- Corresponding Author:
- S. M. Taghizadeh
Department of Novel Drug Delivery Systems, Polymer Science Faculty, Iran Polymer and Petrochemical Institute, P.O. Box: 14965-115, Tehran, Iran. E−mail: s.m.taghizadeh@ippi.ac.ir
| Date of Submission | 08 December 2012 |
| Date of Revision | 24 February 2013 |
| Date of Acceptance | 25 February 2013 |
| Indian J Pharm Sci 2013;75(2):221-226 |
Abstract
A simple isocratic reversed-phase high performance liquid chromatographic method was developed for determination of released desmopressin from chitosan nanoparticles in the in vitro media. The chromatographic separation was achieved with acetonitrile/water (25:75, v/v), in which water contained 0.1% v/v trifluoroacetic acid with pH=2.5 as mobile phase, a Chromolith® Performance RP-18e column (150×4.6 mm; 5 μm) kept at 40° and ultraviolet detection at 220 nm. The compound was eluted isocritically at a constant flow rate of 1.6 ml/min. The method was validated according to the International Conference on Harmonisation guidelines. The validation characteristics included accuracy, precision, linearity rang, selectivity, limit of detection, limit of quantitation and robustness. The calibration curve was linear ( r>0.9999) over the concentration rang 0.5-100 μg/ml. The limit of detection and limit of quantitation in the release media were 0.05 and 0.5 μg/ml, respectively. The proposed method had an accuracy of and intra- and inter-day precision <4.2. Furthermore, to evaluate the performance of the proposed method, it was used in the analysis of desmopressin level in real samples containing chitosan nanoparticles in the in vitro media.
Keywords
Desmopressin, high−performance liquid chromatography, in vitro, method validation, nanoparticle
Desmopressin (1−deamino−8−D−arginine vasopressin; [DDAVP]) is a synthetic nine−amino acid cyclic peptide analogue of vasopressin [1,2]. It is useful for treating bleeding disorders, such as mild forms of haemophilia A, von Willebrand’s disease, polyuria related to central diabetes insipidus and nocturnal enuresis especially in young children [3,4]. Currently, DDAVP is administered orally in tablet formulation (Minirin, Ferring Pharmaceuticals), but its bioavailability is lower than 1% [5]. Several techniques including viscous formulation [6], metered−dose nasal spray instead of nose drops [7], lipophilic pro−drug formulation [8] as well as bio−adhesive [9], liposome [10] and transdermal delivery systems [11] have been employed to enhance desmopressin nasal absorption. Recently, nanoparticulate delivery systems were proposed to have the potential to improve drug stability, to increase the duration of the therapeutic effect and to allow administration through nonparenteral routes [12]. In 2010, Taghizadeh and Safaei have been active in design and development of chitosan nanoparticles for an oral drug delivery system to increase the efficiency and bioavailability of oral drugs [13].
Also, many analytical techniques including high−performance liquid chromatography (HPLC) [14,15], liquid chromatography/mass spectrometry [16,17] and capillary electrophoresis [18] have been used for assaying desmopressin in drug delivery systems. Among these methods, the chromatographic procedure has been repeatedly cited in the United States pharmacopeia (USP) and British Pharmacopeia as an officially validated method using DDAVP chronic rhinosinusitis (CRS). However, most of these methods result in long retention times and use buffers with high concentration salt as mobile phase.
In this study, a simple, fast, accurate and precise reversed−phase HPLC (RP-HPLC) method for DDAVP assay in chitosan nanoparticles in the release media (i.e., phosphate buffer with pH=7.4 and ionic strength=0.03) was evaluated. Evaluation of sensitivity, precision and accuracy of the method yielded best results. Furthermore, this method was very friendly with the type of release media and was tested in an in vitro environment.
