposed method was determined by superimposing the spectrum of both standard and sample peaks and confirmed for its purity. The samples were spotted in triplicate on a TLC plate and developed. The peak of mangiferin comes at Rf of 0.39 in formulation, whereas berberine in the formulation at Rf of 0.80. The quantified amount of mangiferin and berberine from the marketed ayurvedic formulation of Setebid tablet was found to be 0.21 % w/w and 0.097 % w/w, respectively. In the present study, HPTLC method was developed and validated for the estimation of mangiferin and berberine in a marketed formulation. The method was found simple, rapid, accurate, specific and robust for the analysis of mangiferin and berberine in marketed formulation. The developed method can be adopted by any laboratory for the quality control of crude drugs and formulation that contains mangiferin and berberine as active markers.
| Parameters | Mangiferin | Berberine |
|---|---|---|
| Rf | 0.39 | 0.8 |
| Linearity range (ng/spot) | 400-800 | 100-500 |
| Correlation coefficient (r2) | 0.9995 | 0.9991 |
| Regression equation | y=12.05x+399.47 | Y=10.949x+408.47 |
| LOD (ng/spot) | 26.18 | 18.5 |
| LOQ (ng/spot) | 79.35 | 56.08 |
| Percent recovery (n=3) | 1.012 | 1.0103 |
| Precision (% RSD) | Precise | Precise |
| Robustness | Robust | Robust |
| Specificity | Specific | Specific |
Note: Rf: Retention factor; RSD: relative standard deviation; LOD: limit of detection; LOQ: limit of quantification and n=3 represents each is an average of three observations
Table 1: Results of Method Validation Studies
| Compound | Concentration (ng/spot) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Mean area (AU) | SD | % RSD | Mean area (AU) | SD | % RSD | ||
| Mangiferin | 400 | 4601.33 | 23.947 | 0.517 | 1277.56 | 18.189 | 1.423 |
| 600 | 7245.67 | 93.723 | 1.293 | 3459.78 | 7.726 | 0.223 | |
| 800 | 9031.67 | 159.085 | 1.727 | 5256.44 | 18.090 | 0.344 | |
| Berberine | 100 | 5291.11 | 75.381 | 1.424 | 1359.56 | 21.838 | 1.606 |
| 300 | 7808.56 | 154.828 | 1.982 | 3605.89 | 5.102 | 0.141 | |
| 500 | 9613.44 | 75.833 | 0.788 | 5231.67 | 17.704 | 0.338 | |
Note: Each result is an average of three observations. Concentration levels used for precision parameter was 400, 600, 800 ng/spot for mangiferin and 100, 300, 500 ng/spot for berberine, SD is standard deviation and RSD is relative standard deviation
Table 2: Intraday and Interday Precision Results for Mangiferin and Berberine
| Drug | % level | Initial amount (ng) | Total amount (ng) | Amount of drug recovered (ng) | % recovery |
|---|---|---|---|---|---|
| Mangiferin | 80 | 249 | 448 | 454.89 | 1.015 % |
| 100 | 249 | 498 | 505.46 | 1.014 % | |
| 120 | 249 | 547 | 551.19 | 1.007 % | |
| Berberine | 80 | 595 | 1071 | 1068.93 | 0.998 % |
| 100 | 595 | 1190 | 1210.8 | 1.0175 % | |
| 120 | 595 | 1309 | 1329.33 | 1.0155 % |
Note: Each result is an average of three observations performed at 80, 100 and 120 % levels
Table 3: Recovery Study of Mangiferin and Berberine
Conflict of interests:
The authors declared no conflict of interests
References
- Mirzaeian R, Sadoughi F, Tahmasebian S, Mojahedi M. The role of herbal medicines in health care quality and the related challenges. J Herbmed Pharmacol 2021;10(2):156-65.
- Kshirsagar VB, Deokate UA, Bharkad VB, Khadabadi SS. HPTLC method development and validation for the simultaneous estimation of diosgenin and levodopa in marketed formulation. Asian J Res Chem 2008;1(1):36-9.
- V. P. Kamboj. Herbal medicine. Current Science Association; 2000;78(1). P. 35-9.
- https://www.eayur.com/ayurvedic/vati-tablets/avn-setebid-tablets.htm (Accessed on July 14, 2021).
- Du S, Liu H, Lei T, Xie X, Wang H, He X, et al. Mangiferin: An effective therapeutic agent against several disorders. Mol Med Rep 2018;18(6):4775-86.
- Morozkina SN, Nhung Vu TH, Generalova YE, Snetkov PP, Uspenskaya MV. Mangiferin as new potential anti-cancer agent and mangiferin-integrated polymer systems-A novel research direction. Biomolecules 2021;11(1):79.
[Crossref] [Google Scholar] [PubMed]
- More P, Jadhav AP. Development and validation of analytical method for simultaneous estimation of ellagic acid and berberine in polyherbal formulation by RP-HPLC. Indian Drugs 2021;58(05):52-7.
- Narade S, Pore Y. Assessment of permeability behavior of berberine chloride across goat intestinal membrane in presence of natural Biopotentiator curcumin. Indian Drugs 2021;58(4):23-7.
- Dange SV, Shende SS, Rane BT, Tilak AV, Vaidya MU, Limaye MV. An observational study of the antidiabetic activity of berberine in newly diagnosed type 2 diabetes mellitus patients. J Pharm Biomed Sci 2016;6(4):230-3.
- Pirillo A, Catapano AL. Berberine, a plant alkaloid with lipid-and glucose-lowering properties: From in vitro evidence to clinical studies. Atherosclerosis 2015;243(2):449-61.
[Crossref] [Google Scholar] [PubMed]
- He CC, Luo Z, Wang LL. Mangiferin ameliorates hyperglycemia by inhibiting oxidation and α-glucosidase activity. TMR Mod Herbal Med 2018;1(1):4-10.
- Parekh KP, Jadhav AP. Simultaneous HPTLC estimation of Berberine and Curcumin in Gruhadhoomadi Churna. Indian J Pharm Sci 2018;80(3):570-4.
- ICH. Q2A harmonization tripartite guidelines, test on validation of analytical procedures, IFPMA. In: Proceedings of the international conference on harmonization. Geneva; 1994. p. 1-5.