- *Corresponding Author:
- R. Yanamandra
Department of Organic Chemistry, Food, Drugs and Water, A. U. College of Science and Technology, Andhra University, Visakhapatnam-530 003, India
E-mail: ramesh.yanamandra@gvkbio.com
| Date of Submission | March 19, 2012 |
| Date of Revision | March 14, 2012 |
| Date of Acceptance | May 18, 2011 |
| Indian J Pharm Sci, 2012, 74 (2): 116-121 |
Abstract
A rapid, simple, sensitive and selective analytical method was developed by using reverse phase ultra performance liquid chromatographic technique for the simultaneous estimation of bambuterol hydrochloride and montelukast sodium in combined tablet dosage form. The developed method is superior in technology to conventional high performance liquid chromatography with respect to speed, resolution, solvent consumption, time, and cost of analysis. Elution time for the separation was 6 min and ultra violet detection was carried out at 210 nm. Efficient separation was achieved on BEH C18 sub-2-μm Acquity UPLC column using 0.025% (v/v) trifluoro acetic acid in water and acetonitrile as organic solvent in a linear gradient program. Resolutions between bambuterol hydrochloride and montelukast sodium were found to be more than 31. The active pharmaceutical ingredient was extracted from tablet dosage from using a mixture of methanol, acetonitrile and water as diluent. The calibration graphs were linear for bambuterol hydrochloride and montelukast sodium in the range of 6.25-37.5 μg/ml. The percentage recoveries for bambuterol hydrochloride and montelukast sodium were found to be in the range of 99.1-100.0% and 98.0-101.6%, respectively. The test solution was found to be stable for 7 days when stored in the refrigerator between 2-8?. Developed UPLC method was validated as per International Conference on Harmonization specifications for method validation. This method can be successfully employed for simultaneous estimation of bambuterol hydrochloride and montelukast sodium in bulk drugs and formulations.
Keywords
Bambuterol hydrochloride, column liquid chromatography, montelukast sodium, Ultra Performance Liquid Chromatography (UPLC), validation and simultaneous estimation
Ultra Performance Liquid Chromatography (UPLC) system is an innovative product that brought revolution in high performance liquid chromatography by outperforming conventional high performance liquid chromatography (HPLC). UPLC decreases sample run times up to a factor of 10, uses up to 95 percent less solvent and significantly improves productivity in the lab. UPLC achieves the speed by using novel sub two-micron particles that reduces chromatographic run times and improves resolution. UPLC was designed as a total system to leverage both ultra-high pressure and small particle separation attributes that result in uniquely superior performance with significant improvements in resolution, sensitivity and speed.
UPLC system will eliminate significant time and cost per sample from analytical process while improving the quality of results, the system allows chromatographers to work at higher efficiencies, flow rates, and backpressures. UPLC photodiode array (PDA) detector detects and quantifies lower concentrations of sample analyte, trace impurities with maximum sensitivity and compares spectra across wavelengths and broad concentration ranges. The present study was conducted to separate and quantify bambuterol hydrochloride and montelukast sodium in combined tablet dosage form by using RP-UPLC technique is described in this investigation.
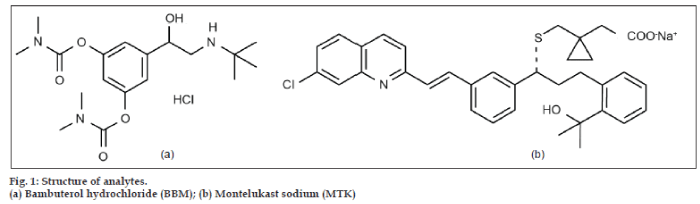
Bambuterol hydrochloride (BBM) (fig. 1a) is used in the treatment of asthma [1,2], is a direct acting sympathomimetic with predominantly-adrenergic activity (β2-agonist) [3]. It is a prodrug of terbutaline [4], after absorption the drug is metabolized via hydrolysis and gets converted into its active metabolite terbutaline. BBM is official in BP [5]. Various HPLC methods [6-8] for dosage, in plasma [9,10], and in combined dosage form [11,12] have been reported for the estimation of BBM in pharmaceutical dosage form by UV detection.