Desmopressin (DDAVP) was purchased from Macfarlan Smith (Edinburgh, UK). HPLC−grade acetonitrile, potassium hydrogen phosphate and trifluoroacetic acid (TFA) obtained from Romil chemicals (Loughborough, UK). Acetic acid, paraffin and span 80 were obtained from Merck Company (Darmstadt, Germany) and glutaraldehyde from Aldrich Chemical Company (USA). Chitosan (M: 780,000 DD: 61.5) was obtained from Fluka Company (Switzerland) and modified as a required DD: 75 (3). HPLC−grade water was produced locally using a Milli−Q system from Millipore (Billerica, MA, USA).
Briefly, in order to prepare the chitosan nanoparticle drug delivery system, the aqueous phase containing 0.045 g chitosan and 0.5 mg DDAVP dissolved into 4.5 ml acetic acid 1% v/v dropped into water−oil (w/o) emulsion. The oil phase contained 29.6 ml paraffin and 0.4 ml span 80 with 0.08 ml glutaraldehyde 25% v/v as the cross−linker to provide the stability of emulsion. The mixing rate was modified to 2000 rpm to get smaller nanoparticles at the 40° temperature during the emulsification. The resulted emulsion was centrifuged at high speed and washed twice with petroleum ether and dried in room temperature. Another sample consisting of 1 g of the polymeric carrier formulation containing no DDAVP in 40 ml of the release medium was also prepared for spiking and as the negative control sample.
To study the in vitro drug release, the chitosan nanoparticles (0.006 g) were put into dark glasses with a capacity of 20 ml, containing 10 ml phosphate buffer (pH=7.4, ionic strength=0.03). The glasses were shaken horizontally and constantly at the temperature of 37° by controlled shaker at 100 rpm. At specified time intervals up to 48 h, samples (3 ml) were withdrawn and replaced with fresh medium. The resulting solution was filtered using 0.45 μm polytetrafluoroethylene filter into standard analytical glass vials and injected in to the HPLC. Three such samples were prepared (0.006 g) according the USP criteria and injected triplicate.
Analysis of standards and real samples were performed on a Younglin SP930D low pressure gradient pump, ultraviolet (UV 730D) dual wavelength UV detector, CTS30 column oven controlled by Autochro™ 2000 software (Kyungki−do, Korea), a Rheodyne 7725i (PerkinElmer, USA) injector, along with a 20 μl sample loop. Chromolith® Performance RP-18e column (150×4.6 mm; 5 μm) was employed for all separations. The mobile phase was a mixture of 0.1% TFA (pH=2.5) in water and acetonitrile (75/25) at a flow rate of 1.6 ml/min in isocratic elution mode. The injection volume was 20 μl for all samples. The detection was performed at the wavelength of 220 nm. Refrigerated centrifuge model 2−16 KC (Sigma, Germany) was employed for phase separation.
A standard stock solution of DDAVP (1000 μg/ml) was prepared in phosphate buffer (pH=7.4). Calibration standards at seven levels by further diluted stock standard solution in the concentration rang of 0.5−100 μg/ml. Similarly, quality control (QC) standard solutions were prepared daily by diluting the stock standard solution for the final QC concentration of 5, 50 and 100 μg/ml. All of these solutions were stored in a fridge (4°) and brought to ambient temperature just prior to use. Samples in triplicates were made for each concentration and peak areas were plotted against the corresponding concentration to obtain the calibration graphs.
The HPLC method was evaluated according to the International Conference on Harmonisation (ICH) guideline [19]. The following validation characteristics were addressed: linearity, accuracy, precision and specificity, limit of detection (LOD) and limit of quantification (LOQ) and robustness. System suitability standard solution, which contained 100 μg/ ml DDAVP. System suitability was determined from six replicate injections of the system suitability standard before sample analysis. The acceptance criteria for DDAVP were <2% relative standard deviation (RSD) and a signal−to−noise ratio of at least ten for the corresponding peak area.
