- *Corresponding Author:
- R. M. Singh
Research and Development Division, Indian Pharmacopoeia Commission, Sector-23, Rajnagar, Ghaziabad-201 002,India
E-mail: raman19662002@yahoo.co.in
| Date of Submission | 9 August 2010 |
| Date of Revision | 4 August 2011 |
| Date of Acceptance | 8 September 2011 |
| Indian J Pharm Sci, 2011, 73 (5): 577-579 |
Abstract
A simple, precise, rapid, accurate and economic reverse phase high performance liquid chromatographic method has been developed for the estimation of prulifloxacin in tablet dosage form. The separation was achieved by using octadecylsilane column (C18) and KH2PO4 buffer: acetonitrile adjusted to pH 7.3 with triethyl amine in proportion of 10:90 v/v as mobile phase, at a flow rate of 1.0 ml/min. The detection was carried out at 278 nm. The retention time of prulifloxacin was found to be 2.4 min. The limit of detection and limit of quantitation were found to be 0.14 μg/ml and 0.42 μg/ml respectively. The accuracy and reliability of the proposed method was ascertained by evaluating various validation parameters like linearity, precision, accuracy and specificity according to ICH guidelines. The proposed method provides an accurate and precise quality control tool for routine analysis of prulifloxacin in tablet dosage form.
Keywords
HPLC, method development and validation, prulifloxacin
Prulifloxacin is the lipophilic prodrug of ulifloxacin [1], is an oral fluoroquinolons with a broad spectrum in vitro activity against various Gram-positive and Gram-negative microorganisms. It inhibit Topoisomerase IV and used to treat simple cystitis, acute exacerbation of chronic bronchitis, and complicated lower urinary tract infection in children and adults [2-3]. Prulifloxacin is chemically known as 6-fluoro-1-methyl-7-{4-[(5-methyl-2-oxo-1,3- dioxol-4-yl)methyl] piperazin-1-yl}-4-oxo-4H- [1,3] thiazeto[3,2-.alpha] quinoline-3-carboxylic acid [4].
It is inhibitor of Topoisomerase IV which produce nicking and negative supercoiling in DNA segment. Literature survey reveals that very few methods have been reported for the analysis of prulifloxacin based on electrochemical [5], spectroscopic [6-7] mass spectroscopy [8] and other [9-11]. The present study illustrates development and validation of a simple, accurate and precise procedure for determination of prulifloxacin by RP-HPLC in tablet dosage form.
Prulifloxacin working standard and tablets (600 mg) were obtained from Macleods Laboratories, Mumbai, India. All chemicals and reagents used were of HPLC grade and purchased from Merck Specialities Pvt. Ltd and S. D. Fine Chemicals, Mumbai, India.
Waters HPLC system containing 600 controller pump, 717 plus auto-sampler, inline degasser, 486 tunable absorbance detector and temperature control device with operating Millennium32 software were used in the study. A Sunfire C18 column (250×4.6 mm, 5 μm particle size) was used as stationary phase. The mobile phase was optimized as KH2PO4 buffer: acetonitrile adjusted to pH 7.3 with triethyl amine in proportion of 10:90, v/v as mobile phase and detection was carried out at 278 nm, at a flow rate of 1.0 ml/min.
About 50 mg of prulifloxacin standard was weighed accurately and transferred to 100 ml volumetric flask. The volume was made up to mark with the mobile phase to obtain a concentration of 500 μg/ml. Further dilutions were made to obtain the concentration in the range of 40-120 μg/ml of prulifloxacin. For analysis in tablet dosage form, twenty tablets were weighed. The tablets were finely powdered and powder equivalent to 10 mg of prulifloxacin was accurately weighed and transferred into a 100 ml volumetric flask. The volume was made up to mark with the mobile phase to obtain a concentration of 100 μg/ml and filtered through 0.45 μm membrane filter.
The system suitability was checked by injecting 20 ml of standard solution results were founded within the range. The relative standard deviation in five replicate injections were obtained 0.5%, and tailing factor was found to be 1.62. Twenty microlitre of standard and sample solutions were separately injected on HPLC system. From the peak area of prulifloxacin the amount of drugs in the sample were computed. The analysis was repeated in triplicate. The % assay of the tablet was found to be 102.11%.
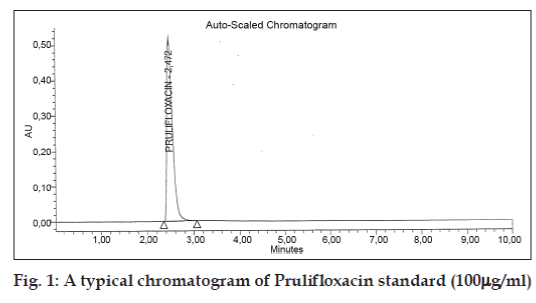
The developed method was validated in terms of specificity, linearity, accuracy, limit of detection, limit of quantification, intra-day, inter-day precision and robustness for the assay of prulifloxacin as per ICH guidelines [12]. Specificity was studied for the examination of the presence of interfering components. Prulifloxacin standard solution of 100 mg/ml was injected and no impurity was detected in its assay. The retention time obtained for Prulifloxacin by HPLC is about 2.4 min as shown in (fig. 1).