Montelukast Sodium (MTK) (fig. 1b) is a leukotriene receptor antagonist, used in the treatment of asthma. It is not official in IP, BP, and USP. Various liquid chromatographic analytical methods [11-14] for dosage forms and in plasma by UV detection [15-17], by fluorescence detection [18-20], stereo selective enantiomeric high performance liquid chromatography [21], and stability indicating method [22] have been reported. The combination of an oral bronchodilator (BBM) and an agent (MTK) would take care of both the components of asthma, bronchoconstriction and inflammation which give greater clinical efficacy. The combination dosage form of BBM and MTK are available in the market for the prophylaxis and treatment of chronic asthma and chronic bronchitis in pediatrics. One daily dose is recommended for adults during bedtime.
Two reverse phase HPLC methods [11,12] have been reported for the simultaneous determination of BBM and MTK in dosage form. But the reported methods have either longer chromatographic run time or lacks in chromatographic resolution, sensitivity and peak symmetry. A review of the literature did not reveal the presence of simultaneous estimation by using UPLC technique. Present study involves development and validation of UPLC method for the simultaneous estimation of BBM and MTK in combined tablet dosage form, which is fast, simple, sensitive, with better resolution and peak symmetry.
Materials and Methods
Bulk sample of BBM was obtained from Aarti Industries Ltd., (Mumbai, India), MTK was obtained from Inogent Laboratories Ltd., (Hyderabad, India). Commercially available Montair Plus® (Cipla Ltd.) was purchased from local pharmacy containing BBM (10 mg), MTK equivalent to montelukast (10 mg). HPLC grade acetonitrile, methanol was obtained from Apchem, Ashonuj Chem Pvt. Ltd., (Navi Mumbai, India). Trifluoro acetic acid (TFA) spectroscopy grade was purchased from Merck, Darmstadt, Germany. High purity water was obtained from Millipore Milli-Q Plus water purification system. Acquity BEH-C18 and BEH-Shield RP18 columns were purchased from Waters. The LC system used for method development and method validation consisted of a Waters Acquity UPLC with auto sampler and PDA Detector. Empower software (Waters) was used for data handling installed on a Pentium computer (Lenovo).
Chromatographic conditions
The analysis was carried out on Acquity BEH C18 column (100×2.1 mm, 1.7 μ). The mobile phase composition was 0.025% TFA in water as aqueous and 0.025% TFA in acetonitrile as organic solvent system with a linear time gradient program [Time/% solvent: 0/30, 1.5/40, 3/90, 6/90, 6.1/30] at a flow rate of 0.30 ml/min, detection was monitored at 210 nm, and chromatographic run time of 6.0 min. The injection volume was 3.0 μl.
Preparation of diluents and stock solutions
A mixture of methanol and water (10:90, v/v) was used as diluent 1, acetonitrile and water (30:70, v/v) was used as diluent 2. Stock solutions of BBM and MTK 500 μg/ml was prepared separately by dissolving the appropriate amount of each analyte in 25 ml of diluent 1 and diluted to the required concentrations with diluent 2 to obtain a concentration of 25 µg/ml.
Analysis of marketed formulations
Ten tablets were weighed to determine the average tablet weight and powdered in a mortar. Powder equivalent to 10 mg of BBM and MTK was transferred into a 100 ml volumetric flask. About 25 ml of diluent 1 was added and kept on a rotary shaker for 20 min to disperse the material completely followed by sonication for 10 min, cooled to room temperature, make up to mark with diluent 2 and mixed well. About 25 ml of sample solution was centrifuged for 15 min at 2,500 rpm. The sample solution was filtered through a 0.45 μm Nylon-66 membrane filter and 12.5 ml of this solution was taken in a 50 ml volumetric flask and made up to volume with diluent 2.
Method validation
Method validation was performed according to ICH guidelines [23] with respect to precision, linearity, accuracy, detection limit, quantification limit, specificity and robustness. The precision of the developed method was evaluated by six replicate injections of the above standard mixture. The RSD of two drugs was calculated for peak areas, USP tailing factor and plate count. The intermediate precision of the method was also evaluated on a different column.
The solutions for linearity were prepared at six concentration levels ranging from 25 to 150% of the target concentration 25 μg/ml from 500 μg/ml stock solutions. The correlation coefficients, slopes and Y-intercepts of the calibration curve were determined. Standard addition and recovery experiments were conducted to determine accuracy of the method for the quantification of two drugs. The study was carried out at 50, 100 and 150% for three replicate injections of each concentration of the analyte followed by calculation of the percentage recovery. The limit of detection (LOD) and limit of quantitation (LOQ) is calculated by signal to noise ratio method of 3:1 and 10:1, respectively.