Standard calibration curve was prepared with seven calibrators over a concentration rang of 0.5−100 μg/ml for DDAVP. The data of peak area versus concentration was treated by linear least square regression analysis. The standard curve was evaluated for intra−day and inter−day linearity. To study the reliability and suitability of the developed method, recovery experiments were carried out. Placebo samples were spiked with different amount of DDAVP at 50, 100 and 150% in duplicate for each one (n=6) over the theoretical values. Measured values were compared with the theoretical concentration. The percentage RSD of individual measurements was also determined and <5% was set as the limit for acceptance.
The precision of the developed method was assessed in terms of repeatability and intermediate precision by analysing three replicate QC standard samples at 50, 100 and 150% levels that cover the calibration rang for DDAVP. The % RSD values of the results corresponding to the peak area and retention time were expressed for intra−day precision and on 3 days for intermediate (inter−day) precision. The LOD and LOQ for the procedure were performed on samples containing very low concentration of analytes under the ICH guidelines. By applying the visual evaluation method, LOD was expressed by establishing the minimum level at which the analyte can be reliably detected. LOQ was considered as the lowest concentration of the analytes in standards that can be reproducibly measured with acceptable accuracy and precision (RSD<2%). The robustness of the method was evaluated by analysing the system suitability standards and evaluating system suitability parameter data after varying, individually, the HPLC pump flow rate (±10%), organic solvent content (±6%) and column compartment temperature (±14%).
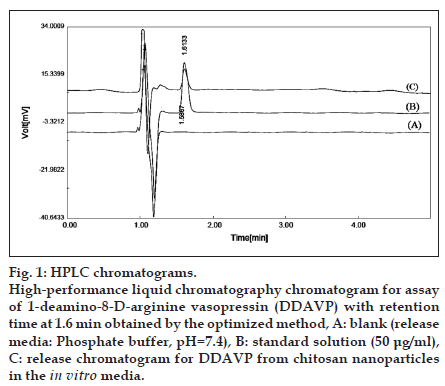
First, HPLC method was investigated for DDAVP assay in the release media. Detection wavelength was optimized at wavelength of 220 nm according to UV spectrum of the analyte in the presence of the release media (aqueous phosphate buffer with pH=7.4 and ionic strength of 0.03). In order to establish optimized HPLC conditions for DDAVP assay in the real samples and DDAVP CRS, systematic variations of the effective parameters comprising mobile phase composition, such as aqueous pH, ionic strength, ratio of aqueous to organic mobile phase constituents, flow rate and column packing were investigated. Based on the results, the best chromatographic conditions considering total run time, retention time, solvent elution time and peak shape (i.e., symmetry and analytical power) were fixed at a flow rate of 1.6 ml/min and mobile phase containing of acetonitrile/water (25:75, v/v), in which water contained 0.1% (v/v) TFA with pH=2.5. Separation was carried out on the Chromolith® Performance RP-18e (150×4.6 mm; particle size: 5 μm) at 40°. As previously described, the detection wavelength was set at 220 nm. The optimized method was running in all stages of method validation, including laboratories experiments. Chromatograms obtained from the analysis of blank, standard and real samples are demonstrated in fig. 1.
Fig. 1:HPLC chromatograms. High−performance liquid chromatography chromatogram for assay of 1−deamino−8−D−arginine vasopressin (DDAVP) with retention time at 1.6 min obtained by the optimized method, A: blank (release media: Phosphate buffer, pH=7.4), B: standard solution (50 μg/ml), C: release chromatogram for DDAVP from chitosan nanoparticles in the in vitro media.