Linearity was studied by preparing standard solutions of prulifloxacin at different concentration levels. The linearity ranges were found in the range of 40-120 mg/ml. The standard calibration curve was generated using regression analysis with Microsoft excel. The assay was judged to be linear as the correlation coefficient was greater than 0.995 by the least-square method as shown in (Table 1).
| Parameter | Prulifloxacin |
|---|---|
| Linearity range (µg/ml) | 40-120 |
| Correlation coefficient (r) | 0.999 |
| Regression equation (y=mx+c) | 119467 |
| Slope (m) | |
| Intercept (c) | 351138 |
| Limit of detection (LOD) (µg/ml) | 0.1404 |
| Limit of quantification (LOQ) (µg/ml) | 0.4255 |
Table 1: Validation Parameters of The Proposed Method
Recovery studies of the drug were carried out for the accuracy parameter at three different concentration levels i.e. multiple level recovery studies. A known amount of prulifloxacin standard was added into preanalysed sample and subjected them to the proposed HPLC method. The % recovery was found to be within the limits as listed in (Table 2).
| Label claim mg/tablet | Total amount added (mg) | Amount Recovered* (mg) | %Recovery |
|---|---|---|---|
| Prulifloxacin | 8.0 | 8.1±0.18 | 101.25 |
| 600 | 10.00 | 9.9±0.21 | 99.0 |
| 12.00 | 11.9±0.12 | 99.16 |
*Each value is a mean± deviation of three determinations.
Table 2: Recovery Studies of Prulifloxacinin Tablet
Precision was studied to find out intra and inter day variations in the test methods of prulifloxacin in the concentration range of 40-80 mg/ml for three times on the same day and different day. Precision was determined by analysing corresponding standard daily for a period of three days. The intra-day and inter-day precision obtained was % RSD (< 2) indicates that the proposed method is quite precise and reproducible as shown in (Table 3).
| Concentration (µg/ml) | Intraday precision | Interday precision | ||
|---|---|---|---|---|
| Mean | % | Mean | % | |
| area | R.S.D | area | R.S.D | |
| 40 | 4473357 | 0.88 | 4334389.3 | 1.03 |
| 60 | 6796148 | 0.92 | 66798412 | 0.92 |
| 80 | 8878567 | 0.95 | 9108180 | 1.02 |
Table 3: Result from Intraday and Interday Pricision of Prulifloxacin
The limit of detection (LOD) and limit of quantitation (LOQ) was calculated based on the standard deviation (SD) of the response and the slope (S) of the calibration curve at levels approximating the LOD and LOQ, LOD = 3.3 (SD/S) and LOQ = 10 (SD/S) is shown in (Table 1). Robustness was done by small changes in the chromatographic conditions like mobile phase, flow rate, temperature, mobile phase composition etc. and found to be unaffected.
A sensitive accurate, fast and precise isocratic reverse phase high performance liquid chromatographic method has been developed for the determination of prulifloxacin in bulk and in tablet dosage form. The developed method was found to be simple and have short run time which makes the method rapid. Statistical analysis proves that method is repeatable and selective for the analysis of prulifloxacin. This method may be employed for the quality control analysis the advantages are low cost of reagents, speed and simplicity of sample treatment.
References
- Giannarini G, Tascini C, Selli C. Clinical studies of a broad-spectrum quinolone agent. Future Microbiol 2009;4:13-24.
- Cai T, Mazzoli S, Nesi G, Boddi V, Mondaini N, Bartoletti R. 14-day prulifloxacin treatment of acute uncomplicated cystitis in women with recurrent urinary tract infections: a prospective, open-label, pilot trial with 6-month follow-up. J Chemother 2009;21:535-41.
- Blasi F, Aliberti S, Tarsia P, Santus P, Centanni S, Allegra L. Prulifloxacin: A brief review of its potential in the treatment of acute exacerbation of chronic bronchitis. Int J Chron Obstruct Pulmon Dis. 2007;2:27-31.
- Available from; http://www.faqs.org/patents/app/20100113783. [Last accessed on 2010 July 05].
- Yang Z , Wang X, Qin W, Zhao H. Capillary electrophoresis-chemiluminescence determination of norfloxacin and prulifloxacin. Anal ChimActa 2008;623:231-7.
- Huang YB, Wang SJ, Zi YQ, Yu Z, Gao XY, Tang YC, et al. Investigation on the interaction of prulifloxacin with human serum albumin: A spectroscopic analysis. Chem Pharm Bull 2010;58:582-6
- Huang Y, Yan J, Liu B, Yu Z, Gao X, Tang Y, et al. Investigation on interaction of prulifloxacin with pepsin: A spectroscopic analysis.SpectrochimActa A 2010;73:1024-9.
- Guo L, Qi M, Jin X, Wang P, Zhao H. Determination of the active metabolite of prulifloxacin in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B AnalytTechnol Biomed Life Sci 2006;832:280-5.
- Yu F, Liu W, Chen F, Cai P. Highly sensitive spectrofluorimetric determination of trace amounts of prulifloxacin using the aluminium(III)-prulifloxacin system. Luminescence 2008;23:429-33.
- Wang X, Shen B, Zhao H, Jin L. Determination of ulifloxacin by terbium-sensitized fluorescence with second-order scattering and its applications. Anal Sci 2007;23:1373-6.
- Zhang L, Wen J, Pan Y, Li Z, Fan G, Fan G, et al. Determination of ulifloxacin, the active metabolite of prulifloxacin, in human plasma by a 96-well format solid- phase extraction and capillary zone electrophoresis. J Chromatogr B AnalytTechnol Biomed Life Sci 2008;872:172-6.
- International Conference on Harmonisation, Guidance for Industry In: Q2B Validation on Analytical Procedures: Methodology. Switzerland: IFPMA;1996. p.1-8