For robustness evaluation the experimental conditions were deliberately altered and resolution between BBM and MTK was evaluated. The impact of flow rate on resolution, tailing factor and plate count was studied by the alteration of ±0.05 ml/min. The mobile phase composition and injection volume was kept constant. The effect of different column, different analyst, and different system was also studied. Solution stability of two drugs solution in a tightly capped volumetric flask for 7 days when stored in a refrigerator at 2-8° temperature was studied. The contents of two drugs were determined in 8 h intervals. Mobile phase stability was assessed over a period of 72 h by injecting the freshly prepared sample solutions in 8 h interval as well. The contents of two drugs were determined in the test solutions.
Forced degradation studies
Forced degradation studies of both the drugs were carried out under conditions of acid hydrolysis (0.1 N HCl), base hydrolysis (0.1 N NaOH) and peroxide treatment (0.3% H2O2). Aliquot quantities of mixture of two drugs were weighed in different volumetric flasks and added 3.0 ml of 0.1 N HCl, 3.0 ml of 0.1 N NaOH, and 1.0 ml of 0.3% H2O2, respectively and diluted to 50 ml with diluent 1. These solutions were refluxed at 80° for 8 h, cooled to room temperature, made up to 100 ml with diluent 2 and analyzed by UPLC.
Results and Discussion
The primary target in developing UPLC method was to achieve simultaneous separation and estimation of two drugs in tablet dosage under common conditions that are applicable for routine quality control, research and development of these drugs in ordinary laboratories. The development of RP-UPLC methods for the determination of drugs has received more attention recently because of their speed, resolution, sensitivity, less solvent consumption, cost effectiveness and more productivity which is important in the quality control of drugs and drug products. This work was intended to develop a rapid, precise and reliable method in reverse-phase UPLC separation combined with UV detection for simultaneous estimation in bulk samples and in dosage formulations.
Development of a rapid, rugged and suitable UPLC method for the separation of BBM and MTK required a number of trials to be carried out using different mobile phase compositions. As part of the preliminary work, separation was attempted using BEH C18 (100×2.1 mm, 1.7 μm) column with 0.025% TFA in water as aqueous and 0.025% TFA in acetonitrile as organic in different gradient programs at a flow rate of 0.3 ml/min, at a common UV maxima 210 nm. To improve the resolution between two drugs, attempts were made with different percentages of acetonitrile in the beginning of the gradient program. Trials were also done at different flow rates and different temperatures to optimize the peak shape, sensitivity, tailing factor and resolution between two drugs.
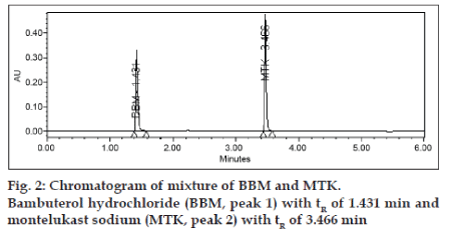
The typical retention times of BBM and MTK is 1.4 and 3.4 min, respectively in a total chromatographic run time of 6 min (fig. 2). The resolution (Rs) between BBM and MTK was ~40. The system suitability test results are summarized in Table 1. The repeatability was checked by repeatedly injecting (n=6) solution of BBM and MTK and the RSD values for peak areas of BBM and MTK were found to be within 2.0% confirming a suitable precision of the method. The correlation coefficient obtained was greater than 0.99 for both bulk and tablet dosage form which confirmed the linear relationship between peak areas and concentrations. Slope and Y-Intercept values were 14763.2 and 65783 for bulk, 6294.4 and 10789 for dosage form for BBM, and 9622.1 and 47214 for bulk and 9069.9 and 62949 for dosage form for MTK. Linearity was determined for six concentrations of each in three replicate injections. The Linearity test results are summarized in Table 2. The percentage recovery obtained for BBM and MTK ranged from 98.4-100.1% and 98.0-101.6%, respectively. The Accuracy test results are summarized in Table 1. The LOD values for BBM and MTK were found to be 0.04 and 0.05, 0.06 and 0.05 for bulk and dosage form, respectively. The LOQ values for BBM and MTK were found to be 0.12 and 0.15, 0.18 and 0.15 for bulk and dosage form, respectively and the results were summarized in Table 1.