When a method has been optimized it must be validated before practical use. By following the ICH guidelines for analytical method validation, Q2 (R1), the system suitability testing (SST) was performed and the validation characteristics were addressed. The system suitability test ensures the validity of the analytical procedure. All critical parameters tested met the acceptance criteria on all days. According to the monograph, analyte is eluted by forming symmetrical single peak well−separated from the solvent front (Table 1). For the construction of calibration curves, seven calibration standard solutions were prepared over the concentration rang of 0.5−00 μg/ml. The results, summarised in Table 2, show a good correlation between analyse peak and concentration with r>0.9999 (n=7).
| Parameter (units) | DDAVP |
|---|---|
| Theoretical plates | 8668 |
| Asymmetry (As) | 1.1 |
| Repeatability, tR (% RSD) | 1.07 |
| Repeatability, A (% RSD) | 0.6 |
| Precision (% RSD) | 3.1 |
| Accuracy (% RSD) | 4.1 |
| Accuracy (% recovery) | 102.8 |
| Selectivity | No interference |
| LOD (µg/ml) | 0.05 |
| LOQ (µg/ml) | 0.5 |
RSD=relative standard deviation, DDAVP=1−deamino−8−D−arginine vasopressin, LOD=limit of detection, LOQ=limit of quantification
Table 1: Method validation results for the studied compound
| Parameters (units) | DDAVP |
| Linearity rang (µg/ml) | 0.5−100 |
| Slope | 9.74582±0.26 |
| Intercept | 0.91304±1.52 |
| Correlation coefficient (r) | 0.9999±0.0002 |
| Residual sum of squares | 0.00033 |
DDAVP=1−deamino−8−D−arginine vasopressin, values are reported as mean±SD of tree calibration curves generated on three consecutive days (n=3), seven concentrations in the linearity rang were evenly distributed
Table 2: Linearity parameters for the estimation of DDAVP
Accuracy and precision were established across the analytical range for DDAVP. The intra− and inter−day accuracy and precision were calculated from the QC samples (Table 1). Repeatability (intra−day precision) of the analytical method was found to be reliable based on % RSD (<2%) corresponding to the peak areas and retention times. Intermediate precision (inter−day accuracy) was demonstrated on different days and evaluating the peak area data at three QC standards that cover the assay method range. The % RSD values were <5% and illustrated the good precision for the analytical method. For determining accuracy, placebo solutions spiked with reference standards were used. The recovery was 100±5% for all samples with % RSD <5%.
Specificity is the ability to assess the analyte unequivocally in the presence of components expected to be present. Typically, these components might include impurities, degradants and matrix. Therefore, only one peak was observed and specificity of the method was one. The LOD and LOQ values were found to be 0.05 and 0.5 μg/ml.
To ensure to insensitivity of the developed HPLC method to minor changes in the experimental conditions, it is important to demonstrate its robustness. None of the alterations caused a significant change in peak area RSD, USP tailing factor and theoretical plates (Table 3). Although the changes in retention times were more significant, separation was sufficient and quantitation was still possible.
| Parameter altered | Retention time, | Asymmetry |
|---|---|---|
| tR (min) | (As) | |
| Optimised chromatographic conditionsa | 1.46 | 1.1 |
| Increased organic solvents (26:74) | 1.44 | 1.09 |
| Decreased organic solvents (24:76) | 1.51 | 1.2 |
| Increased flow rate (1.7 ml/min) | 1.38 | 1.15 |
| Decreased flow rate (1.4 ml/min) | 1.57 | 1.05 |
| Decreased−column temperature (35°) | 1.49 | 1.03 |
a Chromatographic conditions were acetonitrile/water (25:75 v/v), in which water contained 0.1% (v/v) trifluoroacetic acid with pH=2.5, maintained with flow rate 1.6 ml/min, temperature at 40°and detection at 220 nm
Table 3: Method validation data for robustness study
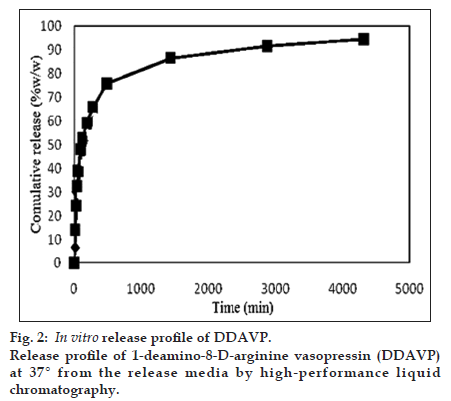
The efficiency of the presented method was evaluated by determining the desmopressin concentration from chitosan nanoparticles in the release media (phosphate buffer, pH=7.4). The results of three−replicate analysis of each sample were obtained using the proposed method. The release curve is depicted in fig. 2.