| Parameter (Units) | BBM | MTK |
|---|---|---|
| Retention time±allowable time (min.) | 1.40±0.1 | 3.42±0.1 |
| Resolution | - | 44.4 |
| Theoretical polates | 10269 | 172498 |
| Tailing factor (asymmetry factor) | 1.72 | 1.54 |
| Capacity factor | 1.8 | 5.8 |
| Precision (%RSD, n=6) | 0.93 | 1.27 |
| Linearity range (µg/ml) | 6.25–37.5 | 6.25–37.5 |
| Correlation coefficient | 0.999 | 0.992 |
| LOD (µg/ml) | 0.05 | 0.05 |
| LOQ (µg/ml) | 0.15 | 0.15 |
| Recovery (%) | 99.47 | 103.1 |
BBM is Bambuterol Hydrochloride, MTK is Montelukast Sodium, SST stands for system suitability test, LOD stands for limit of detection, LOQ stands for limit of quantification
Table 1: Summary of sst and validation parameters.
| Parameter (Units) | BBM | MTK |
|---|---|---|
| Linearity range (µg/ml) | 6.25–37.5 | 6.25–37.5 |
| r2±%RSD | 0.999±0.02 | 0.992±0.06 |
| Slope±%RSD | 6294.4±0.3 | 9069.9±0.2 |
| Intercept±%RSD | 10789±1.29 | 62949±1.80 |
BBM is Bambuterol Hydrochloride, MTK is Montelukast Sodium, r is regression coefficient, RSD stands for relative standard deviation
Table 2: Linear regression data for calibration curves
Deliberate changes in chromatographic conditions (flow rate, column, and column temperature) resulted in resolution values greater than 35 between BBM and MTK illustrating a good robustness of the method. The Robustness and Ruggedness test results are summarized in Table 3.
| Parameter | Variation | BBM (% Assay±%RSD) | MTK (% Assay±%RSD) |
|---|---|---|---|
| Different flow rate (0.30±0.05 ml/min) | 0.25 ml/min | 99.08±0.35 | 100.58±1.18 |
| 0.35 ml/min | 98.89±0.30 | 99.35±0.62 | |
| Different column temperature (25±2º) | 23º | 99.72±0.34 | 101.5±0.30 |
| 27º | 99.49±0.39 | 101.2±0.32 | |
| Different column (100x2.1 mm, 1.7 μm) | BEH C18 | 101.61±1.08 | 96.05±1.15 |
| BEH Shield RP18 | 97.17±0.24 | 102.41±1.46 | |
| Different system | System 1 | 100.32±0.65 | 98.96±0.37 |
| System 2 | 98.16±0.36 | 96.88±1.43 | |
| % of Acetonitrile (v/v) | 27% | 99.21±0.78 | 100.65±0.65 |
| 33% | 99.95±0.42 | 100.73±1.18 | |
| Intraday (n=3) | Same day | 98.79±0.32 | 99.46±0.16 |
| Interday (n=3) | Different day | 97.53±0.35 | 98.58±1.2 |
Concentration level used for robustness evaluation was 25 µg/ml, RSD stands for relative standard deviation for n=3 observations
Table 3: Robustness and ruggedness by the proposed method
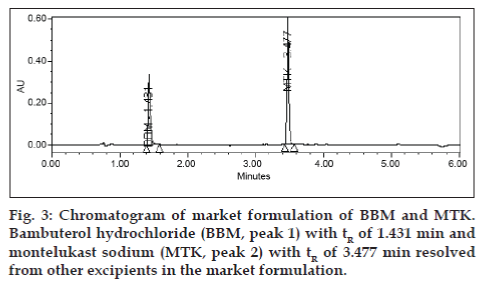
Potential interferences from excipients (dibasic calcium phosphate anhydrous, pregelatinized starch, croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, povidone, hypromellose, titanium dioxide, sodium lauryl sulfate, triacetin, titanium dioxide, red oxide of iron and yellow oxide of iron) were also investigated. No interferences from excipients were observed (fig. 3). Analysis was performed for two different batches of pharmaceutical dosage form (each n=3). In all batches the contents of BBM and MTK were well within the limits of 90.0-110.0% (w/w). The Assay results for tablet dosage form are summarized in Table 4. For intra-day and inter-day precision five replicate injections of assay concentration were analyzed. The stability of BBM and MTK were assessed during analysis and storage. No significant changes were observed in the content of BBM and MTK during solution and mobile phase stability experiments. Due to the sensitivity of MTK to the light, the standard and sample solutions prepared in amber color volumetric flasks, wrapped with aluminum foil were stable up to 7 days when stored in a refrigerator between 2 to 8°.
| Formulation | Label claim (mg) | Amount found (mg) | % Label claim±SD* | |||
|---|---|---|---|---|---|---|
| BBM | MTK | BBM | MTK | BBM | MTK | |
| B. No: 1 | 10 | 10 | 10.16 | 9.605 | 101.61±1.08 | 96.05±1.15 |
| B. No: 2 | 10 | 10 | 9.657 | 10.369 | 96.57±0.38 | 103.7±1.28 |
BBM is Bambuterol Hydrochloride, MTK is Montelukast Sodium, SD* is standard deviation for n=3 observations
Table 4: Assay results for tablet dosage form
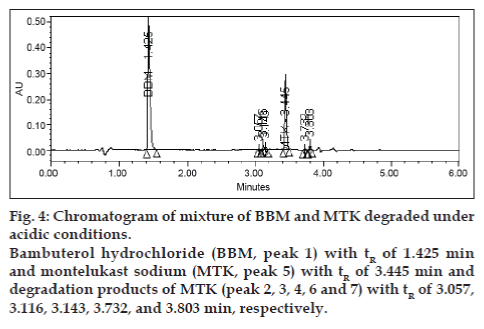
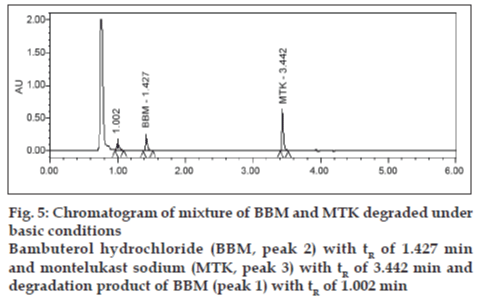
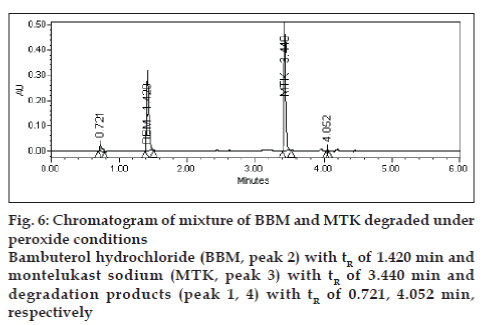
Degradation was not found in acid hydrolysis for BBM, but 17% degradation was found for MTK with a maximum individual degradation product of 6.0% (fig. 4). Degradation was not found in base hydrolysis for MTK, but 40% degradation was found for BBM (fig. 5). Degradation was found in peroxide treatment for BBM and MTK of 9.8% and 0.9%, respectively (fig. 6). Degradants that are formed have base line chromatographic resolution with main components and peak purity flags passes for both drug products, hence the method is specific.
Fig. 4: Chromatogram of mixture of BBM and MTK degraded under
acidic conditions.
Bambuterol hydrochloride (BBM, peak 1) with tR of 1.425 min
and montelukast sodium (MTK, peak 5) with tR of 3.445 min and
degradation products of MTK (peak 2, 3, 4, 6 and 7) with tR of 3.057,
3.116, 3.143, 3.732, and 3.803 min, respectively.
In the proposed study, Reverse Phase UPLC method with UV detection was described and validated for the simultaneous separation and estimation of BBM and MTK for tablets. Statistical analysis proved that method was accurate, precise and reproducible. The developed method was found to be simple, rapid, sensitive and selective for analysis of BBM and MTK for bulk and tablet dosage form without any interference from the excipients. The method was successfully used for estimation of drugs in a pharmaceutical formulation. As the chromatographic run time is short and the method is economical, it can be applied in routine and in quality control analysis where there is no need of two different methods. The proposed UPLC method is non hazardous to human health and also to the environment and is more economic due to the reason that a couple of hundreds of samples can be analyzed in a single day.
Acknowledgements
The authors wish to thank the management, Dr. Mohammed Sharfuddin and other colleagues of GVK Biosciences Private Ltd. Hyderabad, India, for their support to carry out this investigation.
References
- Sweetman SC. Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 768.