In this study, we have evaluated a simple, sensitive, specific and rapid HPLC method that it is very friendly with the type of release media. This method exhibited best sensitivity, accuracy and precision for inter− and intra−day analysis. Finally, the proposed method is recommended as the routine analysis technique for desmopressin assay in in vitro media.
References
- Cvetković RS, Plosker GL. Desmopressin: In adults with nocturia. Drugs 2005;65:99−107.
- Zaoral M. DDAVP (Desmopressin) and solid phase peptide synthesis. Biopolymers 2008;90:213.
- Nichols R, Hohenhaus AE. Use of the vasopressin analogue desmopressin for polyuria and bleeding disorders. J Am Vet Med Assoc 1994;205:168−73.
- Vilhardt H. Basic pharmacology of desmopressin. Drug. Invest 1990;2:2−8.
- Vilhardt H, Lundin S. Biological effect and plasma concentrations of DDAVP after intranasal and peroral administration to humans. Gen Pharmacol 1986;17:481−3.
- Harris AS, Svensson E, Wagner ZG, Lethagen S, Nilsson IM. Effect of viscosity on particle size, deposition, and clearance of nasal delivery systems containing desmopressin. J Pharm Sci 1988;77:405−8.
- Harris AS, Nilsson IM, Wagner ZG, Alkner U. Intranasal administration of peptides: Nasal deposition, biological response, and absorption of desmopressin. J Pharm Sci 1986;75:1085−8.
- Kahns AH, Buur A, Bundgaard H. Prodrugs of peptides. 18. Synthesis and evaluation of various esters of desmopressin (dDAVP). Pharm Res 1993;10:68−74.
- Critchley H, Davis SS, Farraj NF, Illum L. Nasal absorption of desmopressin in rats and sheep. Effect of a bioadhesive microsphere delivery system. J Pharm Pharmacol 1994;46:651−6.
- Law SL, Huang KJ, Chou HY. Preparation of desmopressin−containing liposomes for intranasal delivery. J Control Release 2001;70:375−82.
- Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedlearray patch system. J Control Release 2004;97:503−11.
- Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res 2007;24:2198−206.
- Taghizadeh SM, Safaei R. Preparation and investigation of chitosan nanoparticles including salicylic acid as a model for an oral drug delivery system. E−Polymers 2010;036:1−7.
- Dudkiewicz−Wilczyńska J, Snycerski A, Tautt J. Determination of the content of desmopressin in pharmaceutical preparations by HPLC and validation of the method. Acta Pol Pharm 2002;59:163−8.
- Jiskra J, Pacakova V, Ticha M, Stulik K, Barth T. Use of capillary electrophoresis and high−performance liquid chromatography for monitoring of glycosylation of the peptides dalargin and desmopressin. J Chromatogr 1997;761:285−96.
- Getie M, Neubert RH. LC−MS determination of desmopressin acetate in human skin samples. J Pharm Biomed Anal 2004;35:921−7.
- Schmitz T, Huck CW, Bernkop−Schnürch A. Characterisation of the thiol−disulphide chemistry of desmopressin by LC, mu−LC, LC−ESI−MS and Maldi−Tof. Amino Acids 2006;30:35−42.
- Qin XZ, Ip DP, Chang KH, Dradransky PM, Brooks MA, Sakuma T. Pharmaceutical application of LC−MS. 1 – Characterization of a famotidine degradate in a package screening study by LC−APCI MS. J Pharm Biomed Anal 1994;12:221−33.
- ICH guidelines topic Q2 (R1) validation of analytical procedures: Methodology, obtained. Available from: http://www.emea.europa.eu/ htms/human/ich/ichquality.htm. [last accessed on 24 February 2012]