- Budavari S. The Merck Index. 12th ed. New Jersey: Whitehouse Station; 1996. p. 163.
- Sweetman SC. Martindale: The Complete Drug Reference. 33rd ed. London: Pharmaceutical Press; 2002. p. 761.
- Budavari S. The Merck Index. 12th ed. New Jersey: Whitehouse Station; 1996. p. 1070.
- The British Pharmacopoeia, Vol. 1. London: Stationery Office Books; 2001. p. 174-5.
- Jing Pz, Yan L. Determination of bambuterol hydrochloride and its related substances in bambuterol hydrochloride capsules by HPLC. Chin Pharm Affairs 2008;11:994-6.
- Bartolincic A, Druskovic V, Sporec A, Vinkovic V. Development and validation of HPLC methods for the enantioselective analysis of bambuterol and albuterol. J Pharm Biomed Anal 2005;36:1003-10.
- Wannerberg O, Persson B. Liquid chromatographic methods for the determination of bambuterol hydrochloride and related compounds. J Chromatogr A 1988;435:199-203.
- Rosberg B, Schroder C, Nyberg L, Rosenborg J, Wiren JE. Bambuteroland terbutaline in human cerebrospinal fluid and plasma. Eur J Clin Pharm 1993;45:147-50.
- Chen XY, Xu HY, Zhong DF, Yang HY, Zhang YF. Determination of bambuterol in human plasma by liquid chromatography-electrospray tandem mass spectrometry: Application to pharmacokinetic study. Yao XueXueBao 2001;36:762-5.
- Patil S, Pore YV, Kuchekar BS, Mane A, Khire VG. Determination of montelukast sodium and bambuterol hydrochloride in tablets using RP HPLC. Indian J Pharm Sci 2009;71:58-61.
- Patel SA, Patel SK, Patel DJ, Patel NJ. Analytical method development and validation of montelukast sodium and bambuterol hydrochloride in combined dosage form by RP-HPLC. Int J Pharm Tech Res 2010;2:1767-71.
- Kumar SJV, Ramachandran D, Saradhi VS. RP-HPLC method for the estimation of montelukast sodium in pharmaceutical dosage forms. CurrTrends Biotech Pharm 2010;4:943-6.
- Singh RM, Saini PK, Mathur SC, Singh GN, Lal B. Development and validation of a RP-HPLC method for estimation of montelukast sodium in bulk and in tablet dosage form. Indian J Pharm Sci 2010;72:235-7.
- Challa BR, Awen BZ, Chandu BR, Khagga M, Kotthapalli CB. Method development and validation of montelukast in human plasma by HPLC coupled with ESI-MS/MS: Application to a Bioequivalence Study. SciPharm 2010;78:411-22.
- Amin RD, Cheng H, Rogers JD. Determination of montelukast in human plasma by liquid chromatography. J Pharm Biomed Anal 1995;13:155-8.
- Robert P, Pauline L, Wayne M, Elizabeth K. A rapid and sensitive method for the quantification of montelukast in sheep plasma using liquid chromatography/tandem mass spectrometry. J Chromatogr B 2007;858:282-6.
- Al-Rawithi S, Al-Gazlan S, Al-Ahmadi W, Alshowaier IA, Yusuf A, Raines DA. Expedient liquid chromatographic method with fluorescence detection for montelukast sodium in micro samples of plasma. J Chromatogr B Biomed SciAppl 2001;754:527-31.
- Ochiai H, Uchiyama N, Takano T, Hara K, Amei T. Determination of montelukast sodium in human plasma by column-switching high performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed SciAppl 1998;713:409-14.
- Chauhan B, Rani S, Nivsarkar M, Padh H. New liquid liquid extraction method for determination for montelukast in small volume human plasma samples using HPLC with fluorescence detector. Indian J Pharm Sci 2006;68:517-20.
- Liu L, Cheng H, Zhao JJ, Rogers JD. Determination of montelukast (MK-0476) and S-enantiomer in human plasma by stereo selective high performance liquid chromatography with column switching. J Pharm Biomed Anal 1997;15:631-8.
- Alsarra I. Development of a stability-indicating HPLC method for the determination of montelukast in tablets and human plasma and its applications to pharmacokinetic and stability studies. Saudi Pharm J 2004;12:136-43.
- International Conferences on Harmonization Q2 (R1). Validation of analytical Procedures: text and methodology, 2005.